Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) has advanced to a common procedure for treating also older patients with malignancies and immunodeficiency disorders by redirecting the immune system. Unfortunately, cure is often hampered by relapse of the underlying disease, graft-versus-host disease, or severe opportunistic infections, which account for the majority of deaths after HSCT. Enhancing immune reconstitution is therefore an area of intensive research. An increasing variety of approaches has been explored preclinically and clinically: the application of cytokines, keratinocyte growth factor, growth hormone, cytotoxic lymphocytes, and mesenchymal stem cells or the blockade of sex hormones. New developments of allogeneic HSCT, for example, umbilical cord blood or haploidentical graft preparations leading to prolonged immunodeficiency, have further increased the need to improve immune reconstitution. Although a slow T-cell reconstitution is regarded as primarily responsible for deleterious infections with viruses and fungi, graft-versus-host disease, and relapse, the importance of innate immune cells for disease and infection control is currently being reevaluated. The groundwork has been prepared for the creation of individualized therapy partially based on genetic features of the underlying disease. We provide an update on selected issues of development in this fast evolving field; however, we do not claim completeness.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) has become a common procedure for the therapy of hematologic malignancies and immune disorders. Donor bone marrow and mobilized peripheral blood stem cells are routinely used for the reconstitution of immune function in leukemia and lymphoma patients after radiation and/or chemotherapy. Recently, there have also been increasing attempts to treat solid tumors by immunotherapeutic approaches to induce a graft-versus-tumor effect, reviewed by Demirer et al.1
Tempo of immune reconstitution
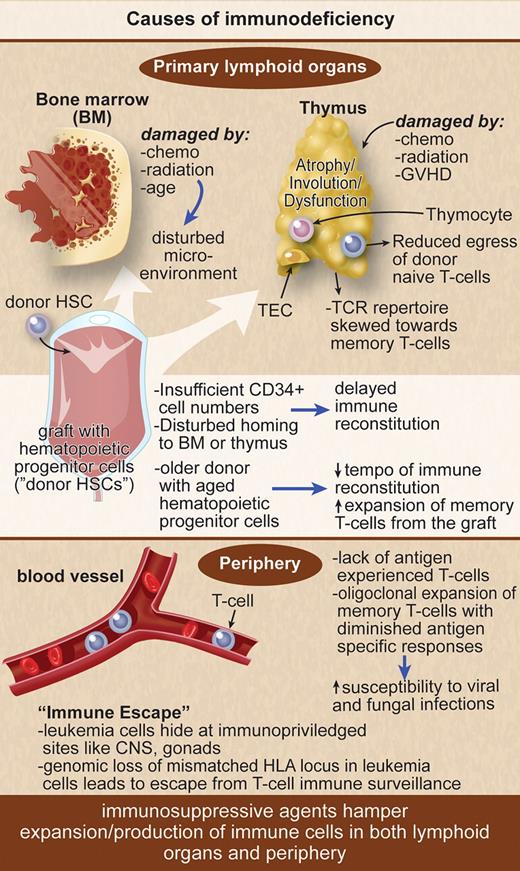
The reconstitution of different immune cell subsets after an allogeneic HSCT occurs at different tempos. After the conditioning regimen there is the “aplastic phase” (neutropenia) until neutrophils recover at approximately 14 days after peripheral blood stem cell transplantation, approximately 21 days after bone marrow transplantation, and approximately 30 days after umbilical cord blood transplantation (UCBT). The infections encountered during the aplastic phase do not differ from those found in other neutropenic patients and consist primarily of bacterial infections. The first 100 days after HSCT are characterized by cellular immune deficiencies with a reduced number of cytotoxic lymphocytes, natural killer (NK) cells of the innate immune system, and T cells of the specific immune system (Figure 1). This renders the patient especially susceptible to viral and fungal infections. Rapid recovery of NK cells after HSCT is based on an expansion of the cytokine-producing CD56bright NK-cell subset. This early expansion of CD56bright NK cells gradually declines but may persist for 1 year. Initial recovery of the T-cell compartment relies on peripheral expansion of memory T cells, driven by cytokines and the presence of alloreactive antigens, before the production of naive T cells in the thymus starts. This is especially true for CD4+ T cells that reconstitute later than CD8+ T cells and rely more on thymic production of naive T cells after HSCT, leading to an inversion of the CD4/CD8 ratio. T-cell receptor rearrangement excision DNA circles (TRECs) are used as markers for naive T-cell reconstitution occurring in the thymus. TREC levels remain low until 3 to 6 months after allogeneic HSCT. Increasing patient age is associated with thymic atrophy leading to decreased thymic output of naive T cells resulting in low TREC levels. The lack of naive T cells with a broad T-cell receptor (TCR) repertoire leads to an increased risk for opportunistic infections and leukemia relapse and is aggravated by graft-versus-host disease (GVHD; Figure 1).
Immunosuppressive agents hamper expansion/production of immune cells in lymphoid organs and periphery.
Immunosuppressive agents hamper expansion/production of immune cells in lymphoid organs and periphery.
The reconstitution of the B-cell compartment representing the humoral immunity may take up to 2 years after HSCT. First, transitional CD19+CD24++CD38++ B cells occur in the peripheral blood, before their percentage progressively decreases, whereas the mature B-cell proportion rises.2 The lack of memory B cells, decreased levels of circulating immunoglobulins, impaired immunoglobulin class switching, and a loss of complexity in immunoglobulin gene rearrangement patterns leave allogeneic HSCT patients vulnerable to encapsulated bacteria such as Streptococcus pneumoniae and Haemophilus influenzae. The patients may obtain protective titers of pathogen-specific antibodies through vaccination. The optimal timing of immunization depends on the time elapsed since HSCT, the type of the graft, and the presence or absence of GVHD, as recently reviewed in extenso elsewhere.3
Aspergillosis and CMV reactivation as major obstacles after HSCT
Notable for recent developments to improve T-cell immune reconstitution, we reviewed invasive aspergillosis and cytomegalovirus (CMV) reactivation, as it poses major obstacles after HSCT despite promising new approaches. A retrospective European Group for Blood and Marrow Transplantation (EBMT) study has determined the following risk factors for invasive aspergillosis: length of neutropenia, the status of the underlying disease, myeloablative conditioning, CMV disease, GVHD grades II-IV, and bone marrow or cord blood as a source of stem cells compared with peripheral blood stem cells.4 A risk model (low, intermediate, high) for progression of aspergillosis was developed based on this retrospective EBMT survey and needs to be validated in future trials on invasive aspergillosis. As the transfer of naive T cells is associated with delayed immune reconstitution, adoptive transfer of functionally active Aspergillus fumigatus–specific T cells might be a therapeutic option to restore immune effector mechanisms. Recently, a method for the rapid clinical scale generation of functionally active A fumigatus–specific T cells has been developed.5 Validation in clinical trials is pending. CMV reactivation may cause different diseases affecting lung, liver, gut, or central nervous system after allogeneic HSCT. CMV seropositivity remains a major risk factor for transplant-related mortality (TRM). Seropositive recipients obtaining a graft from a seropositive donor have improved survival and reduced TRM compared with those who received a transplant from a seronegative donor because of lack of transfer of CMV-specific T cells in the graft. Lack of CMV-specific T cells after the first episode of CMV reactivation is associated with multiple subsequent reactivations. This topic has recently been reviewed in depth.6 T cell–depleted (TCD) grafts have been introduced into allogeneic HSCT to reduce the risk of T cell–driven GVHD,7 at the expense of a higher incidence of fungal or viral infections after HSCT. However, a recent study demonstrated that TCD HSCT may be as successful as regular HSCT if antithymocyte globulin is excluded from the conditioning regimen, leading to durable engraftment with a low incidence of GVHD.8 TCD grafts supplemented with committed progenitor cells may enhance immune reconstitution of different immune cell subsets in the absence of GVHD.9 In addition, in TCD allogeneic HSCT, NK cells appear to play a major role in the control of viral reactivation. Chen et al10 have determined in a retrospective single-center study of 131 HLA-identical sibling donor/recipient pairs that additional activating killer immunoglobulin-like receptor (KIR) genes in the donor compared with the recipient were associated with lower TRM, increased survival, and lower incidence of CMV reactivation. Validation in a larger prospective clinical trial is warranted.
RIC regimens and increasing age of allogeneic hematopoietic stem cell transplant recipients
With the development of reduced-intensity conditioning (RIC) regimens in allogeneic transplantation, it has become possible to include disabled and older patients.11 The age limits are steadily rising, and some transplant physicians even question whether age limits still apply. The damage done to the thymus as production site for T cells is less severe in RIC compared with myeloablative conditioning.11 However, performing HSCT in older patients introduces new problems, as the lymphoid structures of the immune system also age.12 The immune system takes longer to rebuild and is more likely to be insufficient after transplantation. The older the patient, the more the T-cell recovery stems from expansion of memory T cells in the graft (Figure 1). The de novo production of naive T cells in the thymus with a broad T-cell receptor repertoire necessary for a powerful control of pathogens and of malignancies is limited. Therefore, the susceptibility to viral and fungal infections is elevated. The prediction of immune reconstitution in older patients is difficult, as the speed of immune system aging is very variable. Furthermore, the age not only of the recipient, but also of the donor is decisive as the graft of an older donor has decreased immune-reconstituting ability in the recipient.13 Rejuvenating the aging immune system is an area of rising importance considering the predicted increased percentage of aged patients in the population of developed countries.
Immune reconstitution after transplantation is also influenced by the occurrence of acute or chronic GVHD and by the immunosuppressive treatment selected (Figures 1 and 2). In general, potentially life-threatening GVHD leads to delays in immune reconstitution. Even so, the risk of GVHD is reduced if a TCD graft has been infused and/or antithymocyte globulin conditioning chosen; both approaches create, on the other hand, a deficit in T-cell immunity, leaving the recipient more susceptible to infections and/or leukemia relapse.14
The source of hematopoietic stem cells matters
There is consensus that the ideal donor for an allogeneic HSCT is a matched related donor. Unfortunately, a matched related donor is found in only approximately 25% of all cases; as an alternative, a matched unrelated donor (MUD) is found in only 30% (range, 10%-60% depending on the ethnic background). Luckily, the options of hematopoietic stem cell (HSC) transplant grafting have broadened. The numbers of haploidentical transplants from a sibling donor15 or the application of one or more umbilical cord blood (UCB) units for adult patients is steadily increasing. The advantage of a family donor (haploidentical or other) and, to an even greater extent, a cord blood donor is that they are, in general, immediately available, which is essential in high-risk leukemia with no time to search for an MUD. In addition, a second graft of the original family donor or from another family member is easily obtainable in the case of graft rejection, which is not true for a UCBT. However, this development poses new problems, which have not yet been sufficiently solved: for example, 40% of nonrelapse causes for posttransplantation mortality in high-risk leukemia patients after haploidentical transplantation are from CMV or Aspergillus infections.16
Umbilical cord blood transplantation in adults
The number of adult patients treated with UCB is increasing as it is generally abundant, and no serious ethical dilemmas exist in terms of donor collection. UCB has become a valuable alternative to other hematopoietic stem cell sources. However, UCB often bears the disadvantage of insufficient cell numbers for immune reconstitution in adult patients because of the recipient's body weight, thymopoietic failure, or lack of antigen-experienced T cells, which leads to an impaired response toward cognate antigens and deficits in T-cell signal transduction mechanisms.17 In addition, UCB contains T regulatory (Treg) cells with more potent suppressor function than adult Treg cells. Many infections that afflict transplant patients are particularly frequent and more severe in the context of UCB transplantation. Therefore, current research attempts focus on transplanting more than a single UCB unit or on expanding selected subpopulations from UCB in vitro. UCB transplantation and its immune reconstitution features that apply to adult recipients has been extensively reviewed.18
Haploidentical HSCT in adults
Because of HLA disparity, the risk of fatal GVHD in haploidentical transplantation is high. Different approaches to minimize this risk have been developed. The graft is depleted of T cells either by a positive CD34+ cell selection aiming for high numbers of hematopoietic progenitor cells including so-called “veto cells” to overcome major genetic barriers and enable rapid and durable engraftment (mega-dose concept)15 or by a positive CD3+/CD19+ cell depletion to ensure that engraftment-facilitating cells such as dendritic cells or NK cells are transplanted.19,20 Attempts in haploidentical transplantation to infuse T cell–repleted grafts after an intensive myeloablative conditioning and myelosuppressive treatment have led to incidences of GVHD grades II-IV of 78%.21,22 In T cell–depleted haploidentical transplantation, the graft-versus-leukemia (GVL) effect is provided primarily by NK cells if killer immunoglobulin ligand (KIR) incompatibility in the GVH direction is present. KIR ligand incompatibility led to improved outcomes in human haploidentical HSCT as shown by the Velardi group (Ruggeri et al).23 Other groups could not show the beneficial effects of NK cells,24 which are probably explained by the lack of T-cell depletion in that setting as the potency of the NK-cell effect appears to be diminished by the presence of T cells. KIRs are specific for allotypic determinants that are shared by different HLA class I alleles (referred to as KIR ligands). They may be activating or inhibitory. NK-cell infusion has the advantage of inducing no GVHD while maintaining GVL effects.23,25 Thus far, the features of NK-cell alloreactivity are not completely understood, so that still more research is necessary to ensure the selection of the optimal donor. Likewise, γδ T cells are thought to promote antileukemic effects and are capable of recognizing malignant cells through mechanisms that require no prior antigen exposure or priming.26 Current data on immune reconstitution of this T-cell subset are limited and it remains unclear whether the observed antileukemic effects are related to graft processing or donor factors.
Immunomodulatory treatments: strengthening the endogenous immune system
The thymus involutes with age, leading to a gradual loss of newly produced naive T cells resulting in a restricted T-cell receptor repertoire skewed toward memory T cells. Thymic tissue has been considered a requirement for the generation of T cells with a broad antigen repertoire. The need for the thymus to repopulate the peripheral T-cell pool in adults has been acknowledged only since the advent of myeloablative therapy in HSCT and the treatment of AIDS patients (Figure 1). Different attempts to strengthen the endogenous immune system have been made by protecting the thymus either with keratinocyte growth factor or through sex hormone blockade. In addition, growth hormone might have a positive effect on the immune system, which would be useful in HSCT.
Application of keratinocyte growth factor, growth hormone, or androgen blockade
Keratinocyte growth factor (KGF; palifermin, Kepivance; Biovitrum, formerly Amgen) is a member of the fibroblast growth factor family that mediates epithelial cell proliferation and differentiation in a variety of tissues such as gut, skin, and thymus. Because of its protective effects on mucosa, KGF has entered the clinic for the prophylaxis of severe mucositis. The administration of KGF before conditioning and after transplantation resulted in enhanced thymopoiesis and peripheral T-cell numbers in different animal models. The KGF receptor is expressed on thymic epithelial cells (TECs) producing interleukin-7 (IL-7), suggesting preservation of IL-7 production is a possible mechanism.27 KGF's function as a protective and tropic factor for TECs ensures thymocyte proliferation and maturation. KGF-treated mice showed preservation of the thymic microenvironment, allowing normal thymopoiesis during acute GVHD after allogeneic HSCT.28 These findings are important as TECs are targets of the GVHD reaction. Enhancement of T-cell reconstitution after HSCT has also been demonstrated in an autologous nonhuman primate transplantation model for clinically relevant specific immune responses toward a T cell–dependent neoantigen.29 Unfortunately, in patients who underwent an allogeneic HSCT, the incidence of acute GVHD was not reduced and engraftment and early survival remained unaffected.30 In a long-term follow-up of these patients, no significant differences between the KGF-treated versus the untreated patients were observed regarding CMV or invasive fungal infection, chronic GVHD, or long-term survival.31
The age-related atrophy of the thymus is accompanied by decreased thymic output. This coincides with increased amounts of circulating sex steroids from puberty. Surgical or pharmacologic sex hormone blockade has been demonstrated in murine models and in prostate cancer patients to induce a regeneration of the thymus leading to a restoration of peripheral naive T-cell phenotype (Figure 2). It also reversed a decline in B-cell production by increasing bone marrow cellularity. The Boyd group (Sutherland et al)32 reported enhanced immune system regeneration in humans after allogeneic or autologous transplantation. Although the differences in TREC production, T-cell repertoire regeneration, and amount of naive CD4+ and CD8+ T cells were minor in the early posttransplantation period, the authors found a significant survival advantage in the patients treated with luteinizing hormone–releasing hormone agonist (goserelin, Zoladex; AstraZeneca) compared with the control group in the autologous but not in the allogeneic setting. Despite enhanced T-cell responses in vitro, no increase in GVHD rates was observed in the luteinizing hormone–releasing hormone agonist–treated group. Overall, the patient numbers per group were small, and the diseases and conditioning regimens rather heterogeneous, so that no final conclusions should be drawn. Larger clinical trials are necessary. However, the combined use of KGF with sex hormone blockade in patients undergoing an allogeneic transplantation after myeloablative conditioning also led to a supranormal thymopoiesis and thymic output, a broad Vbeta repertoire, and decreased homeostatic T-cell proliferation.33 The right approach may thus be the combination of both attempts.
Neuroendocrine hormones have been shown to affect numerous immunologic responses after in vivo administration. The importance of endogenous growth hormone for the immune system has been demonstrated in growth hormone–deficient mice with a defective cellular immunity and thymus atrophy reversible when growth hormone was substituted.34 Recombinant human growth hormone (rhGH) led to an overall increase in thymocytes in a murine allogeneic transplantation model, but had no effect on the proportion of thymocyte subsets or TREC levels.35 Its beneficial effect on thymopoiesis might be because of the promotion of pluripotent HSCs or common lymphoid precursors homing to the thymus. This hypothesis is supported by observations in a murine fetal thymic organ culture model, in which an increase in thymocyte progenitors derived from bone marrow was reported.36 Furthermore, rhGH has been shown in HIV-infected patients to enhance thymic function and peripheral immune responses in humans (Figure 2).37 RhGH (Genotropin; Pfizer) is being evaluated in patients undergoing a UCBT to accelerate the immune reconstitution in a phase 1 trial (http://ClinicalTrials.gov identifier NCT00737113, “RhGH for accelerating immune reconstitution post unrelated cord blood transplant”).
Tyrosine kinase inhibition to facilitate thymic engraftment
The Mackall group (Krauss et al)38 demonstrated in a murine transplant model that tyrosine kinase inhibition by sunitinib facilitated thymic engraftment by modulating thymic niche accessibility (Figure 2). Sunitinib inhibits the important thymocyte growth factors c-kit and FLT-3.39 This effect was much more distinct compared with treatment with a monoclonal anti-CD25 antibody to open the CD4−CD8− double-negative 3 (DN3) niche for thymocytes. Synergistic effects applying sunitinib and anti-CD25 antibody to open DN1-DN3 niches occurred. Currently, studies are ongoing in murine models to determine the effects of the combination on stem cell engraftment, durability of engraftment, and activity in minor mismatched models. The option to open up niches for naive T cells might be of particular interest to older patients as an increasing proportion of niches in peripheral immune tissues become occupied by terminally differentiated cells.40 Thus, the few naive T cells produced by the deteriorating thymus might not be able to take residence in the larger number of available niches.
Notch-based culture systems to promote T-cell reconstitution
Notch signaling is required for cellular differentiation processes. Four different Notch receptors (Notch 1-4) and 5 ligands (Jagged 1 and 2 and Delta-like 1, 3, and 4) have been identified. The inhibition of Notch 1 results in a partial inhibition of thymocyte differentiation41 and accumulation of precursors in the thymus. T-cell lineage committed precursor cells for adoptive cellular therapies can be obtained in large quantities applying Notch-based culture systems. They have been proven to be very effective in enhancing T-cell reconstitution and antitumor activity after allogeneic TCD HSCT in murine models (Figure 2).42,43 Currently, there is an observational trial ongoing in the United States to evaluate Notch-induced NK-cell activity in blood and bone marrow samples of cancer patients versus healthy controls (http://ClinicalTrials.gov identifier NCT00918658, “Study of NK cells in BM and blood samples from patients with hematologic cancer and from patients who do not have cancer”). Preliminary results of a phase 1 study evaluating the effect of applying Notch-based culture system for infusion of engraftment facilitating cells in human allogeneic HSCT were presented at the American Society of Hematology 2008 annual meeting.44
Improved immune reconstitution after transplantation with exogenous cytokines
Application of IL-2.
IL-2 (Proleukin; Chiron Corporation) is a pleiotropic cytokine with a central role in immune responses. Its application has been evaluated as safe in small trials with a short follow-up. Lower doses of IL-2 appeared to enhance NK-cell numbers without significant effects on T cells.45 Recombinant IL-2 administered as consolidating immunotherapeutic agent early after HSCT at a time of minimal residual disease might reduce the relapse rate and increase the immunocompetence of these patients. This could be because of a lymphoid orientation of primitive CD34+CD105+ cells, which express high-affinity IL-2 receptors. Exogenous IL-2 might thus lead to an enhancement of the autologous GVL effect.46 The best evidence for IL-2 enhancement of donor T-cell function after allogeneic HSCT comes from reports of patients who failed to respond to donor lymphocyte infusion (DLI) for relapsed disease but achieved complete remission after treatment with IL-2.47,48 The role of IL-2, when added to DLI, needs to be further elucidated. At present, there is no general agreement on the optimum dosage or route of administration, and clinical trials in HSCT have led to conflicting results. The determination of optimum dosage schedules and methods of administration should enable a better assessment of the role of IL-2 in the treatment of these patients. Currently, there are several clinical trials just completed or still ongoing in the allogeneic HSCT setting, which it is hoped will define the use of IL-2 (eg, http://ClinicalTrials.gov identifiers NCT00003962, “IL-2 following BMT in treating patients with hematologic cancer,” and NCT00941928, “Haploidentical NK cells with epratuzumab [IL-2] for relapsed ALL”).
Application of IL-7.
IL-7 (CYT 99007; Cythris) plays a key role in the development of T cells and promotes thymic-dependent and -independent pathways.49,50 It is the most potent cytokine identified so far, promoting thymopoiesis by enhancing proliferation of immature thymic progenitors.51 Its impact on T-cell immune reconstitution after murine HSCT is still a controversial issue, depending on the characteristics of the transplantation, the model used, and the dose and length of IL-7 treatment. In some murine models, the application of IL-7 for 1 to 2 weeks early after HSCT led to an enhanced proliferation of immature thymic progenitors,51 however appeared to be transient.52 Alpdogan et al demonstrated in an allogeneic murine model that IL-7 increased the homeostatic proliferation of nonalloreactive T cells, but had no effect on alloreactive T cells and the development of GVHD.53 Others have found the opposite results in mice confirmed by IL-7Rα blockade experiments.54,55 In an autologous nonhuman primate model, treatment with IL-7 led to an increase of the CD4+ cell counts through peripheral expansion rather than de novo generation. In line with this observation were increased volumes of spleen and lymph nodes but not of the thymus in the IL-7–treated animals compared with the controls.56 Similarly, in a nontransplantation trial in human cancer patients, IL-7 application led to an increase of naive T cells with a broad TCR repertoire (Figure 2).57 Importantly, in the autologous nonhuman primate model with application of IL-7 for 6 to 10 weeks after transplantation, a GVHD-like gut infiltration by T cells was observed. Thus, this agent needs to be applied with caution in patients to avoid deleterious effects. Currently a phase 1 trial in allogeneic HSCT is ongoing (http://ClinicalTrials.gov identifier NCT00684008, “Safety Study of IL-7 in Recipients of a Hemopoietic Stem Cell Transplant Peripheral Blood Stem Cell Transplant”).
Application of IL-15.
IL-15 as member of the IL-2 cytokine family stimulates proliferation of T cells, NK cells, and B cells through a receptor consisting of IL-15Rα, IL-2Rβ, and the common γ chain. It has been found to be less toxic than IL-2 in murine transplantation models58 and shows potential as adjuvant for immunotherapy and tumor vaccination strategies.59,60 It has also been observed to improve T-cell engraftment in murine models.61 As the primary survival and growth factor for NK cells, it may promote GVL but not GVHD effects.62 IL-15 has been applied as adjuvant in vaccination, but thus far there have not been any trials with IL-15 in the HSCT setting.
Cellular therapy after HSCT
Adoptive transfer of ex vivo–expanded immunomodulatory cells such as Treg cells, NK/Treg cells, donor-derived NK cells, and mesenchymal stem cells (MSCs) and adoptive transfer of allogeneic T cells specific for viral63,64 or tumor65,66 antigens appears promising to improve immune reconstitution after transplantation. In our review, we focus primarily on adoptive transfer of T cells67,68 and the cotransplantation of MSCs.
Adoptive transfer of viral and leukemic-specific T cells and use of regulatory T cells after HSCT
Increasing research activity is ongoing to enhance GVL-promoting cytotoxic T cells while suppressing the unwanted GVHD effects based on differential requirements for functionality, for example, tumor necrosis factor–related apoptosis-inducing ligand expression in murine models.69,70 Adoptive transfer of tumor-specific cytotoxic T cells is associated with different hurdles, as induction of unwanted GVHD renders DLI a potential toxic and insufficient controllable treatment regimen. To complicate issues further, the expansion of high-avidity tumor or leukemia-specific T cells in vitro is difficult and leads to a low efficacy in vivo based on, for example, exhaustion and loss of function in vitro.64 One promising approach to decrease the risk of GVHD is the genetic manipulation of T cells with herpes simplex virus thymidine kinase suicide gene as developed in Milan, Italy.71,72 If GVHD develops, transduced cells can be eliminated by ganciclovir treatment. However, difficulties remain because of low and transient transgene expression, unpredictable pairing of the exogenous and endogenous TCR chains, and poor survival and expansion potential of gene-modified effector T cells. A novel approach to increase functionality of the suicide gene–modified T cells with a central memory phenotype is therefore in vitro pretreatment with IL-7 and IL-15.73 Viral infection and reactivation contribute significantly to mortality and morbidity after allogeneic HSCT. There have been many attempts to selectively improve immune reconstitution of virus-specific T cells after HSCT. Recent approaches are the infusion of CMV, Epstein-Barr virus, or adenovirus-specific T cells selected from the donor and transferred to the recipient.74-78 In addition, CMV-specific T cells can be activated and expanded in vitro by stimulation with antigen-presenting cells loaded with specific proteins or peptides,79 as recently reviewed by our group.80 Essential for the impact of CMV reactivation on the HSCT outcome regarding triggering of GVHD and/or promoting GVL effects appears to be the quality of the T-cell response. Furthermore, there have been multiple approaches to selectively improve immune recovery by attenuating GVHD, for example, by expansion of T regulatory T cells (Treg cells), T-cell anergy induction, or selective reduction of alloreactivity as reviewed elsewhere.81-84
GVL promotion by minor histocompatibility antigen–specific T cells
Increasing evidence suggests that CD8+ T cells recognizing minor histocompatibility antigens (mHags) on leukemic cells also play an essential role promoting GVL effects after allogeneic HSCT.85,86 These mHag-specific T cells may be generated applying a HLA/mHag multimer-guided approach, for example, coating autologous dendritic cells with HLA-A2/mHag complexes for in vitro generation of mHag-specific T cells.
Adoptive transfer of NK cells
The adoptive transfer of NK cells that do not promote GVHD but do promote GVL reactions is particularly useful in haploidentical HSCT where the risk of fatal GVHD is increased.87 In the case of a KIR mismatch, NK cells kill allogeneic cells that lack a class I MHC ligand for clonally distributed KIR as outlined in “Haploidentical HSCT in adults.”23,88
Cotransplanting mesenchymal stem cells
The bone marrow microenvironment is increasingly being recognized as a potential target for immunotherapeutic attempts during or after an allogeneic HSCT. The results of applying mesenchymal stem cells (MSCs) are so far contradictory. Although there is consensus that they are pluripotent, have immunomodulatory abilities, and are a tool for managing or preventing GVHD as well as promoting clinical transplantation tolerance, it is not yet clear under which circumstances they support hematopoietic progenitor cell engraftment and show immunosuppressive properties in vivo.
In small case series and phase 1/2 clinical trials, hematopoietic engraftment has been accelerated when MSCs were cotransplanted in the haploidentical as well as in the UCB setting.89,90 Whether MSCs lead to a proliferation induction or inhibition of T cells in vitro appears to be dose dependent.87,91 When MSCs interact with NK cells, they inhibit IL-2–induced proliferation of resting NK cells, whereas they only partially affect the proliferation of activated NK cells in vitro.92 Several studies either as clinical case series or in animal models have advocated that MSCs may be transplanted across allogeneic barriers without eliciting an immune response. However, the Moretta group (Spaggiari et al92 ) demonstrated in vitro that IL-2–activated NK cells (but not freshly isolated NK cells) efficiently lyse autologous and allogeneic MSCs. A recent evaluation in a murine model questioned the immune privilege of MSCs but supported the hypothesis that they induce rejection in the allogeneic setting, which is followed by an immune memory potentially challenging long-term survival of allogeneic MSCs.93 Larger clinical trials with sufficient statistical power and an adequate scientific program are needed to further clarify the role of MSCs in allogeneic HSCT.
Immune escape mechanisms
Unfortunately, many patients develop a relapse of their underlying disease after allogeneic HSCT. Although the majority of relapses occur in the periphery, isolated relapses at extramedullary sites as chloromas or plasmacytomas or at immunologically privileged sites including central nervous system and gonads are observed.9 Leukemia and other tumor cells escape from the immunologic attack by changes in antigen presentation and production of inhibitory cytokines.94 Recently, Vago et al95 demonstrated that the genomic loss of the mismatched HLA locus in leukemic cells is another major mechanism of in vivo escape from T-cell immune surveillance after haploidentical HSCT. They demonstrated that in 5 of 17 patients relapsing with AML, patient-specific HLA alleles could not be detected in bone marrow samples harvested at disease relapse. They showed that donor T cells were able to recognize the original HLA-heterozygous leukemia but not the mutant variant of the leukemia harvested at the time of relapse. The genomic rearrangement granted the disease an in vivo selective advantage in escaping from an established donor T-cell response (Figure 1). These findings support the hypothesis that major HLA antigens are principally responsible for the GVL effect. T-cell effector functions may be influenced by leukemic cells. Disappearance of high-avidity T cells in patients with active CML disease has been described as well as the reappearance of these cells in interferon-α responders.96
Future developments
Recently, monoclonal antibodies such as the anti-CD20 antibody rituximab (Mabthera; Roche) have been shown to be useful in several clinical trials for the treatment of GVHD or Epstein-Barr virus–associated lymphoproliferative diseases, and thus have a positive influence on immune reconstitution after HSCT.97 Furthermore, proteasome inhibitors such as bortezomib (Velcade; Ortho Biotech) and immunomodulatory drugs (so-called IMIDs) such as lenalidomide (Revlimid; Celgene), which are well established in cancer treatment because of their direct antitumor effects, also modulate the tumor microenvironment. They appear to suppress GVHD and potentially enhance GVL effects when used alone or in combination with DLI.98-100
Summary and outlook
HSCT offers the opportunity for successfully treating malignancies and immune disorders. New therapies and better supportive care continue to improve outcome after HSCT. Nevertheless, delayed immune reconstitution and disease relapse remain major limitations to the widespread application of allogeneic transplantation. Understanding the causes of immune escape and hampered immune reconstitution provides the rationale to develop powerful immunotherapies. It is important to develop scores to identify those patients who would benefit most from immunomodulatory treatments during and after allogeneic transplantation.
Acknowledgments
The authors thank Torsten Steinbrunn for critical reading of the paper. The authors apologize to all authors whose work could not be cited because of length restrictions of this update on immune reconstitution after allogeneic HSCT.
Authorship
Contribution: R.S. and H.E. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hermann Einsele, Department of Internal Medicine II, Julius-Maximilians-University, Oberdürrbacher Strasse 6, Bldg A3, Rm A3.-1.918, 97080 Würzburg, Germany; e-mail: einsele_h@klinik.uni-wuerzburg.de.