Abstract
Mutation of the p53 tumor suppressor is associated with disease progression, therapeutic resistance, and poor prognosis in patients with lymphoid malignancies and can occur in approximately 50% of Burkitt lymphomas. Thus, new therapies are needed to specifically target p53-deficient lymphomas with increased efficacy. In the current study, the specific impact of inhibition of the small GTPase Rac1 on p53-deficient B- and T-lymphoma cells was investigated. p53 deficiency resulted in increased Rac1 activity in both B-cell and T-cell lines, and its suppression was able to abrogate p53 deficiency–mediated lymphoma cell proliferation. Further, Rac targeting resulted in increased apoptosis via a p53-independent mechanism. By probing multiple signaling axes and performing rescue studies, we show that the antiproliferative effect of Rac1 targeting in lymphoma cells may involve the PAK and Akt signaling pathway, but not the mitogen-activated protein (MAP) kinase pathway. The effects of inhibition of Rac1 were extended in vivo where Rac1 targeting was able to specifically impair p53-deficient lymphoma cell growth in mouse xenografts and postpone lymphomagenesis onset in murine transplantation models. Because the Rac1 signaling axis is a critical determinant of apoptosis and tumorigenesis, it may represent an important basis for therapy in the treatment of p53-deficient lymphomas.
Introduction
Lymphoma is the fifth most diagnosed cancer in the United States each year, with its incidence increasing by 84% from 1974 to 2004. Burkitt lymphoma (BL) is an aggressive form of non-Hodgkin lymphoma that accounts for 30% to 50% of pediatric lymphomas and only 1% to 2% of adult lymphomas.1,2 BL is a B-cell tumor that occurs in several clinical forms. The endemic disease most often affects children and young adults in Africa infected with the Epstein-Barr virus, whereas the sporadic form of the disease is primarily not Epstein-Barr associated and is reported in Europe and North America. The third type of BL is associated with HIV infection. However, common among all types of BL is the propensity to lose p53 tumor suppressor function. A majority of BL lines and at least 30% of BL biopsies carry p53 mutations.3-7 Similar to other tumor types, p53 mutations in BL cluster in the core domain and include residues that affect its function, including Arg175, Arg248, and Arg273.8
Treatment of BL is centered around standard DNA-damaging chemotherapies. However, p53 mutation is predictive of resistance to these types of therapies among lymphoid malignancies and often contributes to disease progression and poor prognosis.9,10 Thus, pathways that contribute to the progression of p53-deficient tumors need to be revealed so that new therapies may be developed to specifically target these tumors.
Rac1, a member of the Rho family of GTPases, is an intracellular transducer known to regulate multiple signaling pathways that influence actin organization, apoptosis, proliferation, migration, and transformation.11-15 Deregulated expression or activation patterns of Rac1 can result in aberrant cell signaling and tumorigenesis. Rac1 is ubiquitously expressed and exists in 2 conformational states, an inactive GDP-bound form and an active GTP-bound form. In response to extracellular signals, the interconversion of these states occurs via guanine nucleotide exchange factors (GEFs), which convert Rac1 to its active form, and GTPase-activating proteins (GAPs), which inactivate Rac1.16,17 The importance of Rac1 activity hinges on its ability to interact with its specific effectors. Many of these effectors impinge upon antiapoptotic programs or on cell-cycle machinery to promote growth and survival of cancer cells that would normally undergo apoptosis. Because up-regulation of expression or activity, but rarely mutation, of Rac1 GTPase is associated with human tumorigenesis, it can be envisioned that Rac1 may serve as a signal modifier of primary genetic hits, such as p53 mutation, to regulate tumor progression. In support of a possible functional relationship between Rac1 signaling pathway and p53, p53 deficiency has been shown to increase Rac1 activity in primary mouse embryonic fibroblasts, and this collaboration is sufficient to promote transformation in these cells.11
Here, we tested the role of Rac1 in both p53-deficient B- and T-lymphoma cell proliferation and apoptosis. Increased Rac1 activity was evident in the absence of functional p53, and Rac1 targeting was able to abrogate p53-deficient hyperproliferation and induce apoptosis in both cell types. These data were recapitulated by in vivo xenografts that displayed decreased tumor development when Rac1 was suppressed. Last, our results from genetic mouse models of p53−/− lymphomagenesis suggest that targeting Rac1, but not closely related Rac2, can significantly increase animal survival time.
Methods
Cell culture and infection
Wild-type (p53-mutant) and p53ts mutant (p53-add back) BL41 (human BL cells) and J3D (murine T-cell lymphoma cells) cells created as described in Ramqvist et al18 were a kind gift from Drs K. G. Wiman (Karolinska Institute, Stockholm, Sweden) and Y. Wang (Oak Ridge National Laboratory, Oak Ridge, TN). Genetically identical, matched p53 wild-type and mutant human B-lymphoma cells (TK6 and NH32, respectively) were a generous gift of Dr H. L. Liber (Harvard University, Boston, MA). All lymphoma cell lines were propagated in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 2mM l-glutamine, and 100 U/mL penicillin/streptomycin at 37°C in air containing 5% CO2. Cells were infected with recombinant retrovirus encoding MIEG3-Rac1 dominant-negative mutant (Rac1 N17) produced according to standard protocol. Rac1 knockdown (shRac1) or scrambled control (shSCR) cells were created through infection with lentivirus encoding either an shRNA plasmid directed against Rac1 as previously described19 or a scrambled control plasmid. Retroviruses encoding a constitutively active PAK1 T423E mutation (caPAK) and a constitutively active Akt mutant containing N-terminal Src myristolyation sequences (caAkt) were constructed as previously described20 and were used in combination with retrovirus encoding pBabe-GFP-Akt kinase dead (Akt-KD; generously provided by Dr Davis Plas, Univeristy of Cincinnati College of Medicine) and retrovirus encoding MIEG3 as controls. All infected cell populations were sorted 48 hours after infection before experiments were carried out.
Immunoblot analysis and Rac pull-down assay
Whole-cell lysates were prepared by cell extraction using lysis buffer containing 20mM Tris-HCl (pH 7.6), 100mM NaCl, 10mM MgCl2, 1% Triton X-100, 0.2% sodium deoxycholate, 1mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 1 μg/mL aprotinin, and 1mM dithiothreitol for 30 minutes. Equal amounts of protein, as determined by Bradford assay, were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Specific proteins were detected by standard immunoblotting procedures using the following primary antibodies: (Cell Signaling Technologies; 1:500 dilution) phospho-PAK1 (Ser144)/PAK2 (Ser141), PAK1, phospho-p44/42 mitogen-activated protein (MAP) kinase (Thr202/Tyr204), p44/42 MAP kinase, phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, phospho-p38 MAP kinase (Thr180/Tyr182), p38 MAP kinase antibody, phospho-Akt (Tyr326), phospho-Akt (Ser473), Akt, p53, HA, cleaved caspase-3 (Asp175), cytochrome c (Upstate; 1:500 dilution), Rac1 (Novus Biologics; 1:1000 dilution), Rac2 (BD Biosciences; 1:1000 dilution), pan-Rac (catalog no. 610651, Research Diagnostics Inc; 1:10 000 dilution), Gapdh (Sigma-Aldrich; 1:500), and β-actin.
For Rac pull-down assay, cell lysates were incubated with purified glutathionine-agrose immobilized fusion proteins (10 μg each) at 4°C for 45 minutes. After incubation, the beads were washed twice in the lysis buffer, and the bound proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted as previously described.21 Densitometry was performed on the immunoblots using ImageJ software (National Institutes of Health).
The LY294002 (9901; Cell Signaling Technologies) or wortmannin (W3144; Sigma-Aldrich) P13K inhibition experiments were performed by incubating cells with the respective inhibitor for 3 hours before cell harvest for Rac1-GTP pull-down assay.
Flow cytometry
Cells (5 × 105) were harvested and processed for Annexin V/ 7AAD staining according to the manufacturer's protocol (BD Biosciences). Flow cytometric data were acquired on a FACSCanto bench-top flow cytometer (BD Biosciences), and the cell-cycle distributions were determined using FlowJo software (TreeStar). Blood samples from mice that received transplants were analyzed monthly by flow cytometry for chimerism. Staining was performed using antibodies to CD45.1 and CD45.2 (BD Biosciences).
Xenograft studies and mouse models
For xenograft experiments, 2- to 3-month-old nonobese diabetic/Shi-scid/IL-2Rγnull (NOG) mice were injected with 200 μL of a phosphate-buffered saline (PBS) and phenol red–free Matrigel matrix basement membrane (Becton Dickinson) solution (3:1) containing 2 × 106 cells subcutaneously into the flank in a contralateral manner. Tumor volume was measured with calipers every 7 days using the following equation: V = 0.52 (width)2 × (length). Xenograft tumors were weighed before a portion was taken for genomic polymerase chain reaction (PCR) of Rac1 deletion, or for Rac1 immunoblot. Tumors were then fixed in 10% neutral buffered formalin. For transplantation studies, p53−/−Mx1CreTg/+ or p53−/−Mx1CreTg/+Rac1flox/floxRac2+/− (referred to as p53−/− MxRac1 or p53−/− MxRac1 polyinosinic-polycytidylic acid [PolyIC]) compound 129Sv and C57BL/6J background mouse was generated as previously described.12 Total bone marrow of a p53−/−MxRac1 mouse was isolated and injected by tail vein into lethally irradiated (1175 cGy, split-dose) BoyJ recipients, which specifically differ from the donors by expression of a cell-surface protein, CD45.1 (recipient) versus CD45.2 (donor). Blood chimerism was assessed 1 month after transplantation, and mice containing approximately 90% or more donor cells received 3 × 300 μg of intraperitoneal PolyIC (Amersham Biosciences) injections every other day to delete the Rac1 gene in hematopoietic cells in vivo. Animals were then bled monthly to monitor genetic deletion, chimerism, and complete blood counts. p53−/− mice (a generous gift of Dr S. Lowe, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) were crossed to generate p53−/−Rac2−/− mice as previously described12 and used in the study. Upon death, the lymph nodes, liver, spleen, and thymus of the mice were retrieved aseptically. Housing, care, and all experimentation were performed according to protocols approved by the Institutional Animal Care and Use Committee of Cincinnati Children's Hospital Medical Center.
Rac1 genetic deletion genotyping
DNA was isolated from mouse peripheral blood and analyzed for prevalence of the knockout band compared with the loxP band as preformed previously.22
Results
p53 deficiency causes an increase of Rac1 activity in lymphoma cells
Analyses in primary mouse embryonic fibroblasts (MEFs) have revealed that endogenous Rac1 activity is subject to functional p53 regulation such that reintroduction of wild-type p53 into p53-deficient cells suppressed Rac1-GTP levels.13 To determine whether such an inverse functional relationship between Rac1 GTPase and p53 occurs in the context of malignancy, we measured Rac1 activity in both the p53-deficient BL41 human BL and J3D murine T-cell lymphoma lines in the presence or absence of functional p53 expression. For this purpose, a temperature-sensitive p53 mutant that is active at the permissive temperature of 32°C but inactive at the nonpermissive temperature of 37°C was expressed in the cells,18 and the relative levels of Rac1-GTP were estimated by GST-effector domain pull-down assays under both the permissive and nonpermissive conditions. As shown in Figure 1, functional p53 expression at 32°C effectively suppressed Rac1-GTP formation compared with the 37°C condition where p53 is nonfunctional in both the BL41 (Figure 1 lane 2 vs lane 4) and J3D (Figure 1 lane 6 vs lane 8) cells. These results indicate that expression of functional p53 restrains Rac1 activity and loss of p53 causes elevated Rac1 activity in both B- and T-lymphoma cells.
p53 deficiency causes elevated Rac1 activity. BL41 and BL41ts cells (left panel) and J3D and J3Dts cells (right panel) were cultured for 24 hours at 32°C or 37°C. Cell lysates were subjected to GST-PAK pull-down assay and processed for Western immunoblot with anti-p53 or anti-Rac antibodies. β-actin served as a loading control. The J3D bands displayed were part of the same gel, yet were separated by other lanes. The bands from at least 2 independent experiments were quantified by densitometry and displayed below. Error bars represent SD.
p53 deficiency causes elevated Rac1 activity. BL41 and BL41ts cells (left panel) and J3D and J3Dts cells (right panel) were cultured for 24 hours at 32°C or 37°C. Cell lysates were subjected to GST-PAK pull-down assay and processed for Western immunoblot with anti-p53 or anti-Rac antibodies. β-actin served as a loading control. The J3D bands displayed were part of the same gel, yet were separated by other lanes. The bands from at least 2 independent experiments were quantified by densitometry and displayed below. Error bars represent SD.
Rac1 targeting abrogates p53-deficient lymphoma cell proliferation
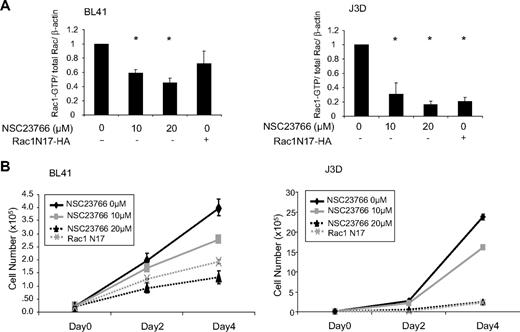
To examine the requirement of elevated Rac1 activity in the p53-deficient lymphoma cells for tumor cell growth, we have applied a dominant-negative mutant of Rac1, Rac1N17, and a pharmacologic inhibitor of Rac1, NSC23766, to the cells. The efficacy of the dominant-negative Rac1 mutant or the Rac-specific small-molecule inhibitor on Rac1 inhibition in the lymphoma cells was measured in the p53-deficient BL41 and J3D cells following either Rac1 targeting agent treatment. As shown in Figure 2A, Rac1 activity in both cell types was inhibited by NSC23766 in a dose-dependent manner. In parallel, Rac1 activity was also reduced by Rac1N17 expression. The inhibitory effect on Rac1 activity in J3D cells appeared to be more potent than that in BL41 cells, possibly due to species- or cell type–specific differences.
Rac1 activity contributes to p53-deficient lymphoma cell hyperproliferation. (A) Cell lysates were subjected to GST-PAK pull-down assay and processed for Western immunoblot to determine Rac1 activity in BL41 (left panel) and J3D (right panel) cells after 4-day treatment with NSC23766 or transduction with a Rac1 N17 mutation. Anti-HA or anti-Rac antibodies were used and β-actin served as a loading control. Immunoblot bands from at least 3 independent experiments were quantified by densitometry and displayed. *P < .05, significant with respect to untreated control. (B) BL41 (left panel) or J3D (right panel) cells were treated with NSC23766 or transduced with Rac1N17 as in panel A and cell number was monitored by trypan blue exclusion. Error bars represent SD.
Rac1 activity contributes to p53-deficient lymphoma cell hyperproliferation. (A) Cell lysates were subjected to GST-PAK pull-down assay and processed for Western immunoblot to determine Rac1 activity in BL41 (left panel) and J3D (right panel) cells after 4-day treatment with NSC23766 or transduction with a Rac1 N17 mutation. Anti-HA or anti-Rac antibodies were used and β-actin served as a loading control. Immunoblot bands from at least 3 independent experiments were quantified by densitometry and displayed. *P < .05, significant with respect to untreated control. (B) BL41 (left panel) or J3D (right panel) cells were treated with NSC23766 or transduced with Rac1N17 as in panel A and cell number was monitored by trypan blue exclusion. Error bars represent SD.
In p53−/− MEFs, active Rac1 GTPase was found to contribute to the promotion of hyperproliferation.11,23 Next, we asked if the proliferation phenotype of p53-deficient lymphoma cells could be inhibited by Rac1 suppression. Treatment of either BL41 or J3D cells with NSC23766 or Rac1N17 was able to inhibit cell growth in a dose-dependent manner over a 4-day period (Figure 2B). This effect was also seen in genetically identical, matched p53-mutant and p53-reconstituted human B-lymphoma cells, NH32 and TK6, respectively. In this cell system, the p53-mutant NH32 cells have a distinct proliferative advantage over a 4-day period, which is completely restored to a level of cell growth comparable with the wild-type (wt) p53 expressing TK6 cells upon treatment with 20μM NSC23766 (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Whereas, NSC23766 treatment had a much less profound effect upon the growth of the p53 wt TK6 cells over the same time period. Together, these data indicate that Rac1 targeting may serve as a strategy to inhibit p53-deficient lymphoma cell proliferation.
Rac1 suppression sensitizes p53-deficient lymphoma cells to apoptosis
The p53 tumor suppressor and several Rac1 effectors are known to be critical for cell survival and induction of apoptosis, and p53-deficient cells often exhibit resistance to commonly used therapies.9 Therefore, we interrogated the effect of Rac1 targeting on apoptosis induction in the specific context of p53 deficiency. Treatment of p53-deficient cells with NSC23766 or Rac1N17 led to a dose-dependent increase in the rate of apoptosis compared with that of untreated BL41 or J3D cells by Annexin V and 7AAD staining and flow cytometry analysis (Figure 3). Similarly, expression of an exogenous p53ts− add-back mutant in these cells at the permissive temperature (32°C) caused increased apoptosis and a decrease in the S-phase cell population compared with the p53-deficient parental cells (data not shown). Similarly, in the genetically identical, p53-matched cell types NH32 (parental; p53-mutant) and TK6 (p53-reconstituted) Rac1 knockdown collaborated with p53 deficiency to increase apoptosis 3-fold over the p53-reconstituted cells (supplemental Figure 2). These data indicate that there is a collaborative effect of loss of both p53 and Rac1 function to promote apoptosis.
Rac1 targeting modulates apoptotic response of p53-deficient lymphoma cells. BL41 or J3D cells treated with NSC23766 or transduced with Rac1N17 were harvested for flow cytometry to determine the rate of apoptosis by staining with Annexin V and 7AAD. Error bars represent SD.
Rac1 targeting modulates apoptotic response of p53-deficient lymphoma cells. BL41 or J3D cells treated with NSC23766 or transduced with Rac1N17 were harvested for flow cytometry to determine the rate of apoptosis by staining with Annexin V and 7AAD. Error bars represent SD.
To further analyze the effect of Rac1 inhibition on apoptosis in human cells, BL41 cells were transduced with a lentivirus encoding human Rac1 shRNA (shRac1) or scrambled shRNA (shSCR) and sorted based on yellow fluorescent protein (YFP) expression after 48 hours and used in experiments after 24 hours in culture. These cells exhibited elevated levels of cleaved caspase-3 and cytochrome c by immunoblot when efficient Rac1 knockdown was achieved, and levels of the Rac1 downstream effector phospho-Pak1 became undetectable (Figure 4A). These cells contained unchanged levels of Rac2 upon Rac1 knockdown, indicating that the shRac1 construct is specific. Further, in the absence of an effective Rac3 antibody we probed the level of pan-Rac as a surrogate. These cells contained similar levels of pan-Rac to Rac1, indicating that Rac3 does not play a significant role in this cell system. Pull-down assays to assess the levels of active pan-Rac–GTP and Rac2-GTP did not reveal any effects of Rac1 knockdown (data not shown). These data reveal that a compensatory mechanism among Rac2 and Rac3 does not exist, and that the proliferation and apoptosis effects are indeed caused by Rac1 loss. Analysis of these cells containing Rac1 knockdown by flow cytometry revealed an almost 4-fold increase in apoptosis in the absence of Rac1 (Figure 4B). Collectively, these results indicate that Rac1 targeting may specifically suppress the hyperproliferation of p53-defective tumor cells by stimulating a p53-independent apoptotic signal.
Rac1 knockdown induces apoptosis in human lymphoma cells. (A) BL41 cells were transduced with either a YFP-tagged shRac1 construct or scrambled sh control (shSCR) and sorted 48 hours after infection. Cells were harvested 24 hours after sorting and immunoblotted with antibodies to Rac1, Rac2, pan-Rac, pPAK1, cleaved caspase-3, cytochrome c, and Gapdh. (B) Cells from panel A were analyzed by flow cytometry to determine the Annexin V/ 7AAD-positive population. Error bars represent SD.
Rac1 knockdown induces apoptosis in human lymphoma cells. (A) BL41 cells were transduced with either a YFP-tagged shRac1 construct or scrambled sh control (shSCR) and sorted 48 hours after infection. Cells were harvested 24 hours after sorting and immunoblotted with antibodies to Rac1, Rac2, pan-Rac, pPAK1, cleaved caspase-3, cytochrome c, and Gapdh. (B) Cells from panel A were analyzed by flow cytometry to determine the Annexin V/ 7AAD-positive population. Error bars represent SD.
Rac1 targeting in p53-defective lymphoma cells involves the downstream PAK and Akt pathway
To begin to unveil the molecular pathways affected by targeting Rac1 in p53-deficient lymphoma cells, we have examined specific Rac1 effectors that function in cell growth control. Treatment of BL41 or BL41-p53ts cells with the Rac1 inhibitor, NSC23766, or Rac1N17 mutant led to dose-dependent inhibition of phospho-PAK1 and phospho-Akt levels that correlate with their respective activities (Figure 5A; left panel). However, the activities of several other potential Rac1 effectors of the MAP kinase module (ie, ERK, JNK, and p38) were not affected by Rac1 inhibition (Figure 5A right panel). This was also observed even more dramatically in the J3D and J3D-p53ts cells (supplemental Figure 3). Next, to address whether active Akt and active PAK1 were capable of rescuing the apoptosis phenotype induced by Rac1 inhibition, BL41 cells were cotransduced with YFP-tagged shSCR or shRac1 and retrovirus encoding either GFP-tagged constitutively active PAK (caPAK), GFP-tagged constitutively active Akt (caAkt), GFP-tagged Akt kinase-dead (Akt-KD), or GFP alone (MIEG3). Cells were harvested 48 hours after transduction, and the GFP and YFP copositive cells were analyzed by flow cytometry for apoptosis by Annexin V and 7AAD staining (Figure 5B). Both caPAK1 and caAkt, but not Akt-KD, were able to completely reverse the apoptosis phenotype observed in Rac1-suppressed cells. These results suggest that the Rac1 targeting strategy can modulate the PAK1-Akt signaling pathway, but not the MAP kinases, to affect the lymphoma cell survival and proliferation properties.
Effects of Rac1 targeting on cell cycle and apoptosis may involve the PAK and Akt pathway. (A) BL41cells containing the p53ts mutant were treated with the Rac1 inhibitor, NSC23766, or transduced with a Rac1N17 mutant and immunoblotted for phospho-PAK1 and phospho-Akt (left panel), and potential effectors of the MAP kinase module (ie, ERK, JNK, p38; right panel) and their respective total protein levels. Both left and right panels were from the same immunoblot, and β-actin served as a loading control for both panels. Immunoblot bands were quantified by densitometry, and untreated controls were set to 1. (B) BL41 cells were transduced with lentivirus encoding either a YFP-tagged shRac1 construct or scrambled sh control (shSCR) in conjunction with retrovirus encoding either GFP-tagged caPAK, GFP-tagged caAkt, GFP-tagged Akt-KD, or GFP alone. Cells were harvested for flow cytometry 48 hours after infection to determine the rate of apoptosis in the YFP/GFP-copositive population by staining with Annexin V and 7AAD. (C) BL41 human B-lymphoma p53 mutant temperature-sensitive cells were incubated at either 32°C (p53 wild-type) or 37°C (p53 mutant) for 24 hours before a 3-hour pulse with 20μM wortmannin or 50μM LY294002. Cells were then harvested and subjected to GST-Pak pull-down assay and immunoblotted on the same day for Rac1, pAkt Ser473, total Akt, and Gapdh. The data are representative of 6 different experiments and error bars represent SD.
Effects of Rac1 targeting on cell cycle and apoptosis may involve the PAK and Akt pathway. (A) BL41cells containing the p53ts mutant were treated with the Rac1 inhibitor, NSC23766, or transduced with a Rac1N17 mutant and immunoblotted for phospho-PAK1 and phospho-Akt (left panel), and potential effectors of the MAP kinase module (ie, ERK, JNK, p38; right panel) and their respective total protein levels. Both left and right panels were from the same immunoblot, and β-actin served as a loading control for both panels. Immunoblot bands were quantified by densitometry, and untreated controls were set to 1. (B) BL41 cells were transduced with lentivirus encoding either a YFP-tagged shRac1 construct or scrambled sh control (shSCR) in conjunction with retrovirus encoding either GFP-tagged caPAK, GFP-tagged caAkt, GFP-tagged Akt-KD, or GFP alone. Cells were harvested for flow cytometry 48 hours after infection to determine the rate of apoptosis in the YFP/GFP-copositive population by staining with Annexin V and 7AAD. (C) BL41 human B-lymphoma p53 mutant temperature-sensitive cells were incubated at either 32°C (p53 wild-type) or 37°C (p53 mutant) for 24 hours before a 3-hour pulse with 20μM wortmannin or 50μM LY294002. Cells were then harvested and subjected to GST-Pak pull-down assay and immunoblotted on the same day for Rac1, pAkt Ser473, total Akt, and Gapdh. The data are representative of 6 different experiments and error bars represent SD.
Previous studies of p53 knockout MEF cells suggest that p53-regulated Rac1 activity may be mediated through PI3K.13 In an attempt to dissect the molecular relationship between P13K, Rac1, and p53 in the lymphoma model, we used the PI3K pathway inhibitors, wortmannin and LY294002, in the temperature-sensitive BL41 and J3D p53-mutant lymphoma cell lines (at 37°C p53 is mutant; at 32°C p53 is wild-type) and genetically matched NH32 (p53-mutant) and TK6 (p53-reconstituted) B-lymphoma lines. Although the results confirmed our observations in Figure 1 that wt p53 inhibits Rac1-GTP, we were unable to detect any effect of PI3K inhibition on Rac1-GTP levels by the GST-Pak1 pull-down assay (Figure 5C). Total Akt and phospho-Akt Ser473 levels served as controls for P13K inhibition. Thus, this experiment reveals that the mechanism by which p53 regulates Rac1 activity in the lymphoma cells appears to be PI3K-independent and differs from that of the fibroblasts.
Rac1 targeting impairs p53-deficient lymphoma growth in a xenograft model
To interrogate the biologic consequence of Rac1 suppression in tumorigenesis, we used xenografts in immunodeficient NOG mice. BL41 cells transduced with shRac1 or shSCR and sorted based on YFP expression were injected (2 × 106) contralaterally into the flanks of NOG mice. Rac1-proficient cells produced measureable tumors earlier than the Rac1-suppressed cells and continued to grow significantly faster (Figure 6A), so that by 70 days the Rac1-proficient tumors had grown to 5 times the size of the knockdown tumors. Tumors were excised and weighed at day 73 (Figure 6B), confirming the larger size of the Rac-proficient tumors. In addition, all 7 of the shSCR BL41 injections developed into tumors, whereas only 2 of the 5 shRac1 BL41 injections were able to support tumor growth. To determine whether Rac1 reactivation or compensation occurred in the 2 shRac1 tumors that developed, the tumors were processed for immunoblot and probed for total Rac1 expression (supplemental Figure 4). The 2 shRac1 tumors that developed were found to express levels of Rac1 comparable with the shSCR control tumors, suggesting that the proliferation of residual cells that contained Rac1 expression in the implanted shRac1 cell population had overtaken the Rac1 knockout cells to form the tumors. This notion is supported by the fact that measurable tumor development was delayed in these 2 tumors compared with the 7 shSCR tumors, as tumor development was initiated from a significantly reduced cell number. The levels of Rac1 in the tumors can be compared with the levels of Rac1 in the cells at the time of xenograft injection (Figure 4A). Together, these data suggest that in the context of established lymphoma cells, Rac1 plays a pivotal role in modifying tumorigenic proliferation and constitutes a useful target.
Rac1 targeting impairs p53-deficient tumor growth in vivo. (A) BL41 cells were transduced with either a YFP-tagged shRac1 construct or scrambled sh control (shSCR) and sorted as in Figure 4A. Cells were harvested 24 hours after sorting and injected (2 × 106) contralaterally into the flanks of NOG mice and tumor growth was monitored. (B) Tumors from panel A were excised and weighed at day 73. Error bars represent SD.
Rac1 targeting impairs p53-deficient tumor growth in vivo. (A) BL41 cells were transduced with either a YFP-tagged shRac1 construct or scrambled sh control (shSCR) and sorted as in Figure 4A. Cells were harvested 24 hours after sorting and injected (2 × 106) contralaterally into the flanks of NOG mice and tumor growth was monitored. (B) Tumors from panel A were excised and weighed at day 73. Error bars represent SD.
Rac1 deletion delays de novo lymphoma onset in a genetic model
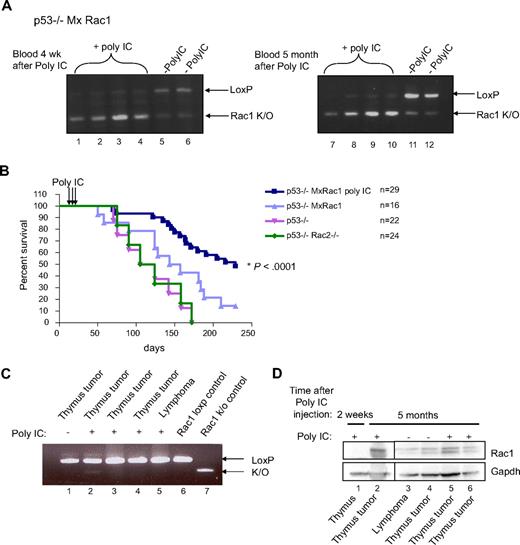
To further probe the influence of Rac1 on p53-deficient lymphomagenesis, we used a mouse model of lymphomagenesis. Because p53-deficient mice develop T- and B-cell lymphomas,24 we have generated p53−/−, p53−/−Rac2−/−, and p53−/−MxRac1 compound mice in the 129Sv × C57BL/6J background to genetically ablate Rac1 or Rac2 combined with the absence of the p53 gene. The hematopoietic phenotypes of the Rac1 or Rac2 knockout mice have been well documented;12 deletion of Rac1 alone from the T- or B-cell lineage does not affect normal lymphoid cell development or function, whereas Rac2 deficiency does not change T-cell development but alters marginal zone and peritoneal B-cell distribution.25,26 Next, total bone marrow from a p53−/−MxRac1 mouse was transplanted into lethally irradiated BoyJ recipients. At 1 month after transplantation, peripheral blood was analyzed for the relative levels of CD45.1 (contributed by BoyJ recipient) and CD45.2 (contributed by BL6 donor) antigens, and chimerism was assessed by flow cytometry to ensure that we had achieved complete hematopoietic replacement with donor cells. Mice containing 90% or more donor cells were split into 2 groups: one that received injections of PolyIC to induce hematopoietic Rac1 deletion and one that received mock injections. We and others have performed these studies multiple times, and we see no detrimental effects of PolyIC on tumorigenesis because PolyIC injection without inducing genetic deletion does not detectably affect mouse long-term survival. Blood chimerism and the genetic deletion of Rac1 in the peripheral blood was monitored monthly after PolyIC injection. Persistent deletion of the Rac1 gene over time was observed as displayed by predominance of a lower knockout allele compared with the upper loxP allele in representative PCR analyses of the same animals 1 month and 5 months after PolyIC injection (Figure 7A). Minor spontaneous Rac1 deletion was observed in the animals not injected with PolyIC, possibly due to a basal level of inflammation present in the mice; however, the amount of Rac1 reduction did not significantly affect these experiments (Figure 7A lanes 5, 6, 11, 12). The PolyIC-induced genetic deletion of Rac1 appeared to persist over time, similar to the transplantation chimerism throughout the lifespan of the recipient mice (supplemental Figure 5). The Rac1-proficient animals began showing signs of disease and dying around day 55 after transplantation, which continued until approximately day 200, whereas the Rac1-deficient animals (p53−/−MxRac1 PolyIC) experienced a significant delay in disease onset and death, with most of the animals dying between days 175 and 250 (Figure 7B). At the end of the study, 15 of the 29 mice in the p53−/−MxRac1 polyIC group remained disease free. Surprisingly, p53−/−Rac2−/− animals did not show any delay of disease onset. Mice in this study manifested disease in several ways, with thymus tumor development and rear leg paralysis being the most common. The mice also developed B-lymphomas and myeloproliferative disorders, although at a reduced frequency. To understand the requirement of Rac1 for tumor development in this model and to test whether residual Rac1 from incomplete PolyIC-induced gene deletion may have a compensatory effect, tumors were excised and processed for analysis of the presence of Rac1 genomic DNA (Figure 7C) and protein (Figure 7D). Rac1 protein was absent in thymus tissue 2 weeks after Poly IC injection, yet present in every thymus tumor or B-lymphoma found. Because most animals of the Rac1-null genotype maintained the Rac1 deficiency in myeloid cells of peripheral blood, these results reveal a specific requirement for Rac1, but not Rac2, for tumor development.
Rac1-deficiency delays p53-deficient lymphoma growth in vivo. (A) Total bone marrow from a p53−/−MxRac1 mouse was isolated and injected (500 000 cells) by tail vein into lethally irradiated (1175 cGy, split-dose) BOYJ recipients. Blood chimerism was assessed 1 month after transplantation and mice containing approximately 90% or greater donor cells received 3 × 300 μg of intraperitoneal PolyIC injections every other day for 4 injections to delete the Rac1 gene in hematopoietic cells in vivo or were mock-injected. Animals were bled monthly to monitor genetic deletion of Rac1 by PCR. Representative PCRs of blood from the same animals 1 month (left panel) or 5 months (right panel) after PolyIC injection are displayed. (B) Survival of animals from panel A are displayed with transgenic p53−/− and p53−/−Rac2−/− animals included. (C) Representative tumors were excised from animals that underwent transplantation from panel A upon death and processed for PCR to assess levels of Rac1 DNA. (D) Tumors from panel C were snap-frozen in liquid nitrogen, lysed, and immunoblotted to determine the expression level of Rac1 protein.
Rac1-deficiency delays p53-deficient lymphoma growth in vivo. (A) Total bone marrow from a p53−/−MxRac1 mouse was isolated and injected (500 000 cells) by tail vein into lethally irradiated (1175 cGy, split-dose) BOYJ recipients. Blood chimerism was assessed 1 month after transplantation and mice containing approximately 90% or greater donor cells received 3 × 300 μg of intraperitoneal PolyIC injections every other day for 4 injections to delete the Rac1 gene in hematopoietic cells in vivo or were mock-injected. Animals were bled monthly to monitor genetic deletion of Rac1 by PCR. Representative PCRs of blood from the same animals 1 month (left panel) or 5 months (right panel) after PolyIC injection are displayed. (B) Survival of animals from panel A are displayed with transgenic p53−/− and p53−/−Rac2−/− animals included. (C) Representative tumors were excised from animals that underwent transplantation from panel A upon death and processed for PCR to assess levels of Rac1 DNA. (D) Tumors from panel C were snap-frozen in liquid nitrogen, lysed, and immunoblotted to determine the expression level of Rac1 protein.
Discussion
Mutation of the p53 tumor suppressor is common among BLs and is associated with disease progression and resistance to DNA-damaging therapies.9,10,27 Thus, a current challenge is to design novel therapeutic strategies that specifically target p53-deficient cells and induce tumor cell death. Taxol-based therapies have been used for their abilities to selectively target p53-defective tumor cells28 ; however, resistance to these therapies can occur through infection by the Epstein-Barr virus, mutation, or drug efflux.29,30 Given that previous studies have linked Rac1 with tumorigenesis, we investigated the role of Rac1 in p53 deficiency–mediated lymphomagenesis and its potential treatment. Our laboratory has found that p53−/− primary MEFs are particularly sensitive to Rac1 gene deletion,11 resulting in an effective induction of apoptosis (S. Akunuru, Y.Z., unpublished observation, August 2009), suggesting the potential utility of such an approach in p53-defective cancer cells. In the present work, we hypothesized that Rac1 inhibition would provide a novel therapeutic approach for p53-deficient lymphomas by exploiting p53 loss to induce cell death. This is based on our observations that Rac1 activity is inversely regulated by functional p53 in both B- and T-lymphoma cells, and that Rac1 contributes to p53 deficiency–induced hyperproliferation. Rac1 is involved in proliferation of p53-deficient cells by modulating both the cell cycle and a p53-independent apoptotic signal. Our study demonstrates that inhibition of Rac1 activity can impact both lymphomagenesis and progression of the disease and, as such, may have implications for the development of novel therapeutic strategies in the treatment of p53-deficient lymphomas.
Recent studies have elegantly shown that reintroduction of wt p53 or p53-regulated signaling components can reverse the growth phenotypes of various tumor types that exhibit mutant p53 in mice.31,32 The effective inhibition of BL41 and J3D cell growth by exogenous expression of wt p53 is consistent with this scenario.18 Typically, such an approach to the human disease requires gene therapy efforts that have been shown to bring on potential complications as exhibited by trials with ONYX-015.33,34 By expressing the dominant-negative Rac1, Rac1N17, or using the small-molecule Rac inhibitor, NSC23766, in the tumor cells, we have shown that Rac1 inhibition abrogates the hyperproliferative phenotype of p53-deficient lymphoma cells (Figure 2B) to an extent that is similar to reconstitution of functional p53 (supplemental Figure 1). Although Rac1 targeting may affect cell growth independent of p53 status, it clearly has a greater impact on p53-deficient cells. This effect on p53-deficient cells could be due to their inherent faster cell cycle compared with the p53-reconstituted or wild-type cells, therefore making them more sensitive to methods of Rac targeting. This scenario would imply that p53 reactivation and Rac inhibition therapies might function in a similar manner in p53-deficient lymphomas, and that the cell-cycle–promoting effects of increased Rac activity could be secondary to the loss of p53. Nonetheless, this study uses several methods of Rac targeting (dominant-negative, shRNA, small-molecule inhibitor, genetic deletion in mice) in lymphomagenesis and treatment to reveal effective suppression of growth of p53-deficient lymphoma cells, and as such may present an alternative means of therapy for this type of tumor. We believe that the Rac-specific small-molecule inhibitor approach merits further analysis in a broader spectrum of tumor models and with pharmacologically optimized inhibitors in an effort to validate Rac1 targeting as a potential therapeutic method.
As opposed to hematopoietic stem cells and their progenitors, previous gene targeting studies in B cells and T cells have shown that Rac1 deletion does not affect normal B- or T-cell development or functions.12,25,26 Rather, the collective dosage of Rac1 and Rac2 activities are required for maintaining normal lymphopoiesis and lymphocyte responses. Thus, Rac1 could be a good therapeutic candidate. Our in vivo genetic data in mouse models where the Rac1 or Rac2 gene was selectively deleted in the p53−/− background provide strong evidence that Rac1, but not Rac2, may serve as a specific target in p53-deficient lymphomas, suggesting that this mode of therapy may have tumor selectivity (Figure 7B). This tumor-specific effect has previously been observed in glioma, where Rac1 suppression was able to induce apoptosis in tumor cells and not normal astrocytes.35 More recently, studies have shed light on the utility of small-molecule Rac targeting for the treatment of lung, breast, prostate, Schwann cell, and hematopoietic malignancies, and also revealed distinct roles of each Rac family member.36-39 Similar to the results of our study, both Rac1 and Rac2 activation in murine models of p210 BCR-ABL–mediated leukemogenesis were shown to be critical for disease development, and small-molecule inhibition of these activities using NSC23766 reduced the proliferative phenotype and delayed disease onset.37 This study, in the context of our finding, would indicate that the activities of Rac family members are cell-type specific, such that Rac1 and Rac2 activities are collectively required for BCR-ABL–induced myeloid proliferative disease progression in hematopoietic stem cells, but only Rac1 is necessary for progression of the B- and T-cell lymphomas examined herein. The fact that Rac GTPase targeting appears to have minimum “side effects” on normal hematopoietic stem cells and lymphoid cells and is selective toward hyperactive Rac activities found in the tumor cells implies that tumor cells have a greater addiction to various Rac signals compared with normal cells.
Our results raise the possibility that the elevated Rac1 activity in p53-defective lymphocytes may act cooperatively with p53 deficiency to promote cell-cycle progression and survival in B- and T-cell transformation. Rac1 ablation is known to induce a p53-dependent apoptotic pathway;14,35,40 however, the molecular mechanism by which apoptosis occurs in the absence of both Rac1 and p53 remains unclear. The possibilities are several-fold. First, Rac1-GTP has been shown to stimulate phosphorylation of Bad on Ser75 to inhibit apoptosis in p53-deficient lymphoma cells via PKA and not Akt in response to DNA-damaging chemotherapies.15 Thus, Rac1-targeting therapy could render suppression of apoptosis to be released by this mechanism. Second, Rac1 has been implicated in the regulation of G1 phase cell-cycle progression.17 In the current study, Rac1 inhibition induced accumulation of cells in G1 (data not shown), suggesting that Rac1 may work in regulating the G1/S phase cell-cycle checkpoint to activate apoptotic programs in the absence of functional p53. Third, it has been shown that BCL2 family members are downstream of Rac1 signaling,41 and a recent study revealed that MLL-rearranged AML cells require Rac1 for growth and survival mediated through Bcl-xL/ Bcl-2,19 which are downstream of Akt. To this end, our study implicates PAK1 and Akt as mediators through which Rac1 signals to prevent apoptosis, and that this action could be occurring more specifically through Bcl-2. Because the role of Rac1 in cell survival is complex and is likely cell- and tumor-type specific, more detailed studies are necessary to elucidate the mechanisms through which Rac1 ablation could promote apoptosis in defined tumor cells.
The mechanism by which p53 deficiency enhances Rac1 activity remains unclear. Previous studies to identify the molecular links between p53 and Rac1 in primary MEFs suggest that PI3K is upstream of Rac1 activation in p53−/− cells, and that p53 transcriptional activity is required for regulation of PI3K-Rac1 signaling.13 Our preliminary studies have revealed that this may not be the case in lymphoma cells, and that the mechanism by which p53 regulates Rac1 activity appears to be PI3K-independent (Figure 5C). Further, Figure 5 demonstrates that suppression of Rac1 activity in the p53 loss-of-function mutant cells inhibits pAkt activity, suggesting that PI3K may function downstream of Rac1. Together, these data would suggest that PI3K may act either up- or downstream of Rac1 in lymphoma cells like in the case of neutrophils.42 Understanding the critical issue of which specific targets of p53 are involved in suppressing the Rac1 signaling axis in normal cells is a goal of future investigations given the vast array of gene targets under the transcriptional control of p53, particularly in the context of lymphoma formation.
In summary, the present study investigates the relationship between Rac1 activity and functional p53 in human Burkitt B-lymphoma BL41 cells and murine T-lymphoma J3D cells. We provide evidence that Rac1 activity is inversely regulated by p53 in p53-deficient tumor cells and that Rac1 suppression in this context can impair cell-cycle progression, induce apoptosis, and retard tumor formation. Similarly, we have shown that genetic Rac1 deficiency in vivo delays disease development in spontaneous lymphoma mouse models. Together, these observations suggest that Rac1 could serve as a valuable target for pharmacologic intervention in the treatment of p53-deficient lymphomas that may be resistant to traditional therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank members of the Zheng laboratory for thought-provoking discussions and Drs K. G. Wiman and Y. Wang for crucial cell lines.
This work was supported by National Institutes of Health grants R01 CA125658, R01 CA125658 S1 (Y.Z.), and T32 CA117846 (E.E.B.).
National Institutes of Health
Authorship
Contribution: E.E.B., W.N., L.W. and F.G. performed experiments, analyzed results, and made the figures; J.F.J handled the mouse work; and E.E.B. and Y.Z. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zheng, Division of Experimental Hematology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: yi.zheng@cchmc.org.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal