Abstract
The adoptive transfer of donor T cells that recognize recipient minor histocompatibility antigens (mHAgs) is a potential strategy for preventing or treating leukemic relapse after allogeneic hematopoietic cell transplantation (HCT). A total of 7 patients with recurrent leukemia after major histocompatibility complex (MHC)–matched allogeneic HCT were treated with infusions of donor-derived, ex vivo–expanded CD8+ cytotoxic T lymphocyte (CTL) clones specific for tissue-restricted recipient mHAgs. The safety of T-cell therapy, in vivo persistence of transferred CTLs, and disease response were assessed. Molecular characterization of the mHAgs recognized by CTL clones administered to 3 patients was performed to provide insight into the antileukemic activity and safety of T-cell therapy. Pulmonary toxicity of CTL infusion was seen in 3 patients, was severe in 1 patient, and correlated with the level of expression of the mHAg-encoding genes in lung tissue. Adoptively transferred CTLs persisted in the blood up to 21 days after infusion, and 5 patients achieved complete but transient remissions after therapy. The results of these studies illustrate the potential to selectively enhance graft-versus-leukemia activity by the adoptive transfer of mHAg-specific T-cell clones and the challenges for the broad application of this approach in allogeneic HCT. This study has been registered at http://clinicaltrials.gov as NCT00107354.
Introduction
The elimination of leukemia after allogeneic hematopoietic cell transplantation (HCT) results in part from a “graft-versus-leukemia” (GVL) effect mediated by lymphocytes contained in or derived from the donor hematopoietic cell graft.1 Clinical observations suggest that the GVL effect is associated with graft-versus-host disease (GVHD), but GVHD is neither necessary nor sufficient for GVL activity.2,3 Despite the potency of the GVL effect, allogeneic HCT ultimately fails in a significant fraction of patients with acute leukemia due to recurrence of the malignancy. Administration of unselected donor lymphocytes to treat relapse after HCT is effective in a minority of patients with acute leukemia, but causes significant morbidity due to GVHD and rarely produces durable remission of disease.4-6 Thus, strategies for enhancing the GVL effect without GVHD are urgently required.
In transplantations where the donor and recipient are matched at the major histocompatibility complex (MHC), minor histocompatibility antigens (mHAgs) encoded by polymorphic genes and presented as peptides bound to MHC molecules on recipient cells are recognized by donor T cells.7-9 A subset of mHAgs are expressed on recipient hematopoietic cells, including leukemic stem cells, but not on tissues affected by GVHD.10 Adoptive transfer of ex vivo–expanded T cells specific for such tissue-restricted mHAgs is effective for treating leukemia in murine models,11 and has been proposed as a strategy to selectively enhance the GVL effect in human HCT.12,13 Ideally, this would be accomplished by the infusion of donor T-cell clones that recognize mHAgs that have been molecularly characterized and for which rigorous analysis of gene expression in tissues has been performed. However, at the present time, few patients would be eligible for T-cell therapy because only a small number of mHAgs have been molecularly defined, and treatment could only be given to antigen-positive patients who have an antigen-negative donor and express the appropriate MHC-restricting allele.14 Previous work in our laboratory has demonstrated that CD8+ cytotoxic T-lymphocyte (CTL) clones specific for mHAgs that are preferentially expressed on hematopoietic cells can be isolated from most patients after myeloablative allogeneic HCT from an MHC-matched, related donor.15 Here, we report the results of a phase 1 clinical trial in which we evaluated the safety of adoptively transferring donor-derived CD8+ CTL clones recognizing mHAgs expressed in recipient hematopoietic cells but not recipient dermal fibroblasts to patients with recurring acute leukemia after myeloablative allogeneic HCT. Molecular characterization of the mHAgs recognized by the CD8+ CTL clones administered to 3 patients in this study who experienced antileukemic activity and/or toxicity was performed to provide insight into the mechanism of the antitumor activity or toxicity, respectively.
Methods
Patient selection and clinical study design
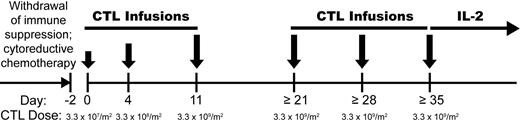
Patients undergoing HCT from a MHC-matched related donor for advanced myelodysplastic syndrome or acute leukemia beyond first remission were eligible for this study (www.clinicaltrials.gov registration: NCT00107354), which was approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center and the U.S. Food and Drug Administration (FDA). All patients and donors provided written informed consent before HCT in accordance with the Declaration of Helsinki. Patients for whom CD8+ mHAg-specific CTL clones meeting selection criteria for use in adoptive therapy were successfully generated became eligible for T-cell therapy upon documentation of posttransplantation relapse. Before administering T cells, an attempt was made to withdraw or reduce immunosuppressive drugs in patients being treated for GVHD. Patients then received cytoreductive chemotherapy, followed by a series of 3 infusions of mHAg-specific CTLs administered at an escalating target dose (3.3 × 107/m2 → 3.3 × 108/m2 → 3.3 × 109/m2) over 11 days (Figure 1). Patients not developing serious toxicity or GVHD were eligible for up to 3 additional infusions at weekly intervals of mHAg-specific CTLs at the target dose of 3.3 × 109/m2, followed by a 14-day course of recombinant interleukin-2 (aldesleukin; IL-2) given subcutaneously at 2.5 × 105 IU/m2 per day. Patients with persistent leukemia after 6 infusions of mHAg-specific CTLs were eligible for additional infusions of the same CTL clone, or of a different CD8+ CTL clone recognizing a distinct mHAg, if available. Patients were evaluated for toxicity, GVHD, and in vivo persistence and migration of adoptively transferred CTLs.
Treatment schema. Patients who relapsed after MHC-matched allogeneic HCT and elected to receive chemotherapy and T-cell therapy first underwent withdrawal of immunosuppression, followed sequentially by appropriate salvage chemotherapy, adoptive T-cell therapy with CD8+ mHAg-specific CTL clones, and a 14-day course of low-dose IL-2.
Treatment schema. Patients who relapsed after MHC-matched allogeneic HCT and elected to receive chemotherapy and T-cell therapy first underwent withdrawal of immunosuppression, followed sequentially by appropriate salvage chemotherapy, adoptive T-cell therapy with CD8+ mHAg-specific CTL clones, and a 14-day course of low-dose IL-2.
Generation, selection, and expansion of mHAg-specific CTL clones
Skin biopsies were obtained from the donor and recipient before transplantation, and blood samples were obtained before and after transplantation. Donor-derived CD8+ CTL clones specific for recipient mHAgs were isolated as previously described.15 Briefly, recipient posttransplantation peripheral blood mononuclear cells (PBMCs) were stimulated 3 times with irradiated recipient pretransplantation PBMCs in RPMI 1640 medium supplemented with 2mM l-glutamine, 25mM HEPES buffer, 1% penicillin/streptomycin, 50μM 2-mercaptoethanol, and 10% heat-inactivated human AB serum (termed CTL medium) in the presence of 5 IU/mL IL-2, then restimulated with irradiated recipient-derived Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines (EBV-LCL) with addition of 5 IU/mL IL-2. The T-cell lines were depleted of CD4+ cells and cloned by limiting dilution, and clones with cytolytic activity against recipient but not donor EBV-LCL were expanded and characterized by flow cytometry and cytotoxicity assays. CD3+/CD4−/CD8+ CTL clones with cytolytic activity against recipient EBV-LCL (specific lysis > 30% at an effector-target ratio [E/T]) of 5:1) but not donor EBV-LCL (specific lysis < 5%) or recipient fibroblasts (specific lysis < 5%) in a 4-hour 51Cr-release assay were defined as specific for a tissue-restricted recipient mHAg (representative data in supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Class I MHC restriction of mHAg-specific CD8+ CTL clones was established as described.15 Up to 3 CTL clones per patient were tested for bacterial and fungal sterility, then cryopreserved as a cell bank for use in adoptive therapy at the time of relapse. The total duration of in vitro culture up to cryopreservation of T-cell clones was 10 to 12 weeks. For infusion, CTL clones were thawed and expanded ex vivo over 14 days in CTL medium with irradiated allogeneic PBMCs and EBV-LCL as feeder cells, 30 ng/mL anti-CD3 monoclonal antibody (OKT3), and 50 IU/mL IL-2, as described.16
Analysis of toxicity, GVHD, and disease response
The incidence and severity of toxicity during and after treatment with cytoreductive chemotherapy, T-cell infusions, and IL-2 were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) Version 3.0. Laboratory evaluations included a complete blood count with differential and a metabolic panel with electrolytes, renal, and liver function testing. GVHD was assessed immediately before and 1 day after each T-cell infusion and at least twice weekly while receiving T-cell therapy, and graded as described.17 Disease response was assessed by monitoring complete blood counts at intervals before and after each T-cell infusion, and by bone marrow exams at intervals before, during, and after the completion of T-cell therapy and whenever clinically indicated. Bone marrow specimens were evaluated by morphology, flow cytometry, and cytogenetics. Complete morphologic remission was defined as the absence of blasts in the peripheral blood and less than 5% blasts in the marrow, flow cytometric remission as the absence of blasts in the marrow with the immunophenotype of the patient's previously documented leukemia, and cytogenetic remission as the absence of clonal (present in 2 or more metaphases) chromosomal abnormalities in a minimum of 20 metaphases examined.
Monitoring persistence and migration of adoptively transferred CTLs
The uniquely rearranged V-D-J region of the T-cell receptor (TCR) β gene of each CTL clone used in adoptive therapy was sequenced, and oligonucleotide primer pairs that specifically amplified each TCRβ were designed. In vivo persistence and migration of adoptively transferred CTLs were assessed with quantitative real-time polymerase chain reaction (PCR) on first-strand cDNA prepared from PBMCs, bone marrow mononuclear cells, or skin biopsy specimens, using the Stratagene Brilliant SYBR Green QPCR Core Reagent Kit and the ABI PRISM 7900HT Sequence Detection System. Standard curves were set up using 1:4 dilutions of cDNA made from CTLs, and dissociation curves were measured after amplification to determine the purity of the product amplified. All samples were normalized with respect to GAPDH expression determined simultaneously by PCR with GAPDH-specific primers.
Molecular characterization of mHAgs
A genome-wide, single nucleotide polymorphism (SNP)–based genotype-phenotype correlation approach18 was used to identify the genes encoding the mHAgs (and causal SNPs) recognized by clones 11C6-109 and 50F5-448 administered to Patient nos. 1 and 6, respectively. Genotyping of mHAg+ and mHAg− EBV-LCL at the causal SNPs was carried out by direct sequencing of genomic DNA. Identification of the antigenic peptides recognized by clones 11C6-109 and 50F5-448 was performed as previously described19 by testing them for recognition of COS-7 cells transiently cotransfected with panels of minigenes encoding candidate epitopes and cDNAs encoding the MHC class I–restricting molecules HLA-A*2902 or HLA-B*5701, respectively. Identification of the gene encoding the mHAg recognized by clone 68H7-331 administered to patient no. 3 has been previously described.20 Expression of mHAg-encoding genes in different tissues and cell types was determined by real-time PCR on commercially acquired cDNA (Clontech Multiple Tissue cDNA Human Panels I and II) or first-strand cDNA prepared from cultured cells or from commercially acquired RNA (human pulmonary alveolar epithelial cell RNA). The primer sequences for PCR analysis of mHAg-encoding gene expression are found in supplemental Table 1.
Flow cytometric analysis of mHAg-specific CTL clones and primary leukemic cells
CD8+ CTL clones were stained with PE- or APC-conjugated monoclonal antibodies (mAbs) specific for human CD3, CD4, CD8, CD45RO, CD45RA, CD27, CD28, CXCR4, CD62L, and CD127, TCR Vβ13, CCR7, and CLA. Leukemic cells obtained from patients treated with T-cell therapy were stained with mAbs for HLA-A, HLA-B, HLA-C, CD44, CD54 (ICAM-1), CD80, CD86, PD-L1, CD58 (LFA-3), and HLA-A29. Flow cytometric data were analyzed with FlowJo or CellQuest software.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm sections of human lung tissue using heat pretreatment (95°C for 60 minutes) in cell conditioner. Tissue sections were blocked for endogenous avidin/biotin and peroxidase with an endogenous biotin blocking kit and peroxidase inhibitor. Sections were treated with affinity-purified rabbit polyclonal antibody to human P2RX7 (1:100), followed by secondary antibody and detection with 3,3′-Diaminobenzidine (DAB) using Benchmark XT (Ventana). IHC pictures were taken with a Leica DM 3000 microscope with 10×/0.40 HC PL APO objective and a Leica DFC290 camera using Leica Application Suite (V3) image processing software.
Results
Characteristics of patients undergoing T-cell therapy
CD8+ CTL clones specific for recipient mHAgs expressed in hematopoietic cells but not in dermal fibroblasts and satisfying all criteria for use in adoptive therapy of relapse were generated from 25 patients who underwent HCT. A total of 13 of these 25 patients relapsed after transplantation, and 7 patients received mHAg-specific T-cell infusions (Table 1). The other 6 patients either elected not to return to Seattle for therapy (3 patients), or were ineligible for T-cell therapy due to active GVHD (3 patients). The 7 patients who received T-cell therapy relapsed at a median of 7 months after transplantation (range, 4-43 months); 6 relapsed in bone marrow, and 1 relapsed in the central nervous system (CNS) only (patient no. 4). All 7 patients had previously developed acute GVHD, 6 had developed clinical extensive chronic GVHD, and 6 were receiving immunosuppressive therapy at the time of relapse.
Identifying and transplant data for patients who underwent T-cell therapy
| Patient . | Dx . | Stage at HCT . | Age at HCT, y . | Conditioning regimen . | GVHD prophylaxis . | Acute GVHD . | Chronic GVHD . | Treatment before T-cell therapy . |
|---|---|---|---|---|---|---|---|---|
| 1 | B-ALL | Refractory | 42 | Cytoxan | Csp, MMF | Skin | Mouth | Vin, Pred, Mito, Ara-C |
| 13.2 Gy TBI | Gut | |||||||
| 2 | MDS | RAEB-T | 46 | Busulfan | Tac, Mtx | Skin | Skin | Ida, Ara-C |
| Cytoxan | ||||||||
| 3 | B-ALL | Remission | 34 | Cytoxan | Csp, Mtx | Skin | Ara-C | |
| 12 Gy TBI | Gut | |||||||
| 4 | B-ALL | Relapse | 41 | Cytoxan | Csp, Mtx | Skin | Eyes | Vin, IT, XRT |
| Mouth | ||||||||
| 12 Gy TBI | BOOP | |||||||
| 5 | B-ALL | Refractory | 20 | Cytoxan | Csp, Mtx | Gut | Eyes | Mtx, Asp, VP-16, Mito |
| 12 Gy TBI | Mouth | |||||||
| 6 | MDS | RAEB | 46 | Busulfan | Csp, Mtx | Skin | Skin | DLI; VP-16, Mito |
| Cytoxan | Gut | |||||||
| 7 | B-ALL | Remission | 34 | Cytoxan | Csp, Mtx | Gut | Skin | FLAG; Mito, Ara-C,VP-16; Dex, Vin, Asp |
| 12 Gy TBI | Mouth |
| Patient . | Dx . | Stage at HCT . | Age at HCT, y . | Conditioning regimen . | GVHD prophylaxis . | Acute GVHD . | Chronic GVHD . | Treatment before T-cell therapy . |
|---|---|---|---|---|---|---|---|---|
| 1 | B-ALL | Refractory | 42 | Cytoxan | Csp, MMF | Skin | Mouth | Vin, Pred, Mito, Ara-C |
| 13.2 Gy TBI | Gut | |||||||
| 2 | MDS | RAEB-T | 46 | Busulfan | Tac, Mtx | Skin | Skin | Ida, Ara-C |
| Cytoxan | ||||||||
| 3 | B-ALL | Remission | 34 | Cytoxan | Csp, Mtx | Skin | Ara-C | |
| 12 Gy TBI | Gut | |||||||
| 4 | B-ALL | Relapse | 41 | Cytoxan | Csp, Mtx | Skin | Eyes | Vin, IT, XRT |
| Mouth | ||||||||
| 12 Gy TBI | BOOP | |||||||
| 5 | B-ALL | Refractory | 20 | Cytoxan | Csp, Mtx | Gut | Eyes | Mtx, Asp, VP-16, Mito |
| 12 Gy TBI | Mouth | |||||||
| 6 | MDS | RAEB | 46 | Busulfan | Csp, Mtx | Skin | Skin | DLI; VP-16, Mito |
| Cytoxan | Gut | |||||||
| 7 | B-ALL | Remission | 34 | Cytoxan | Csp, Mtx | Gut | Skin | FLAG; Mito, Ara-C,VP-16; Dex, Vin, Asp |
| 12 Gy TBI | Mouth |
Identifying and transplant data for the 7 patients who underwent T-cell therapy for treatment of recurrent MDS or acute leukemia after MHC-matched allogeneic hematopoietic cell transplantation.
B-ALL indicates B-lineage acute lymphoblastic leukemia; MDS, myelodysplasia; RAEB(-T), refractory anemia with excess blasts (in transformation); TBI, total body irradiation; Csp, cyclosporine; MMF, mycophenolate mofetil; Tac, tacrolimus; Mtx, methotrexate; Vin, vincristine; Pred, prednisone; Mito, mitoxantrone; Ara-C, cytosine arabinoside; IT, intrathecal therapy; XRT, craniospinal radiation; Asp, asparaginase; FLAG, fludarabine, cytosine arabinoside, G-CSF; Dex, dexamethasone; and DLI, donor lymphocyte infusion.
Treatment and clinical course of patients after diagnosis of relapse
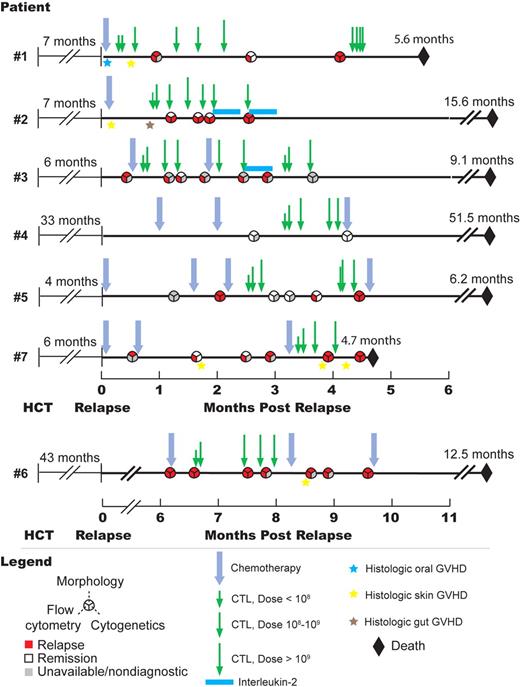
A total of 3 patients (nos. 1-3) underwent treatment with chemotherapy followed by T-cell therapy within 1 month of relapse (Figure 2). A total of 3 patients elected to receive either salvage chemotherapy (nos. 5 and 7) or polyclonal donor lymphocyte infusion (no. 6) as initial therapy for relapse, before being treated with T-cell therapy. One patient (no. 4) with isolated CNS relapse received intrathecal chemotherapy and craniospinal radiation (Table 1). A total of 46 infusions of 8 different CD8+ recipient mHAg-specific CTL clones were administered to the 7 patients, and each patient received at least 4 infusions (range, 4-10 infusions). All patients received at least one infusion with a cell dose greater than 2 × 109, and the highest dose administered to each patient ranged from 2.25 × 109 to 6.6 × 109 cells. A total of 2 patients received subcutaneous IL-2 after T-cell therapy (Figure 2).
Clinical course of the 7 patients who underwent T-cell therapy. Timelines showing chronology of postrelapse treatment, disease status, histologic diagnoses of GVHD, and survival of all 7 patients who were treated for posttransplantation relapse with CD8+ mHAg-specific T-cell therapy.
Clinical course of the 7 patients who underwent T-cell therapy. Timelines showing chronology of postrelapse treatment, disease status, histologic diagnoses of GVHD, and survival of all 7 patients who were treated for posttransplantation relapse with CD8+ mHAg-specific T-cell therapy.
Treatment-related toxicity
All 6 patients receiving multiagent intravenous chemotherapy before T-cell therapy developed grade 3 or 4 neutropenia, thrombocytopenia, or both, and 2 patients developed grade 3 to 4 mucositis. Fever (grades 1-3) and chills in the 24-hour period after each infusion were the most common acute toxicities of CTL infusion, and occurred frequently in all patients after administration of cell doses greater than 1 × 109.
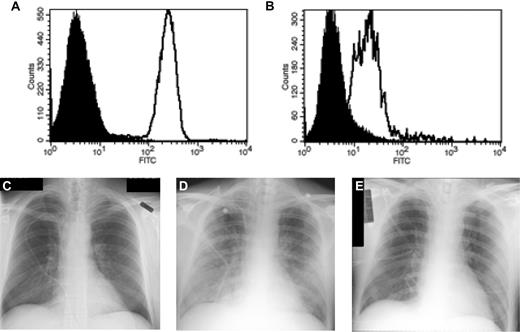
No grade 3 or 4 acute nonhematologic toxicities were observed after infusions of less than 1 × 109 cells. However, 3 patients developed grade 3 or 4 pulmonary toxicity, interpreted as secondary to T-cell therapy, during or within 2 hours of CTL infusions at doses greater than 1 × 109 cells. Patient no. 5 developed transient dyspnea, hypoxemia, and bilateral pulmonary infiltrates while receiving a platelet transfusion administered immediately after a CTL infusion of 6.2 × 109 cells. The symptoms promptly resolved with administration of 2 mg/kg methylprednisolone for 1 day followed by a rapid taper. Patient no. 6 developed grade 3 tachypnea during the third and fourth infusions with 4.4 × 109 and 3.0 × 109 cells, respectively, and was treated on both occasions with supplemental oxygen, furosemide, and acetaminophen with resolution of symptoms. Patient no. 1 developed severe dyspnea, tachypnea, and hypoxemia within 2 hours of infusion of 2.25 × 109 cells, and required intubation for noncardiogenic pulmonary edema. Analysis of bronchoalveolar lavage (BAL) fluid obtained 16 hours after intubation revealed a mononuclear cell infiltrate composed almost exclusively of CD3+CD8+ T cells expressing the unique TCRβ chain of the mHAg-specific CTL clone that had been infused. Flow cytometric analysis revealed that the CD8+ cells recovered from the BAL fluid, in comparison with the CD8+ CTLs before infusion, had uniformly down-regulated their TCR expression (Figure 3A-B), consistent with TCR engagement by specific antigen in vivo. Treatment with methylprednisolone (2 mg/kg/d) led to rapid resolution of the pulmonary edema and safe extubation 40 hours after the T-cell infusion (Figure 3C-E).
Acute pulmonary toxicity in patient no. 1 associated with administration of CD8+ mHAg-specific CTLs. (A-B) Flow cytometric analysis of T-cell receptor expression in CTLs before infusion (A) and after recovery from bronchoalveolar lavage fluid (B) using a human TCR Vβ13-specific monoclonal antibody. (C-E) Chest radiographs taken before (C), 4 hours after (D), and 40 hours after (E) infusion of 2.25 × 109 CTLs.
Acute pulmonary toxicity in patient no. 1 associated with administration of CD8+ mHAg-specific CTLs. (A-B) Flow cytometric analysis of T-cell receptor expression in CTLs before infusion (A) and after recovery from bronchoalveolar lavage fluid (B) using a human TCR Vβ13-specific monoclonal antibody. (C-E) Chest radiographs taken before (C), 4 hours after (D), and 40 hours after (E) infusion of 2.25 × 109 CTLs.
A fourth patient (no. 4) had experienced multiple episodes of corticosteroid-dependent bronchiolitis obliterans with organizing pneumonia (BOOP) before T-cell therapy, and had an exacerbation of BOOP 12 days after the final CTL infusion. At the time of relapse, this patient's pulmonary symptoms were exquisitely prednisone-dependent, and he remained prednisone-dependent due to BOOP until he died, 51.5 months after relapse and 48.5 months after completion of T-cell therapy.
GVHD
Before T-cell therapy, efforts were made to reduce or withdraw immunosuppressive drugs in the 6 patients being treated for previously diagnosed GVHD (5 patients) or BOOP (patient no. 4). Immunosuppression was successfully withdrawn in 2 patients (nos. 3 and 5), and GVHD requiring reinitiation of immune suppression did not recur in either patient. Patient no. 2 had GVHD of skin and gut diagnosed on biopsies obtained before the start of T-cell therapy, and required treatment with tacrolimus and oral beclomethasone; no exacerbation of GVHD was observed during or after T-cell therapy. Patient nos. 1 and 7 were on cyclosporine and prednisone for GVHD at the time of relapse, at which point active GVHD of the mouth (patient no. 1) and skin (patient no. 7) were confirmed on lip and skin biopsy, respectively. Immunosuppression was withdrawn in these 2 patients before the start of T-cell therapy, but both developed multisystem GVHD concurrent with T-cell therapy that required reinitiation of immunosuppression (Figure 2). Clone-specific PCR was used in both patients to assess migration of transferred CTLs to skin, and transferred CTLs were not detected in skin biopsies from either patient (data not shown). Immunosuppression was reduced in patient no. 4, but was increased after completion of T-cell therapy due to recurrence of BOOP. Patient no. 6 had no clinically significant GVHD requiring treatment while receiving T-cell therapy, and a skin biopsy obtained approximately 3 weeks after the final T-cell infusion showed only subclinical chronic GVHD (Figure 2).
Disease response
All 7 patients who received T-cell therapy had advanced disease before myeloablative allogeneic HCT, and all relapsed at a median of 7 months after transplantation. After cytoreductive chemotherapy, withdrawal of or reduction in immune suppression, and 3 or more CTL infusions, 4 of the 6 patients with bone marrow relapse achieved a complete morphologic remission, while 2 failed to respond. A total of 2 of the 4 patients who achieved remission had primary chemotherapy-refractory acute lymphoblastic leukemia (ALL) before transplantation (patient no. 1 and patient no. 5). In 3 patients (patient nos. 1, 3, and 5), cytoreductive chemotherapy given before T-cell therapy failed to clear leukemic blasts from bone marrow samples obtained more than 21 days after chemotherapy was administered. After additional infusions of mHAg- specific CTLs, all 3 patients achieved a complete morphologic remission. All 4 patients who achieved remission in bone marrow subsequently relapsed, however, and 6 of the 7 patients died between 4.7 and 15.6 months after relapse (Figure 2). Patient no. 4, who was treated for isolated CNS relapse, relapsed again in CNS and subsequently in bone marrow at 7 and 34 months, respectively, after T-cell therapy, and died 51.5 months after the initial relapse.
Adoptively transferred T cells migrate to bone marrow but fail to persist in vivo
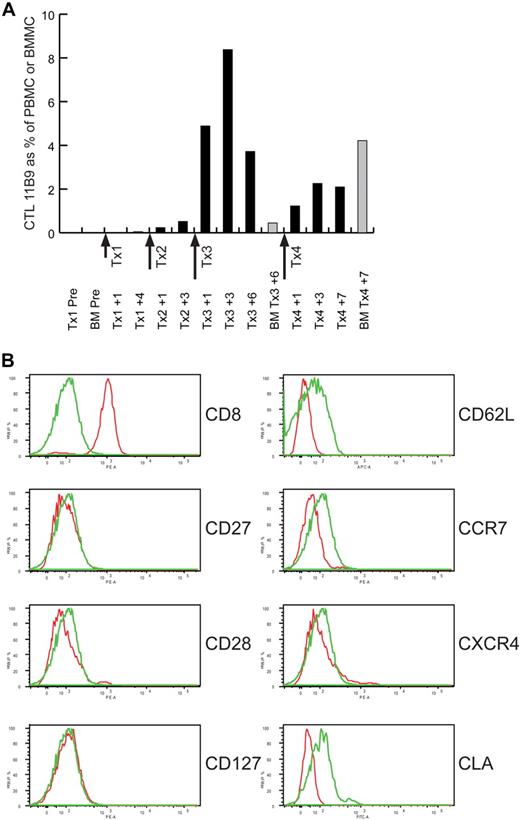
Quantitative reverse transcriptase (RT)–PCR assays with primer pairs specific for each CTL clone's uniquely rearranged TCRβ gene detected adoptively transferred CTLs in the blood and bone marrow after therapy in 5 of the 7 patients. The peak frequencies of transferred CTLs in blood, expressed as a percentage of PBMCs, were observed within 3 to 5 days after cell doses of more than 1 × 109 cells, and ranged from 0.5% to 18.6% (representative data in Figure 4A). The frequencies of adoptively transferred cells decreased rapidly after the peak, and the average half-time of disappearance was less than 7 days in all patients. The peak frequencies of transferred CTLs detected in bone marrow were 4.6% and 4.3% of mononuclear cells, observed in patient no. 3 and patient no. 7, respectively.
Adoptively transferred CTLs migrate to bone marrow but have limited in vivo persistence. (A) Real-time PCR with primers that specifically amplify the uniquely rearranged TCRβ CDR3 region of the mHAg-specific CTL clone 11B9-45 were used to detect and enumerate 11B9-45 CTL in the blood (■) and bone marrow (BM; ▩) of Patient no. 7 at the indicated time points before or during T-cell therapy. The level of 11B9-45 CTLs detected is expressed as a percentage of PBMCs or bone marrow mononuclear cells (BMMCs). The dose (in approximate log10 scale) and timing of each of the 4 T-cell infusions administered to this patient (Tx1, Tx2, Tx3, and Tx4) are indicated. (B) Flow cytometric analysis of 11B9-45 CTLs before adoptive transfer. Cells stained with a mAb specific for the molecule indicated to the right of each histogram are indicated in red, and those stained with an isotype control antibody are indicated in green.
Adoptively transferred CTLs migrate to bone marrow but have limited in vivo persistence. (A) Real-time PCR with primers that specifically amplify the uniquely rearranged TCRβ CDR3 region of the mHAg-specific CTL clone 11B9-45 were used to detect and enumerate 11B9-45 CTL in the blood (■) and bone marrow (BM; ▩) of Patient no. 7 at the indicated time points before or during T-cell therapy. The level of 11B9-45 CTLs detected is expressed as a percentage of PBMCs or bone marrow mononuclear cells (BMMCs). The dose (in approximate log10 scale) and timing of each of the 4 T-cell infusions administered to this patient (Tx1, Tx2, Tx3, and Tx4) are indicated. (B) Flow cytometric analysis of 11B9-45 CTLs before adoptive transfer. Cells stained with a mAb specific for the molecule indicated to the right of each histogram are indicated in red, and those stained with an isotype control antibody are indicated in green.
Flow cytometric analysis of all 8 CTL clones infused in this study revealed a differentiated effector memory phenotype, with negligible cell-surface expression of markers such as CD62L and CCR7 that are present on central memory T cells (representative data in Figure 4B). Most but not all of the clones expressed CXCR4, a chemokine receptor that may be important for homing to bone marrow. Although CTL clone 11B9-45 administered to patient no. 7 did not express CXCR4, the absence of this receptor did not prevent migration of the clone to the bone marrow (Figure 4A). All of the CTL clones infused in this study lacked surface expression of CLA, a molecule associated with T-cell homing to skin.
Molecular characterization of mHAgs and correlation with outcome
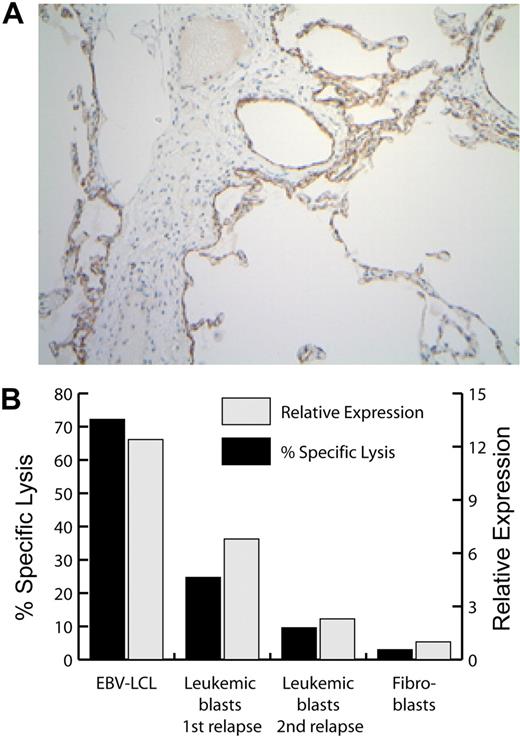
We subsequently identified the genes encoding mHAgs targeted in patients who experienced toxicity after T-cell infusions and/or had evidence of an antileukemic effect. A genome-wide SNP-based genotype-phenotype correlation approach18 was used to identify the gene encoding the HLA-A*2902–restricted mHAg recognized by clone 11C6-109, which was administered to patient no. 1. This patient had persistent Philadelphia chromosome–positive (Ph+) ALL in the bone marrow after chemotherapy and the first 3 CTL infusions, but achieved complete morphologic and cytogenetic remission with subsequent infusions (Figure 2). As previously noted, a serious pulmonary toxicity also developed after the sixth infusion (Figure 3), which was the highest T-cell dose that the patient received. At 3 months after the sixth infusion, the patient relapsed in blood and marrow and then received 4 additional infusions of the same CTL clone at a lower dose (< 1 × 109 cells) without toxicity or response (Figure 2). The P2RX7 gene on chromosome 12q was found to encode the mHAg recognized by clone 11C6-109 (supplemental Table 2). Analysis of P2RX7 minigenes and synthetic P2RX7-derived peptides defined the nonameric P2RX7265-273 peptide WFHHCHPKY as the epitope recognized by clone 11C6-109, with half-maximal lysis observed at a peptide concentration of approximately 1nM (supplemental Figure 2). Differential recognition of antigen-positive and -negative cells is attributable to the nonsynonymous A↔G SNP rs7958311 (supplemental Table 3), which creates a His↔Arg amino acid polymorphism at residue 270 in P2RX7.
Analysis of P2RX7 expression was performed to further investigate both the pulmonary toxicity and the likely antileukemic effect associated with the initial infusions of clone 11C6-109. Real-time PCR showed that the highest levels of P2RX7 transcript were observed in spleen, ovary, lung, and peripheral blood cells, with particularly high levels of expression in CD14+ blood cells (Figure 5; data not shown). Immunohistochemistry of lung tissue revealed that P2RX7 protein expression was restricted to the alveolar epithelium (Figure 6A). P2RX7 transcripts were also detected by PCR in leukemic blasts obtained from patient no. 1 at the time of posttransplantation relapse and before T-cell therapy; these blasts were recognized by 11C6-109 CTL in vitro (Figure 6B). In contrast, leukemic blasts obtained from patient no. 1 at the time of his second posttransplantation relapse—after receiving 6 CTL infusions—showed prominently decreased expression of P2RX7 transcripts by real-time PCR and as well as decreased recognition by 11C6-109 CTLs in vitro (Figure 6B). Analysis of blasts from the first and second relapse showed no significant interval changes in the expression of MHC class I, HLA-A29, CD54 (ICAM-1), CD58 (LFA-3), CD44, or PD-L1 (supplemental Figure 3).
Relative expression of mHAg-encoding genes in different human tissues. Real-time PCR was used to determine the relative expression levels of the P2RX7, DPH1, and DDX3Y genes in different human tissues. Expression of the hematopoietic-specific CD45 gene was also determined to permit estimation of the extent to which each of the tissues examined was contaminated by cells of hematopoietic origin. For each gene, the relative expression level was defined as the expression level of that gene in a specific tissue compared with its expression level in male EBV-LCL. ▩ indicates expression level of each gene in the spleen and PBMCs to facilitate their comparison with the expression levels in nonhematopoietic tissues.
Relative expression of mHAg-encoding genes in different human tissues. Real-time PCR was used to determine the relative expression levels of the P2RX7, DPH1, and DDX3Y genes in different human tissues. Expression of the hematopoietic-specific CD45 gene was also determined to permit estimation of the extent to which each of the tissues examined was contaminated by cells of hematopoietic origin. For each gene, the relative expression level was defined as the expression level of that gene in a specific tissue compared with its expression level in male EBV-LCL. ▩ indicates expression level of each gene in the spleen and PBMCs to facilitate their comparison with the expression levels in nonhematopoietic tissues.
Expression of P2RX7 mRNA, protein, and mHAg in selected tissues. (A) Immunohistochemical analysis of P2RX7 expression in human lung. The width of the image is 936 microns. (B) Expression of P2RX7 (▩) and of the P2RX7-encoded mHAg recognized by CTL clone 11C6-109 (■) in patient no. 1–derived EBV-LCL, dermal fibroblasts, leukemic blasts obtained from patient no. 1 at the time of his first posttransplantation relapse (before T-cell therapy), and leukemic blasts obtained at the time of his second posttransplantation relapse, after the receipt of 6 CTL infusions. P2RX7 expression was assessed by real-time quantitative PCR, and mHAg expression was assessed by a 4-hour 51Cr release cytotoxicity assay at an E/T ratio of 10:1.
Expression of P2RX7 mRNA, protein, and mHAg in selected tissues. (A) Immunohistochemical analysis of P2RX7 expression in human lung. The width of the image is 936 microns. (B) Expression of P2RX7 (▩) and of the P2RX7-encoded mHAg recognized by CTL clone 11C6-109 (■) in patient no. 1–derived EBV-LCL, dermal fibroblasts, leukemic blasts obtained from patient no. 1 at the time of his first posttransplantation relapse (before T-cell therapy), and leukemic blasts obtained at the time of his second posttransplantation relapse, after the receipt of 6 CTL infusions. P2RX7 expression was assessed by real-time quantitative PCR, and mHAg expression was assessed by a 4-hour 51Cr release cytotoxicity assay at an E/T ratio of 10:1.
Patient no. 6 received 4 infusions of CTL clone 50F5-448 and developed grade 3 tachypnea during the third and fourth infusions, but had persistent leukemia despite chemotherapy and T-cell therapy. The genome-wide genotype-phenotype correlation approach was used to determine that the HLA-B*5701–restricted mHAg recognized by clone 50F5-448 was encoded by DPH1, located on chromosome 17p. Recognition of HLA-B*5701–transfected EBV-LCL was perfectly correlated with genotype at rs11653030, located in an intron of DPH1 (supplemental Table 4), which is in complete linkage disequilibrium with rs35394823, a nonsynonymous C↔G SNP in exon 9 of DPH1 that creates a Leu↔Val amino acid polymorphism at residue 335 in DPH1. Analysis of DPH1 minigenes and DPH1-derived synthetic peptides revealed that the mHAg epitope recognized by clone 50F5-448 is the decamer DPH1334-343 with sequence SVLPEVDVW (supplemental Figure 4), which spans the Leu↔Val polymorphism associated with rs35394823. Genotyping of mHAg+ and mHAg− HLA-B57+ EBV-LCL revealed that the G allele at rs35394823, which specifies valine at residue 335, was both necessary and sufficient for recognition by 50F5-448 CTLs (supplemental Table 5).
DPH1 transcripts were detected by real-time PCR in a broad range of hematopoietic and nonhematopoietic tissues, including the lung (Figure 5). Leukemic blasts obtained from patient no. 6 also expressed high levels of the DPH1 transcript, but were poorly recognized by 50F5-448 CTLs (data not shown), despite expression of high levels of MHC class I as well as CD54 (ICAM-1), CD58 (LFA-3), and CD44 (supplemental Figure 5).
Patient no. 3 received sequential T-cell therapy with 2 distinct mHAg-specific CTL clones (Figure 2), the second of which, 68H7-819, was subsequently shown to recognize a male-specific (H-Y) mHAg presented by HLA-B*2705 and encoded by the Y chromosome gene DDX3Y.20 A total of 3 infusions of the DDX3Y-specific clone were administered over an interval of 3 weeks, and the highest dose administered was 2.4 × 109 cells. Although fever and chills occurred after the CTL infusions, patient no. 3 developed no pulmonary toxicity or acute or chronic GVHD either during or after T-cell therapy; the patient died from progressive leukemia 5 months after his final H-Y–specific CTL infusion. Real-time PCR showed that DDX3Y is expressed in both hematopoietic and nonhematopoietic cells, with the highest levels of expression observed in testis20 (Figure 5). The highest level of expression in the extratesticular tissues was observed in the spleen. We previously reported that the H-Y antigen recognized by this clone is uniformly expressed in primary HLA-B*2705+ male leukemic cells, including leukemic cells obtained from patient no. 3 just before his treatment with the DDX3Y-specific CTL clone, and is also expressed in the putative leukemic stem cells that can establish T-lymphoid blast-phase chronic myelogenous leukemia (CML) as well as acute myeloid leukemia (AML) in immune-deficient nonobese diabetic/severe combined immune deficienct (NOD/SCID) mice.20 The DDX3Y-specific CTLs showed poor in vivo persistence in patient no. 3, however, which was likely due to a very high leukemic burden at the time of infusion.
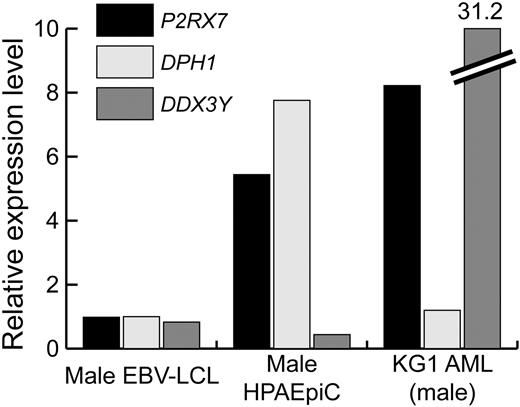
The observation that P2RX7 expression in the lung is limited to alveolar epithelial cells (Figure 6A) prompted us to investigate whether quantitative differences in the expression of P2RX7, DPH1, and DDX3Y in alveolar epithelial cells might be correlated with the degree of pulmonary toxicity experienced by patient nos. 1, 3, and 7, who received P2RX7-, DDX3Y-, and DPH1-specific T cells, respectively. Real-time PCR analysis revealed that the relative expression of both P2RX7 and DPH1 in human male pulmonary alveolar epithelial cells was significantly higher than the expression of DDX3Y (Figure 7). Additional analysis of the expression of these 3 genes in a panel of primary leukemic cells and leukemic cell lines revealed that the expression P2RX7 and DDX3Y was significantly higher in these cells than the expression of DPH1 (representative data with the male leukemic cell line KG1 in Figure 7).
Differential expression of mHAg-encoding genes in pulmonary alveolar epithelial and leukemic cells. Real-time PCR analysis of P2RX7, DPH1, and DDX3Y expression in male EBV-LCL, human male pulmonary alveolar epithelial cells (HPAEpiCs), and the male-derived KG1 AML cell line was performed. The expression level of each gene was computed using the standard curve method, and the expression level in HPAEpiCs and KG1 is referenced to that observed in male EBV-LCL.
Differential expression of mHAg-encoding genes in pulmonary alveolar epithelial and leukemic cells. Real-time PCR analysis of P2RX7, DPH1, and DDX3Y expression in male EBV-LCL, human male pulmonary alveolar epithelial cells (HPAEpiCs), and the male-derived KG1 AML cell line was performed. The expression level of each gene was computed using the standard curve method, and the expression level in HPAEpiCs and KG1 is referenced to that observed in male EBV-LCL.
Discussion
The efficacy of allogeneic HCT as a therapy for leukemia represents a profound example of the potential of the human immune system to eliminate tumors. However, harnessing the GVL effect to improve outcome for patients with advanced disease without aggravating GVHD remains an unresolved challenge. Treatment of posttransplantation relapse in a patient with accelerated-phase CML using T-cell lines selected in vitro for reactivity with recipient leukemic cells has previously been reported,21 but the phase 1 study reported here represents the first investigation of the adoptive transfer of CD8+ mHAg-specific T-cell clones to augment the GVL effect in patients who have relapsed after allogeneic HCT. The primary objectives were to evaluate the feasibility of isolating and expanding CD8+ mHAg-specific T-cell clones that preferentially recognized recipient hematopoietic cells, the safety of infusing such T cells, and their in vivo persistence and migration to bone marrow. The strategy of prospectively isolating and expanding mHAg-specific T-cell clones was technically complex but feasible, although the target maximum cell dose of 3.3 × 109 cells/m2 was achieved in only 3 of the 7 patients.
The data show that selecting CD8+ mHAg-specific CTL clones for therapy based solely on in vitro reactivity with recipient hematopoietic cells but not skin fibroblasts is not always a sufficiently stringent criterion to identify CTLs that can be transferred safely. It was anticipated that toxicities from infusing mHAg-specific CTLs would be a consequence of recognition of recipient cells that expressed the target antigen, and could include GVHD. GVHD requiring treatment occurred in 3 patients, but a role for the infused T cells as a causative factor could not be definitively established. All 3 patients were receiving immunosuppressive therapy for GVHD at the time of relapse, and had undergone rapid withdrawal of immunosuppression just before the start of T-cell therapy. Skin biopsies were obtained in 2 of these patients at the time GVHD recurred and demonstrated histologic features of GVHD, but the transferred CTLs were not detected in the skin by quantitative PCR, and lacked expression of CLA, which is involved in T-cell homing to skin. It remains possible that adoptively transferred T cells contributed to the induction or progression of GVHD in the skin or other involved sites indirectly, such as through the release of inflammatory cytokines. Patient no. 3 did not develop GVHD after the infusion of 2 CTL clones that recognized distinct mHAgs, one of which we later determined was encoded by the Y-chromosome gene DDX3Y.20 Our data suggest that the level of target expression may be a factor in determining the propensity for specific T cells to cause GVHD, and that CD8+ T-cell responses against H-Y antigens may not all be associated with GVHD, as has been suggested previously.22,23 It is also possible that short in vivo persistence or defects in migration or function of the infused T cells were responsible for the absence of GVHD.
Pulmonary toxicity was the most frequent serious toxicity after the infusion of mHAg-specific CTL clones, and occurred in 3 patients after the infusion of cell doses of greater than 1 × 109. Toxicity was not simply a consequence of the cell dose infused, since no toxicity was observed in the other 4 patients who each received infusions of greater than 3.3 × 109 cells. The lung may be especially susceptible to toxicity because this is the initial tissue to which the infused T cells migrate, and prior studies have shown a propensity for transferred T cells to be transiently trapped in the lung.24 In the most serious case (patient no. 1), the mHAg targeted was later shown to be encoded by P2RX7, which is uniformly expressed in pulmonary alveolar epithelium. The accumulation of infused P2RX7-specific T cells in BAL fluid combined with down-modulation of TCR expression provides direct evidence for toxicity as a consequence of target recognition. The administration of methylprednisolone, which has also been used to abrogate local toxicity in patients with melanoma receiving adoptive T-cell therapy,25 coincided with a rapid reversal in pulmonary symptoms. Although our data demonstrate that P2RX7, DPH1, and DDX3Y are all expressed in lung tissue, pulmonary toxicity was observed with infusions of 2 × 109 or more P2RX7- and DPH1-specific, but not DDX3Y-specific, T cells. These findings demonstrate that the presence of RNA transcripts of the gene encoding the target mHAg does not necessarily predict toxicity of mHAg-specific T-cell therapy, and highlight the potential value of a dose-escalation design in future trials to avoid toxicity.
Five of 7 patients on this study achieved complete morphologic remissions after the combination of cytoreductive chemotherapy, withdrawal or reduction of immune suppression, and infusion of mHAg-specific CTLs. Of these, 3 patients had persistent leukemia by morphology more than 3 weeks after completing chemotherapy, and only achieved remission after infusions of mHAg-specific CTLs. These results, combined with direct evidence that the infused T cells migrated to the bone marrow, provide encouraging evidence that transferred CTLs can exert antileukemic activity. However, all 5 of these patients subsequently relapsed. The lack of sustained treatment efficacy may be attributable to multiple factors, including the emergence of antigen loss variants, as suggested by analysis of P2RX7 expression in leukemic blasts obtained from Patient no. 1 before and after treatment with P2RX7-specific T cells, as well as the short in vivo persistence of adoptively transferred cells. Long-term persistence of adoptively transferred T cells has correlated with sustained efficacy in adoptive T-cell therapy of melanoma,25-27 and infused T cells were not detected in the blood beyond 21 days in any of the patients on this trial. The lack of sustained in vivo persistence of transferred CD8+ T-cell clones could be due to differentiation of T cells during in vitro culture to effector cells that are dependent on IL-2 for survival, or to activation-induced cell death in vivo as a consequence of a high antigen load.28,29 In 2 patients in this study, the coadministration of low-dose IL-2 did not promote long-term persistence of transferred T cells. The administration of alternative lymphodepleting chemotherapy before T-cell infusions and higher doses of IL-2 has been effective for improving persistence of transferred T cells and antitumor efficacy in patients with melanoma; such regimens might be useful in leukemia.25,30,31 Additional strategies such as the use of CD8+ effector cells that are derived from central memory cells, IL-15, IL-21, or the coadministration of antigen-specific CD4+ T helper cells, may also improve the outcome.32-35
Our effort to augment the GVL effect without GVHD by adoptive T-cell transfer highlights the challenges in delivering T-cell therapy in the context of allogeneic HCT using T cell–replete hematopoietic cell grafts, which carry a significant risk of GVHD and require administration of posttransplantation immunosuppression. The high incidence of acute and chronic GVHD before T-cell therapy in the patients on our trial complicated the delivery of recipient mHAg-specific T-cell therapy and assessment of the contribution of adoptively transferred T cells to GVHD occurring after infusion. Moreover, the function and in vivo persistence of adoptively transferred T cells are adversely affected by concurrent administration of immunosuppressive agents. Thus, adoptive T-cell therapy targeting recipient mHAgs or leukemia-associated antigens may be more feasible in transplantation settings where the prevalence of GVHD is significantly lower than after administration of T-replete hematopoietic cell grafts. Genetic modification of T cells to endow them with resistance to immunosuppressive agents such as FK50636 would enable adoptive T-cell therapy in cases where GVHD requiring treatment nonetheless occurred.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We acknowledge the expert technical assistance provided by Kimberly A. Boyt, Rudy W. Linterman, Suzanne M. Xuereb, Sharon Lu, Maureen X. Miranda, and Amanda Moklebust in the performance of this study, and the assistance with preparation of figures provided by Katherine E. Warren. Thanks are also due to the patients who participated in this study and to the dedicated staff at the Seattle Cancer Care Alliance who took care of them.
This work was supported by a Lilly Clinical Investigator Award from the Damon Runyon Cancer Research Foundation (to E.H.W.); an award from the Edson Fund for Immunotherapy Research; National Institutes of Health grants CA106512 (to E.H.W.), CA18029 (to S.R.R.), F30 HL093985, and M01-RR-00037; grants from the Japanese Ministry of Education, Culture, Science, Sports, and Technology, for Scientific Research on Priority Areas (B01; no.17016089); and from the Japanese Ministry of Health, Labor, and Welfare, for Research on the Human Genome, Tissue Engineering Food Biotechnology, and the Second- and Third-Team Comprehensive 10-Year Strategy for Cancer Control (no. 26); and a Grant-in-Aid from Core Research for Evolutional Science and Technology (CREST), Japan.
National Institutes of Health
Authorship
Contribution: E.H.W., N.F., Y.A., J.K.M., K.R.L., M.L.B., K.K.W.K., and K.V.R. performed experiments; E.H.W., N.F., Y.A., C.N., T.A.G., S.O., and A.M. analyzed results and made the figures; E.H.W., F.R.A., and S.R.R. designed the research; E.H.W. wrote the paper; and all authors read and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Edus H. Warren, Program in Immunology, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Seattle, WA 98109-1024; e-mail: ehwarren@u.washington.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal