Abstract
Granulocyte/macrophage colony-stimulating factor promotes growth, survival, differentiation, and activation of normal myeloid cells and plays an important role in myeloid leukemias. The GM-CSF receptor (GMR) shares a signaling subunit, βc, with interleukin-3 and interleukin-5 receptors and has recently been shown to induce activation of Janus kinase 2 (JAK2) and downstream signaling via formation of a unique dodecameric receptor complex. In this study we use 2 activated βc mutants that display distinct signaling capacity and have differential requirements for the GMR α-subunit (GMR-α) to dissect the signaling pathways associated with the GM-CSF response. The V449E transmembrane mutant selectively activates JAK2/signal transducer and activator of transcription 5 and extracellular signal-regulated kinase (ERK) pathways, resulting in a high level of sensitivity to JAK and ERK inhibitors, whereas the extracellular mutant (FIΔ) selectively activates the phosphoinositide 3-kinase/Akt and IκKβ/nuclear factorκB pathways. We also demonstrate a novel and direct interaction between the SH3 domains of Lyn and Src with a conserved proline-rich motif in GMR-α and show a selective requirement for Src family kinases by the FIΔ mutant. We relate the nonoverlapping nature of signaling by the activated mutants to the structure of the unique GMR complex and propose alternative modes of receptor activation acting synergistically in the mature liganded receptor complex.
Introduction
The granulocyte/macrophage colony stimulating factor (GM-CSF), interleukin-3 (IL-3), and interleukin-5 (IL-5) receptors are key contributors to the regulation of normal hematopoiesis, mediating growth and survival of hematopoietic progenitor cells and the production and activation of mature hematopoietic cells. GM-CSF in particular can provide both permissive and instructive signals for myeloid differentiation1 and has been shown to play a critical role in dendritic cells.2 Although it is dispensable for steady-state hematopoiesis,3 GM-CSF has an important accessory role in radioprotection by donor hematopoietic cells.4 It also has a nonredundant role in surfactant clearance by alveolar macrophages, resulting in lung disease in GM-CSF–null animals.5 Null animals display compromised antigen-specific and lipopolysaccharide-induced T-cell responses and interferon-γ production, have defects in macrophage function,6 and are susceptible to various infectious agents.7 GM-CSF and IL-3 have important roles in leukemia, with autocrine production and overexpression of their ligand-binding subunits (IL-3R-α and GM-CSF receptor α subunit [GMR-α]) documented in acute myeloid leukemia (AML).8,9 Constitutive activation of GM-CSF survival pathways has been reported in AML,10 and recently GM-CSF also has been shown to play an important role in primary resistance in chronic myeloid leukemia.11
GM-CSF induces activation of Janus kinase 2 (JAK2) and downstream signaling pathways via formation of a unique dodecameric receptor complex.12 Activation of JAK2/signal transducer and activator of transcription 5 (STAT5), Ras-Raf- extracellular signal-regulated kinase (ERK1/2), and phosphoinositide 3-kinase (PI3K)/Akt pathways by the mature GM-CSF receptor have been well characterized both in normal hematopoiesis and in disease where aberrant signaling has been shown to contribute to dysregulated myelopoiesis.13 However, extensive signaling redundancy and cross-talk among cytokine receptors (CRs) has made it difficult to link individual pathways to specific functional outcomes, such as cell survival, proliferation, and differentiation. Activation of one pathway often feeds into another, resulting in a networked signaling response, the final outcome of which is influenced by the cell context, stage of differentiation, transformation, and microenvironment.14 It is also clear that many signaling pathways converge on the same signaling molecules and that some biologic effects can be mediated by multiple effectors.
In part, signaling redundancy of CRs can be attributed to the sharing of receptor subunits. GM-CSF, IL-3, and IL-5 receptors share the common beta subunit (βc), which is the primary signaling subunit that is preassociated with JAK2 and contains several tyrosines which upon phosphorylation provide protein docking sites for activation of signal transduction. Specificity of this class of CRs is achieved through ligand-specific α subunits that enable affinity conversion of the receptor complex in the presence of a specific ligand.15 Although essential for receptor function, determination of the precise signaling contribution of the α subunits has been hampered by the lack of understanding of the role of interacting molecules. Deletion of the short cytoplasmic domain of GMR-α abolishes ligand-induced signaling with no effect on ligand binding.16 The membrane-proximal region, and in particular, the SH3 binding site/PROX-like (SBP) motif,17 is essential for GM-CSF–induced activation of JAK2.18 Some signaling molecules have been shown to associate with the GMR-α cytoplasmic domain, including the p85 regulatory subunit of PI3K,19 and IκKβ20 ; however, the nature and the role of these interactions remain poorly characterized. Nevertheless, it is interesting to note that although GMR-α is essential for GM-CSF receptor activation, it does not signal by itself, highlighting the significance of the GMR-α interaction with βc.
To reduce the level of signaling complexity and dissect the contribution of signaling events activated by GMR, we21,22 have used 2 constitutively activated mutants of βc (V449E and FIΔ) that deliver a subset of the proliferation, survival, and differentiation signals induced by the ligand-activated GMR and display a differential requirement for the GMR-α. Specifically, FIΔ contains a 37 amino acid duplication within the extracellular membrane-proximal region of βc, leading to ligand-independent activation with a strict requirement for GMR-α, consistent with a minimal heterodimeric structure.23 Conversely, V449E contains an amino acid substitution in the transmembrane domain of βc, which is proposed to be associated with ligand- and GMR-α–independent aggregation and activation.23 The differential activity of these 2 mutants also is clear in vivo, where expression of FIΔ induces a chronic myeloproliferative disorder and expression of V449E by mouse bone marrow reconstitution results in an AML.24 To understand the differences in signaling between these 2 classes of activating mutants, we22,25 have used the bipotential murine myeloid cell line, FDB1, which switches between growth and granulocyte-macrophage (GM) differentiation in response to IL-3 and GM-CSF, respectively. Expression of hβc mutants V449E and FIΔ in these cells induces factor-independent proliferation or GM differentiation respectively, mimicking the functional response to cytokines. Here we describe nonoverlapping signaling signatures of 2 βc-activated mutants and propose a model whereby each initiates a subset of the signaling activated by the mature ligand-bound dodecameric GMR. We report an important contribution of JAK2-independent signaling from the FIΔ mutant and demonstrate novel and potentially critical interactions of both Lyn and Src with GMR-α. We propose that these interactions are critical in modulating signals generated by GMR and suggest an important role for accessory subunits in modulating signaling of other CRs.
Methods
Cell culture
The culture conditions of FDB1 cells, the construction of FIΔ and V449E retroviral expression plasmids, and the generation of stable cell lines have been previously described.25 In brief, FIΔ and V449E receptor expression were maintained through the addition of puromycin to culture media and assessed by flow cytometry by use of the anti–flag M2 antibody (Sigma-Aldrich). Before treatment of cells with inhibitors, cells were washed 3 times and starved of growth factor for 12 to 16 hours in medium-containing serum. Stimulation was carried out for 5 minutes at 37°C by the use of 500 bone marrow units (BMU)/mL mouse (m)IL-3 or mouse (m)GM-CSF. Unless otherwise indicated, the final concentration of inhibitors was 50μM PD98059; 25μM AG490, Jak2InhII, U0126, LY294002, PP1, and Lyn peptide inhibitor; 0.5μM wortmannin; and 5μM SU6656. All inhibitors were purchased from Merck with the exception of the Lyn peptide inhibitor, which was obtained from Tocris Bioscience. Transient transfections in HEK293T cells for immunoprecipitation studies were carried out with the use of Lipofectamine 2000 (Invitrogen).
Coimmunoprecipitation and Western immunoblot analysis
Cells were lysed in modified RIPA lysis buffer (MRLB), separated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis, and transferred to nitrocellulose. Proteins of interest were detected by use of the following antibodies and SuperSignal West Pico or West Dura substrates. Rabbit polyclonal anti-JAK2 [pYpY1007/1008] was purchased from Biosource. The mouse monoclonal anti–phospho-STAT5A/B (Tyr694/699), STAT3, and STAT5 antibodies were obtained from Upstate Cell Signaling Solutions. Rabbit polyclonal Phospho-IκBα (Ser32/36), Phospho-Akt (Ser473), Akt, Phospho-p44/42 MAP kinase (Thr202/Tyr204) and p44/42 MAP kinase, phospho-Src family (Y416), Src, Lyn, and p85 antibodies were purchased from Cell Signaling Technology. Secondary immunoglobulin G (IgG) antibodies and SuperSignal West Pico and West Dura detection substrates were obtained from Pierce Biotechnology. The secondary anti–mouse fluorescein isothiocyanate-conjugated antibody was purchased from Sigma-Aldrich. For coimmunoprecipitation, lysates were incubated overnight with 5 μg of our 4H1 antibody26 or IgG1 isotype control and 50 μL of protein A/G Sepharose. The Sepharose was washed with MRLB, boiled in SDS load buffer, and immunoblotted.
Glutathione-S-transferase pull-downs
Glutathione-S-transferase (GST) proteins containing SH3 domains from Src (aa 83-150), Lyn (aa 62-122), and p85 (aa 3-79) were affinity purified on 4MB-glutathione Sepharose (Amersham). Then, 500 μg of cell lysate from HEK293T cells expressing human (h)GMR-α or hGMR-α-APVA was incubated with GST-SH3-Sepharose conjugates overnight at 4°C. Precipitates were washed with MRLB 3 times, and proteins were eluted in SDS-polyacrylamide gel electrophoresis load buffer.
Intracellular flow cytometry
Cells were fixed by the use of a final concentration of 1.6% formaldehyde at 37°C for 15 minutes, permeabilized in 1 mL of ice cold methanol on ice for 30 minutes, and incubated with 5 μg/μL primary antibody (unless otherwise stated) for 30 minutes on ice. After incubation with corresponding fluorescein isothiocyanate–conjugated secondary antibodies on ice for 30 minutes, samples were analyzed by flow cytometry (Beckman-Coulter Epics Elite ESP Flow Cytometer/Cell Sorter).
Fluorescence polarization
GST fusion proteins were prepared for mLyn, mSrc, and mp85 SH3 domains. A black 96-well plate was blocked with casein for 1 hour at 37°C. Samples were incubated in triplicate with 100nM fluorescein-labeled mGMR-α peptide (fluorescein-RLFPPIPGI) and 25 μg of GST-SH3. As controls, mGMR-α-pep only and m-GMR-α-pep + GST were used. The assay plate was incubated for 5 minutes at room temperature before it was read on the FLUOstar OPTIMA (BMG LABTECH) fluorescence plate reader at an excitation wavelength of 520 nm. Data were analyzed with the use of FLUOstar Galaxy software, and polarization values of the controls were subtracted from the other sample values to obtain the relative change in polarization (ie, ΔmP value) for the test samples.
Results
Signaling from the GM-CSF receptor FIΔ mutant is predominantly JAK2 and ERK1/2 independent
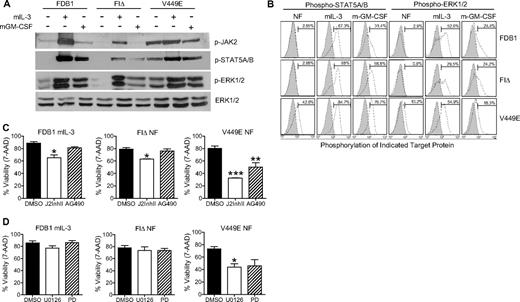
GM-CSF signaling is primarily mediated by activation of JAK2 associated with the cytoplasmic domain of βc. JAK2 phosphorylation is dependent on an intact PXXP motif in the GMR-α subunit which, when mutated, abolishes JAK2/STAT5 signaling.27 Given this requirement for GMR-α in JAK2 signaling, we tested the activation and requirement for JAK2 in signaling from 2 activated βc mutants (FIΔ and V449E) that display a differential requirement for GMR-α.22 Parental FDB1 cells and cell populations stably expressing FIΔ and V449E mutants were withdrawn from growth factor for 16 hours, and lysates were prepared. As a control for signaling pathway activation, cell populations also were treated for 5 minutes with mIL-3 or mGM-CSF. Western immunoblot analysis was used to determine the phosphorylation status of JAK2 and its downstream effector STAT5A/B (Figure 1A).
The hβc FIΔ mutant generates JAK2- and ERK1/2-independent signals. (A) FDB1 cell populations were cultured without growth factor for 16 hours and then stimulated with mIL-3 or mGM-CSF for 5 minutes. Whole-cell lysates were subjected to Western blot analysis with the indicated antibodies. (B) FDB1 cells were cultured in the absence of growth factor (NF), mIL-3, or mGM-CSF and then fixed, permeabilized, and stained with the indicated primary antibodies (open histograms) or isotype-matched control (gray histograms). (C-D) FDB1 cells cultured in 500 BMU mIL-3 and FDB1 cells expressing FIΔ or V449E cultured in the absence of growth factor were treated for 24 hours with dimethyl sulfoxide (DMSO), (C) JAK2 inhibitor II (J2InhII) or AG490, and (D) U0126 or PD98059 (PD). Viability was measured by 7-amino-actinomycin D staining and flow cytometry. Error bars represent SEM, where n = 3; statistical significance was calculated by the use of a Student t test, *P < .05 **P < .01, ***P < .001.
The hβc FIΔ mutant generates JAK2- and ERK1/2-independent signals. (A) FDB1 cell populations were cultured without growth factor for 16 hours and then stimulated with mIL-3 or mGM-CSF for 5 minutes. Whole-cell lysates were subjected to Western blot analysis with the indicated antibodies. (B) FDB1 cells were cultured in the absence of growth factor (NF), mIL-3, or mGM-CSF and then fixed, permeabilized, and stained with the indicated primary antibodies (open histograms) or isotype-matched control (gray histograms). (C-D) FDB1 cells cultured in 500 BMU mIL-3 and FDB1 cells expressing FIΔ or V449E cultured in the absence of growth factor were treated for 24 hours with dimethyl sulfoxide (DMSO), (C) JAK2 inhibitor II (J2InhII) or AG490, and (D) U0126 or PD98059 (PD). Viability was measured by 7-amino-actinomycin D staining and flow cytometry. Error bars represent SEM, where n = 3; statistical significance was calculated by the use of a Student t test, *P < .05 **P < .01, ***P < .001.
As predicted, we observed JAK2 and STAT5A/B phosphorylation in all FDB1 cell populations responding to IL-3 stimulation. Although we observed robust constitutive JAK2 phosphorylation in FDB1 cells expressing the activated V449E mutation, FDB1 cells expressing FIΔ did not display constitutively activated JAK2 or STAT5A/B (Figure 1A). Similarly, we observed negligible constitutive phosphorylation of ERK1/2 in FDB1 FIΔ cells compared with the robust constitutive activation of ERK1/2 observed in FDB1 V449E cells (Figure 1A). Growth factor stimulation of FIΔ- and V449E-expressing cells results in lower levels of tyrosine phosphorylation compared with growth factor stimulated parental FDB1 cells, probably because of a feedback pathway from the constitutive receptors that suppresses wild-type receptor responses. A more sensitive single-cell phospho-profiling approach28 also was used to detect STAT5A/B and ERK1/2 phosphorylation (Figure 1B). Consistent with Western immunoblot analyses, these results confirmed that FIΔ signals independently of STAT5 and ERK1/2 in the absence of growth factor and that V449E activates both STAT5 and ERK1/2, despite its lack of requirement for GMR-α (Figure 1B).
To further assess the requirement for JAK2 and ERK1/2 signaling in the cellular response to the βc mutants, we assessed the viability of FDB1 cell populations treated for 24 hours with JAK inhibitors AG490 and JAK2 inhibitor II (J2InhII) and the mitogen-activated protein/ERK kinase (MEK) inhibitors U0126 and PD98059 (Figure 1C). A 24-hour time point for viability was chosen because parental FDB1 cells die by 24 hours after growth factor withdrawal, and all functional attributes of the cells at this time are presumed to be attributed to signaling from the activated receptors. FDB1 FIΔ cells undergo differentiation; therefore, effects at later time points may not be inhibitor specific and may represent changes that occur as the cells differentiate. Murine IL-3 stimulation after growth factor starvation was used as a control in these experiments because the transient activation of endogenous murine receptors provides an indication of starvation efficiency. Treatment of FDB1 V449E cells with 25μM AG490 and 25μM J2InhII resulted in a significant reduction in the levels of phospho-STAT5A/B as detected by immunoblot analysis (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). An average of 30% and 48% reduction in the viability of FDB1 V449E cells was observed after AG490 and J2InhII treatment, respectively (Figure 1C). In contrast, the JAK inhibitors had a lesser effect on the viability of FDB1 FIΔ cells after growth factor withdrawal, consistent with the generation of JAK2-independent signals by the FIΔ mutant receptor (Figure 1C).
MEK kinases lie upstream of MAPK, and treatment with MEK inhibitors results in inhibition of ERK1/2 activity. We assessed the effect of 2 MEK inhibitors, PD98059 and U0126, on the viability of all FDB1 cell populations. No effect was seen on the viability of parental or FDB1 FIΔ cells after growth factor withdrawal and treatment with 50μM PD98059 or 25μM U0126, consistent with the phosphorylation status of ERK1/2 in these cells (Figure 1D). Conversely, FDB1 V449E cells showed a marked decrease in cell viability to approximately 40% by 24 hours after MEK inhibitor treatment (Figure 1D). Treatment of FDB1 V449E cells with U0126 and PD98059 resulted in a significant reduction in the levels of phospho-ERK1/2 as detected by immunoblot analysis (supplemental Figure 1B). These inhibitor studies suggest a major contribution of both JAK2/STAT5 and ERK1/2 pathways in promoting survival of cells expressing the V449E mutant that is consistent with previous reports implicating these pathways in cell survival and proliferation.29
Differential activation of p85/Akt and IκKβ/nuclear factorκB pathways by the activated βc mutants
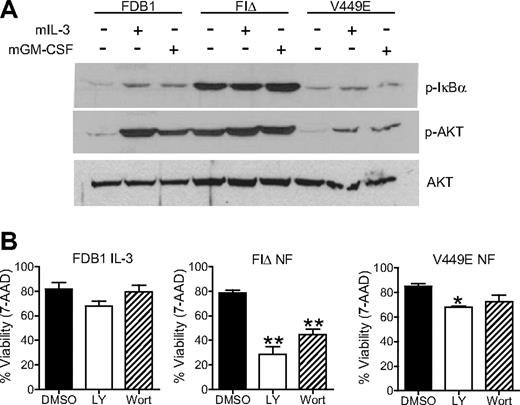
We next assessed activation of Akt, a downstream effector of p85, and of IκBα, a signaling molecule downstream of IκKβ that when phosphorylated derepresses the nuclear factor (NF)κB survival pathway. Lysates from FDB1 cell populations, starved and stimulated with mIL-3 or mGM-CSF, were immunoblotted with phospho-Akt or phospho-IκBα antibodies to measure activation of these pathways. Interestingly, FIΔ cells displayed high levels of constitutive Akt and IκBα phosphorylation in contrast to V449E-expressing cells, which showed minimal constitutive phosphorylation of both Akt and IκBα (Figure 2A). Cells expressing the V449E mutant also showed minimal phosphorylation of both Akt and IκBα when stimulated with mIL-3 or mGM-CSF compared with parental or FDB1 FIΔ cells (Figure 2A).
The hβc FIΔ mutant signals through Akt and NFκB pathways. (A) FDB1 cells were cultured in the absence of growth factor for 16 hours and then stimulated with 500 BMU of mIL-3 or mGM-CSF for 5 minutes. Whole-cell lysates were subjected to Western blot analysis with the indicated antibodies. (B) FDB1 cells cultured in 500 BMU mIL-3 and FDB1 cells expressing FIΔ or V449E cultured in the absence of growth factor were treated with DMSO, LY294002 (LY), or wortmannin (Wort) for 24 hours. Viability was measured by 7-amino-actinomycin D staining and flow cytometry. Error bars represent SEM, where n = 2; statistical significance was calculated by the use of a Student t test, *P < .05 **P < .01.
The hβc FIΔ mutant signals through Akt and NFκB pathways. (A) FDB1 cells were cultured in the absence of growth factor for 16 hours and then stimulated with 500 BMU of mIL-3 or mGM-CSF for 5 minutes. Whole-cell lysates were subjected to Western blot analysis with the indicated antibodies. (B) FDB1 cells cultured in 500 BMU mIL-3 and FDB1 cells expressing FIΔ or V449E cultured in the absence of growth factor were treated with DMSO, LY294002 (LY), or wortmannin (Wort) for 24 hours. Viability was measured by 7-amino-actinomycin D staining and flow cytometry. Error bars represent SEM, where n = 2; statistical significance was calculated by the use of a Student t test, *P < .05 **P < .01.
FDB1 cells expressing FIΔ were sensitive to 2 PI3K inhibitors, showing a significant reduction in the levels of phospho-Akt as detected by immunoblot analysis (supplemental Figure 1C) and a significant decrease in cell viability over the course of 24 hours to 28% with 25μM LY294002 and 42% with 0.5μM wortmannin (Figure 2B). Wortmannin and LY294002 had a less-significant effect on the viability of FDB1 V449E cells, consistent with the reduced Akt phosphorylation in this mutant, and with the presence of an alternative survival signal mediated by JAK2/STAT5 and ERK1/2 pathways. All cells cultured in IL-3 maintained good viability irrespective of inhibitor treatment, emphasizing the redundancy of the multiple pathways activated by IL-3 (Figure 2B).
Role of GMR-α and Src family kinases in FIΔ signaling
Given the strict requirement of the FIΔ mutant for GMR-α23 and the significantly diminished JAK2 activation by this mutant, we next wished to identify molecules associated with GMR-α that may contribute to constitutive activation of this mutant. The regulatory subunit of PI3K, p85, has been previously shown to associate with the GMR-α subunit cytoplasmic domain, an interaction that is dependent upon the SH3 domain of p85.19 In addition, IκKβ has been shown to directly interact with the GMR-α in a yeast-2-hybrid screen.20 With a view to identify the signaling components that are present in the GMR-α:FIΔ complex, we used several approaches to identify proteins binding to the GMR-α–subunit cytoplasmic domain.
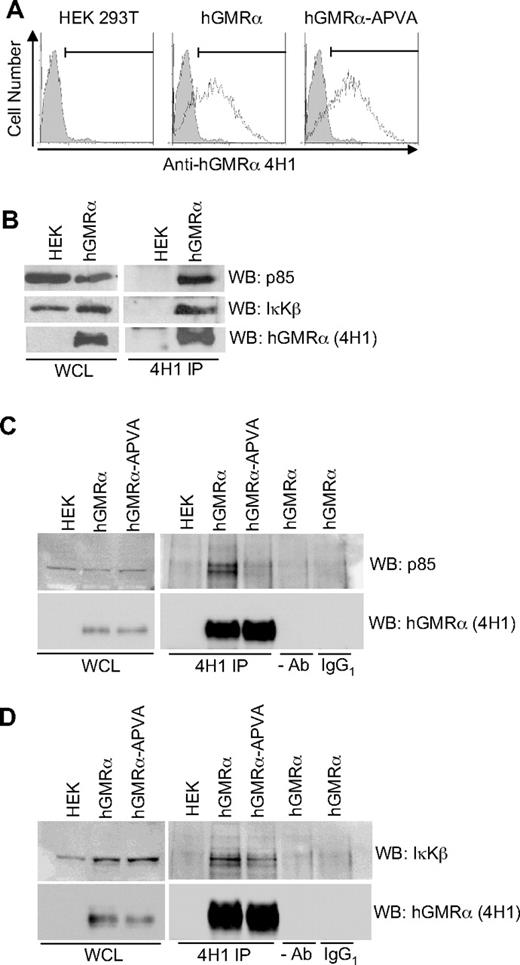
Coimmunoprecipitation was used to confirm previously reported associations of p85 and IκKβ with GMR-α.19,20 Coimmunoprecipitation of GMR-α with p85 and IκKβ was readily observed in HEK293T cells transiently expressing human GMR-α, consistent with published reports (Figure 3A-B). Using a form of GMR-α in which the 2 outer prolines in the PPVP motif are mutated to alanine (APVA), we showed that the interaction of p85 with GMR-α is dependent on the intact SBP motif (Figure 3C). Association of IκKβ with GMR-α is consistent with activation of this pathway by FIΔ and with several reports of NF-κB activation by GM-CSF and IL-3, playing an important role in modulating survival in myeloid cells.20,30 We postulated that the interaction of GMR-α with IκKβ is unlikely to involve the SBP motif because the sequence/structures of IκKβ do not have known proline-interaction domains (SH3 or WW domains). Consistent with this, we found that IκKβ can interact with the GMR-α-APVA mutant (Figure 3D).
IκKβ and p85 interact with hGMR-α. (A) Flow cytometric analysis to detect hGMR-α and hGMR-α-APVA after transfection of HEK293T cells. hGMR-α was detected with 4H1 antibody (open histograms). An isotype-matched control is shown for comparison (gray histograms). (B) hGMR-α, p85, and IκKβ detected in whole-cell lysates (WCL) from HEK293T cells expressing hGMR-α and in 4H1 immunoprecipitates (4H1 IP). (C) hGMR-α and p85 detected in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA and in 4H1 immunoprecipitates (4H1 IP) of the same cells. Control IPs minus antibody (−Ab), or with IgG1 (IgG1) are also shown. (D) hGMR-α and IκB detected in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA, and in 4H1 immunoprecipitates (4H1 IP). Control IPs minus antibody (−Ab), or with IgG1 are also shown.
IκKβ and p85 interact with hGMR-α. (A) Flow cytometric analysis to detect hGMR-α and hGMR-α-APVA after transfection of HEK293T cells. hGMR-α was detected with 4H1 antibody (open histograms). An isotype-matched control is shown for comparison (gray histograms). (B) hGMR-α, p85, and IκKβ detected in whole-cell lysates (WCL) from HEK293T cells expressing hGMR-α and in 4H1 immunoprecipitates (4H1 IP). (C) hGMR-α and p85 detected in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA and in 4H1 immunoprecipitates (4H1 IP) of the same cells. Control IPs minus antibody (−Ab), or with IgG1 (IgG1) are also shown. (D) hGMR-α and IκB detected in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA, and in 4H1 immunoprecipitates (4H1 IP). Control IPs minus antibody (−Ab), or with IgG1 are also shown.
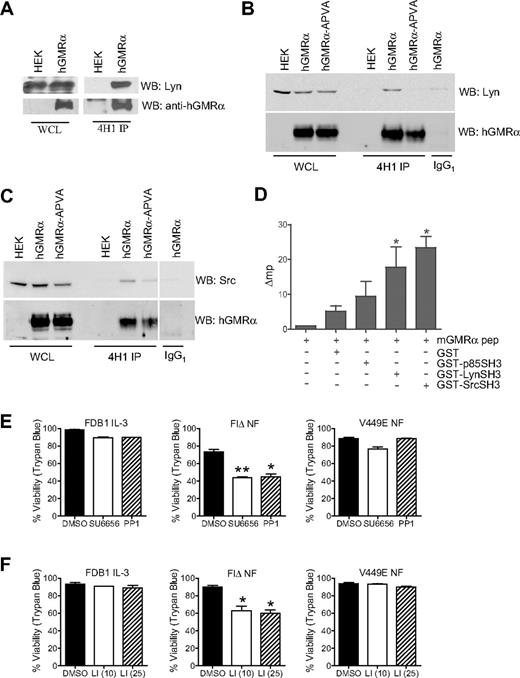
In addition to confirming previously reported interactions with GMR-α, we aimed to identify novel interactions with GMR-α. Because the SBP motif of GMR-α17 includes an excellent match to the SH3 binding consensus, we used a direct binding assay to identify interacting proteins with SH3 domains. Incubation of filters containing immobilized, recombinant SH3 domains (150 proteins; Panomics), with a fluorescently labeled peptide corresponding to the proline-rich core of the human GMR-α SBP motif, identified binding to the SH3 domains of Lyn, Src, CrkD2, and Nck2 (data not shown). Lyn and Src have known roles in CR signaling and were thus analyzed further. Coimmunoprecipitation studies confirmed the interaction of Lyn with GMR-α in HEK293T cells (Figure 4A), and this interaction was lost with the GMR-α–APVA mutant (Figure 4B). Similarly, we show coimmunoprecipitation of Src with GMR-α and reduced binding to GMR-α–APVA (Figure 4C). GST pull-down experiments also were used to demonstrate binding of Lyn and Src with GMR-α. GST fusion proteins of Lyn and Src SH3 domains were generated and used as “bait” to capture GMR-α transiently overexpressed in HEK293T cells. Interactions were confirmed for both Lyn SH3 and Src SH3 domains with GMR-α and were abrogated with GMR-α–APVA (supplemental Figure 2).
Association of Lyn and Src SH3 domains with hGMR-α. (A) Detection of hGMR-α and Lyn in whole-cell lysates (WCL) from HEK293T cells expressing hGMR-α, and 4H1 immunoprecipitates (4H1 IP) of the same cells. (B) Detection of hGMR-α and Lyn in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA, and in 4H1 (4H1 IP) and control IgG1 (IgG1) immunoprecipitates of the same cells. (C) Detection of hGMR-α and Src in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA, and in 4H1 (4H1 IP) and control IgG1 (IgG1) immunoprecipitates of the same cells. (D) Fluorescence polarization analysis of a fluorescein-conjugated mGMR-α peptide alone, with GST, or with p85, Lyn and Src SH3 domain GST fusions proteins. The change in millipolarization (Δmp) is given, where emissions of the mGMR-α peptide alone have been subtracted from the other samples. (E-F) FDB1 cells cultured in 500 BMU of mIL-3 and FDB1 cells expressing FIΔ or V449E cultured in the absence of growth factor were treated with DMSO, (E) 0.5μM SU6656 or 25μM PP1, or (F) 10μM and 25μM Lyn peptide inhibitor (LI) for 24 hours. Viability was measured by trypan blue exclusion. Error bars represent SEM, where n = 2; statistical significance was calculated by the use of a Student t test, *P < .05 **P < .01.
Association of Lyn and Src SH3 domains with hGMR-α. (A) Detection of hGMR-α and Lyn in whole-cell lysates (WCL) from HEK293T cells expressing hGMR-α, and 4H1 immunoprecipitates (4H1 IP) of the same cells. (B) Detection of hGMR-α and Lyn in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA, and in 4H1 (4H1 IP) and control IgG1 (IgG1) immunoprecipitates of the same cells. (C) Detection of hGMR-α and Src in WCL from HEK293T cells expressing hGMR-α or hGMR-α-APVA, and in 4H1 (4H1 IP) and control IgG1 (IgG1) immunoprecipitates of the same cells. (D) Fluorescence polarization analysis of a fluorescein-conjugated mGMR-α peptide alone, with GST, or with p85, Lyn and Src SH3 domain GST fusions proteins. The change in millipolarization (Δmp) is given, where emissions of the mGMR-α peptide alone have been subtracted from the other samples. (E-F) FDB1 cells cultured in 500 BMU of mIL-3 and FDB1 cells expressing FIΔ or V449E cultured in the absence of growth factor were treated with DMSO, (E) 0.5μM SU6656 or 25μM PP1, or (F) 10μM and 25μM Lyn peptide inhibitor (LI) for 24 hours. Viability was measured by trypan blue exclusion. Error bars represent SEM, where n = 2; statistical significance was calculated by the use of a Student t test, *P < .05 **P < .01.
In addition, the use of a fluorescence polarization assay31 with a fluorescently labeled murine GMR-α-SBP peptide demonstrated selective and direct binding of the peptide to GST-Lyn SH3 and GST-Src SH3 domain fusion proteins. We did not detect a significant interaction between p85-SH3 and the GMR-α-SBP peptide in this assay; however, we cannot exclude the possibility of a low-affinity direct interaction (Figure 4D). This finding raised the possibility that the association of p85 with GMR-α may be indirect, via association with Lyn or JAK2; however, using several binding assays we were unable to detect an interaction between JAK2 and GMR-α (data not shown), also reported elsewhere.12,32
The direct association of Lyn and Src with GMR-α suggests that the Src family of tyrosine kinases (SFKs) may play an important role in the response to GM-CSF and be particularly important for the FIΔ-activated mutant. We next used SFK-selective inhibitors to test whether SFKs selectively contribute to FIΔ-mediated survival and differentiation of FDB1 cells. We used 2 independent SFK inhibitors, 25μM PP1 and 5μM SU6656, which are known to reduce the activity of both Src and Lyn (supplemental Figure 1D). Importantly, in the absence of growth factor FDB1FIΔ cells showed sensitivity to SFK inhibitors with a clear reduction in viability (reduced to ∼ 40% with both inhibitors; Figure 4E).
To further assess the role of Lyn specifically, we also used a Lyn-binding peptide inhibitor that comprises a hβc sequence known to interact with Lyn.33,34 Treatment of FDB1 FIΔ cells with the Lyn peptide inhibitor resulted in a significant reduction in viability at the 2 concentrations used (10μM and 25μM) and had no effect on FDB1V449E cells (Figure 4F). The observation that V449E-mediated survival is not affected by treatment with SFK or Lyn-specific inhibitors suggests this pathway is not critical for signaling from this mutant. The inhibitors are presumably not acting via a direct or indirect affect on JAK2 activity because cells expressing GMR-α and hβc maintain robust JAK2 activation in the presence of IL-3 and GM-CSF when treated with SFK inhibitor (data not shown).
In addition, treatment of FDB1 cell populations with SFK inhibitors resulted in a partial reduction in phosphorylated Akt in FIΔ- but not V449E-expressing cells, suggesting the SFK survival signal may at least in part be mediated by Akt (supplemental Figure 1D). The partial reduction of Akt phosphorylation is consistent with Lyn activation of an Akt-independent survival pathways as suggested by Dos Santos et al.35 The increased sensitivity of FDB1 FIΔ cells to SFK inhibition and the selective requirement for GMR-α, together with the reduced sensitivity to JAK inhibition and the lack of JAK/STAT activation, are consistent with an important signaling role for the SFKs in the FIΔ-mediated response. This mutant thus provides a tool for dissecting the roles of SFKs in GM-CSF receptor signaling.
Discussion
GM-CSF has recently been demonstrated to induce a unique receptor subunit configuration involving formation of a dodecameric complex, which results in JAK2 dimerization and activation of JAK/STAT, ERK1/2, PI3K/Akt, and IκB/NFκB downstream pathways.12 In normal cells these pathways cooperate to induce growth, survival, and/or differentiation responses that are cell type and context dependent. In leukemic cells these GM-CSF–induced pathways make an important contribution to proliferation and survival.10 In particular, constitutive serine phosphorylation of GM-CSF receptor has been reported in primary AML cells, associated with an unknown mechanism of receptor activation.36
To dissect the relative contribution of GMR-α and βc to pathway activation and the role of each of these pathways in particular cell responses, we used βc-activated mutants that differentially require GMR-α and that activate nonredundant subsets of pathways and responses, allowing association of signaling with alternative receptor configurations and cellular outcomes. This approach has provided evidence for JAK2-independent and SFK-dependent signals associated with GMR-α. Taken together with evidence of association of GMR-α with the SFKs Lyn and Src, this suggests a novel contribution of GMR-α to responses downstream of GMR.
A comprehensive biochemical analysis revealed nonoverlapping signaling profiles of the βc mutants FIΔ and V449E. In the FIΔ mutant, the PI3K/Akt and IκKβ/NFκB pathways make important contributions to the survival response and are activated in the absence of detectable JAK2 activation. Although highly sensitive to PI3K inhibitors, the FIΔ mutant displayed relative resistance to 2 JAK inhibitors, in keeping with the limited requirement for activation of JAK2. We and others have shown recruitment of the p85 regulatory subunit of PI3K to the GMR-α and this action may facilitate activation of PI3K/Akt signaling in FIΔ in the absence of JAK2 activity.19 In contrast to the FIΔ mutant, signaling from V449E was associated with robust activation of JAK2/STAT5 and ERK1/2, high relative sensitivity to MEK inhibitors, and significantly diminished Akt and IκBα phosphorylation. The poor Akt activation by this mutant, which we have proposed previously to be associated with ligand-independent dimerization,23 is consistent with the observation that PI3K/Akt activity is not observed in BA/F3 cells expressing a βc/JAK2 chimera.37 This finding suggests that either JAK2 activation is not sufficient for the activation of Akt or that Akt activation is suppressed in the absence of additional pathways normally activated by the liganded receptor complex.
A major question from these finding relates to the nature of the primary signaling event associated with the FIΔ mutant. JAK-independent signaling is known to contribute to important outcomes from CRs, including βc.38 JAK2-independent signaling is also consistent with our previous analysis of serial truncations of the FIΔ mutant, which indicate that the primary signal is associated with a region distal to the Box 1 motif responsible for JAK2 association.32 Given the strict requirement of the FIΔ mutant for GMR-α, we explored protein interactions with the GMR-α cytoplasmic domain. We have confirmed here association of GMR-α with the p85 regulatory subunit of PI3 kinase, and with IκKβ, as reported previously.19,20 The finding that Src and Lyn associate with GMR-α directly via the critical proline-rich SBP motif strongly implicates the SFKs as playing a key role in signaling from the FIΔ complex and is consistent with studies demonstrating an important role for SFKs in receptor-mediated responses in the myeloid lineage. Members of the SFK family have been linked to activation of cellular signaling in response to GM-CSF (reviewed in Hibbs and Harder39 ); Lyn in particular is activated by IL-3, IL-5, or GM-CSF and can phosphorylate tyrosine residues in hβc in vitro.34
The selective sensitivity of the FIΔ mutant to a Lyn inhibitory peptide is consistent with Lyn being a key initiator of signaling from the FIΔ mutant. The inhibitory peptide may be acting predominantly by inhibiting Lyn associated with βc; however, the selective sensitivity of the FIΔ mutant to this peptide compared with the V449E mutant, together with the high relative sensitivity of FIΔ to the SFK inhibitors, clearly demonstrates the differential requirement for SFKs in FIΔ signaling. In contrast, the resistance that V449E displays to treatment with SFK inhibitors, including the Lyn inhibitory peptide, indicates that this mutant generates additional or alternative SFK-independent survival signals (probably JAK2 mediated). These survival signals are primarily mediated via the MAPK pathway because treatment with MEK inhibitors dramatically and selectively affects the survival of V449E-expressing FDB1 cells. In the FDB1 system IL-3 survival responses are also relatively resistant to SFK inhibitor treatment, and we propose that both JAK- and SFK-dependent survival pathways are being activated by the wild-type ligand-induced receptor complexes, with extensive redundancy. In vivo studies suggest that JAK2-independent signaling from hβc will support only limited responses and although myeloid progenitors from the fetal liver of JAK2-deficient mice show a lack of growth responsiveness to IL-3 and GM-CSF,37,40 survival responses of JAK2 knockout progenitors in response to these growth factors have not been studied in detail.
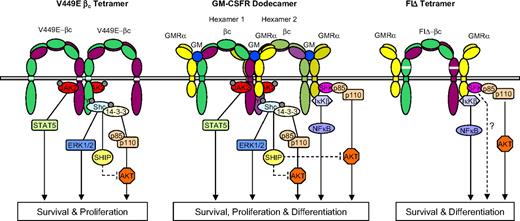
The signaling properties of the 2 activated mutants are consistent with the 2 classes of mutant representing alternative receptor complexes as proposed previously.23 These complexes deliver at least some of the proliferative, survival, self-renewal, and differentiative signals activated by a mature GM-CSF receptor complex. In Figure 5 we summarize the properties of these mutants in the context of the unique GM-CSF dodecameric receptor structure.12 We propose that the FIΔ- and V449E-activated receptor complexes initiate nonoverlapping signaling events, resulting in activation of complementary pathways and nonredundant signaling. These alternative events may be initiated within the wild-type dodecamer complex via the αββα tetramer structure or the αβ heterodimers (Figure 5). This would predict that the αββα complex signals predominantly through activated JAK2. It also raises the possibility that pathways downstream of JAK2 (eg, Shc-initiated activation of SHIP) suppress Akt activation,12 suggesting a mechanism for modulation of signaling within the dodecamer. The tetrameric structure predicted for the FIΔ mutant, together with the lack of detectable association of JAK2 with GMR-α, would preclude JAK2 activation and thus signaling would occur predominantly via JAK-independent mechanisms, including those signals generated from GMR-α. Whether these alternative complexes shown in Figure 5 represent intermediates in formation of the mature GM complex is not clear. The recent demonstration that low concentrations of GM-CSF induce a limited signaling response10,36 is consistent with a role for these alternative receptor configurations in certain contexts, including leukemic cell survival. Lyn frequently is activated in leukemic blasts from AML patients35 and in chronic myeloid leukemia blast crisis41 ; these studies raise the possibility that this is related to aberrant GM-CSF or IL-3 receptor signaling. We suggest that further studies with these mutants will facilitate the identification of the role of SFKs in GM-CSF responses and may shed light on the mechanisms associated with aberrant GM-CSF and Lyn activation in leukemia.
Model for assembly and activation of hβc mutants FIΔ and V449E. The high-affinity complex of the GM-CSF receptor (GMR) is a dodecamer structure (center) comprising 2 ligand bound hexamers.12 The central structure in the dodecamer complex enables JAK2 transphosphorylation and activation of STAT5- and Shc-mediated pathways. Proposed signaling through GMR-α occurs in αβ heterodimers (outer structures in the dodecamer complex), which initiate activation of Akt and NFκB pathways. Also indicated is a possible negative feedback mechanism whereby activation of SHIP downstream of JAK2 may suppress Akt activation. The proposed V449E structure is represented as a βc tetramer, which does not require GMR-α and initiates a subset of the signals generated by the dodecameric GMR complex. V449E-activated pathways are confined to those downstream of JAK2 and receptor tyrosine phosphorylation and support survival and proliferation in the FDB1 cells. The proposed FIΔ structure comprises an αβ tetramer that precludes JAK2 transphosphorylation. This complex generates signals predominantly through GMR-α and results in activation of Akt and NFκB pathways, supporting FDB1 survival and differentiation.
Model for assembly and activation of hβc mutants FIΔ and V449E. The high-affinity complex of the GM-CSF receptor (GMR) is a dodecamer structure (center) comprising 2 ligand bound hexamers.12 The central structure in the dodecamer complex enables JAK2 transphosphorylation and activation of STAT5- and Shc-mediated pathways. Proposed signaling through GMR-α occurs in αβ heterodimers (outer structures in the dodecamer complex), which initiate activation of Akt and NFκB pathways. Also indicated is a possible negative feedback mechanism whereby activation of SHIP downstream of JAK2 may suppress Akt activation. The proposed V449E structure is represented as a βc tetramer, which does not require GMR-α and initiates a subset of the signals generated by the dodecameric GMR complex. V449E-activated pathways are confined to those downstream of JAK2 and receptor tyrosine phosphorylation and support survival and proliferation in the FDB1 cells. The proposed FIΔ structure comprises an αβ tetramer that precludes JAK2 transphosphorylation. This complex generates signals predominantly through GMR-α and results in activation of Akt and NFκB pathways, supporting FDB1 survival and differentiation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mrs Sylvia Nobbs and Mr Sandy McIntyre for their assistance with flow cytometry.
Financial assistance was provided by the National Institutes of Health (NIH; Application ID: R01 HL60657) and by the National Health and Medical Research Council (NH&MRC) of Australia.
National Institutes of Health
Authorship
Contribution: M.P. and R.J.D. wrote the manuscript; M.P. performed the experiments and analyzed the data; D.G.S. provided experimental assistance; G.W.B., C.S., and T.R.H. provided advice and technical assistance; and A.L.B., T.R.H., A.F.L., M.L.H., and T.J.G. were involved in data interpretation and writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A/Prof Richard D'Andrea, The Division of Haematology, Centre for Cancer Biology, SA Pathology, PO Box 14 Rundle Mall, Adelaide, South Australia 5000, Australia; e-mail: Richard.Dandrea@health.sa.gov.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal