Abstract
The evidence of a tight spatial interaction between mast cells (MCs) and B lymphocytes in secondary lymphoid organs, along with the data regarding the abundance of MCs in several B-cell lymphoproliferative disorders prompted us to investigate whether MCs could affect the proliferation and differentiation of B cells. To this aim, we performed coculture assays using mouse splenic B cells and bone marrow–derived MCs. Both nonsensitized and activated MCs proved able to induce a significant inhibition of cell death and an increase in proliferation of naive B cells. Such proliferation was further enhanced in activated B cells. This effect relied on cell-cell contact and MC-derived interleukin-6 (IL-6). Activated MCs could regulate CD40 surface expression on unstimulated B cells and the interaction between CD40 with CD40 ligand (CD40L) on MCs, together with MC-derived cytokines, was involved in the differentiation of B cells into CD138+ plasma cells and in selective immunoglobulin A (IgA) secretion. These data were corroborated by in vivo evidence of infiltrating MCs in close contact with IgA-expressing plasma cells within inflamed tissues. In conclusion, we reported here a novel role for MCs in sustaining B-cell expansion and driving the development of IgA-oriented humoral immune responses.
Introduction
Mast cells (MCs) are the key effector cells in immunoglobulin E (IgE)–mediated allergic diseases such as atopic asthma, allergic rhinitis, and atopic dermatitis, but also participate in a variety of IgE-independent biologic responses.1-4 MCs can produce and secrete mediators influencing various aspects of the biology of dendritic cells, T cells, and B cells, thus acting as regulatory cells.2,4-6 A variety of stimuli can activate MCs; one of the most efficient is the engagement of receptors for the Fc portion of Ig (FcR).7,8 MC activation with IgE and specific antigen (Ag) results in the secretion of a wide spectrum of biologically active mediators including histamine, serotonin, β-hexosaminidase, proteoglycans, proinflammatory lipid mediators, growth factors, cytokines, and chemokines. Some of these products can also be released after the occupancy of MC-FcϵRI by monomeric IgE antibodies. Engagement of the FcϵRI initiates activation of several cytoplasmic protein tyrosine kinases and recruitment of adaptor molecules that subsequently promote the activation of multiple signaling cascades, including phospholipase C, phosphoinositide-3 kinase, mitogen-activated protein kinase, and transcription factors.2,9
MCs are dispersed throughout multiple tissues and are located mainly at the major immunologic interfaces such as skin, gut, and lung. Secondary lymphoid organs including tonsils and lymph nodes show a moderate amount of MCs in physiologic conditions, which increases in the early phases of inflammation, suggesting an active participation of MCs in the orchestration of the immune response.10
Over the past few years, the association between MCs and human cancer has progressively emerged and many studies have highlighted a correlation between the amount of tumor-infiltrating MCs and the degree of tumor aggressiveness (ie, grade) and dissemination (ie, stage), which is in favor of a role of MCs in tumor growth.11 Increased number of MCs have been reported, among the others, in B-cell neoplasms including Hodgkin lymphoma (HL), diffuse large B-cell lymphoma, lymphoplasmacytic lymphoma, and chronic lymphocytic leukemia.11-16 In this setting, MCs might play an important role in tumor-associated inflammation.17 Some studies have demonstrated tumor growth induction secondary to MC activation,11 and diverse mechanisms including the release of proangiogenic factors18-20 have been hypothesized.21-23 However, most of these studies investigating the influence of MCs on the neoplastic clone growth and survival have focused on solid tumors, and therefore little is known on the role of MCs in B-cell neoplasms as well as on MC–B-cell interactions.
On this basis, we aimed to investigate the effects of MCs on the activation and proliferation of B cells by performing coculture assays using primary mouse B cells purified from spleen and bone marrow–derived MCs. We showed that MCs promote both survival and activation of naive B cells as well as proliferation and further plasma cell differentiation of activated B cells through cell-cell contact and soluble factors production.
Methods
Human tissue samples
Formalin-fixed, paraffin-embedded bioptic tissue specimens of inflammatory bowel disease, reactive follicular hyperplasia, and chronic lymphocytic leukemia were retrospectively gathered from the archives of the Department of Human Pathology of the University of Palermo, following the approval and permission of the Institutional Review Board of the University Hospital. All the procedures followed were in accordance with the Helsinki Declaration.
Histopathologic and immunophenotypical analyses on formalin-fixed paraffin-embedded samples
For immunohistochemical analyses on formalin-fixed and paraffin-embedded tissue samples, 4-micrometer–thick sections were cut from paraffin blocks, deparaffinized, and rehydrated to water. Sections underwent microwave-oven heating for antigen retrieval before endogenous peroxidase quenching.
Immunohistochemistry was performed using either the streptavidin-biotin-peroxidase complex (strept-ABC) or the alkaline phosphatase anti–alkaline phosphatase (APAAP) method as previously reported24 and the following primary antibodies: mouse anti–human CD20 (clone L26; Dako), mouse anti–human CD3 (clone LN10; Novocastra), mouse anti–human IgA (clone N1CLA; Novocastra), and mouse anti–human mast cell tryptase (clone AA1; Dako). Aminoethylcarbazole (red signal) and 5-bromo, 4-chloro, 3-indolylphosphate/nitro blue tetrazolium chloride (blue signal) were used as chromogenic substrates.
Double immunohistochemical stainings were performed by 2 consecutive rounds of single immunostaining using strept-ABC and APAAP. Slides were evaluated by an expert pathologist (C.T.) under a Leica DM3000 optical microscope and microphotographs were collected using a Leica DFC320 digital camera.
Cell preparation and culture conditions
Murine B cells were isolated from spleen of C57BL/6 mice by a negative depletion method as previously described,25 and the purity, as percentage of CD19+ cells, was more than 95%. Primary cultures of bone marrow mast cells were obtained by in vitro differentiation of bone marrow cells taken from mouse femur as previously described.26 Bone marrow from C57BL/6 IL-6–deficient mice were kindly provided by J. Rivera (National Institutes of Health). Where indicated, mouse B cells (106 cells/mL) were incubated with purified anti–mouse CD40 monoclonal antibody (mAb) HM40-3 (BD Pharmingen) at 1 μg/mL and anti–mouse IgM Ab (Jackson ImmunoResearch Laboratories) at 0.5 μg/mL for the indicated times.25 Before experiments, 106 cells/mL MCs were sensitized in medium without IL-3 for at least 4 hours with 1 μg/mL dinitrophenol (DNP)–specific IgE, as previously described.25 When B cells were cultured with MCs, B cells (106 cells/mL) were added to IgE-sensitized or nonsensitized MCs (106 cells/mL) in the presence or the absence of 100 ng/mL DNP at 37°C for 72 hours in 96-well, round-bottom plates (Falcon; BD Biosciences).
In some coculture experiments, B cells and MCs were separated by a transwell polyester membrane using 24-well plates (Costar, Euroclone) with 0.4-μm pore size, following the manufacturer's recommendations.
Anti–mouse IL-6R (clone 15A7) and anti-CD40L (clone MR1) antibodies were purified from Hybridoma (a gift from M. Colombo, Immunotherapy and Gene Therapy Unit, Department of Experimental Oncology, Istituto Nazionale per lo Studio e la Cura dei Tumori) and used at 50 μg/mL. To evaluate the role of CD40L/CD40 signaling pathway in cocultured studies, the anti-CD40L blocking Ab was added together with MCs to B cells previously activated with purified anti–mouse CD40 and anti–mouse IgM Ab for 48 hours at the concentration of 50 μg/mL for 48 hours.
Flow cytometric analysis
To assess cell-surface expression of different costimulatory molecules/activation markers, cells were first washed twice with phosphate-buffered saline (PBS) and then incubated at 4°C for 30 minutes with fluorescein isothiocyanate– or phycoerythrin- or biotin-labeled anti–mouse mAbs or isotype control Ab at 10 μg/mL final concentration. For detection of biotin-conjugated Ab, cells were also incubated with Streptavidin–Cy-Chrome (BD PharMingen). All Abs were purchased from eBioscience, except anti–mouse IgA biotin conjugate (BD PharMingen). Anti–mouse CD40 fluorescein isothiocyanate–conjugated Ab was kindly provided by Dr Mario Colombo (Istituto Nazionale per lo Studio e la Cura dei Tumori). Necrotic and apoptotic cells were detected by labeling with annexin V and propide iodide using annexin V-FLUOS staining kit (Roche Applied Science) following manufacturer's instructions. Fluorescence analysis was accomplished on FACScan (Becton Dickinson) using FlowJo software (Version 8.5.2; Treestar Inc).
CFSE assays
Freshly purified mouse B cells were labeled with 1 μM carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) at room temperature for 8 minutes, then washed twice with RPMI 1640 containing 10% fetal calf serum. After stimulation for 3 days, the CFSE profiles of CD19+ B cells were evaluated by flow cytometry. Cell divisions and proliferation index were determined using the Proliferation Wizard algorithm ModFit LT software (Verity Software House). The proliferation Wizard module of ModFit LT V2.0 indicates the proliferation index as the sum of the cells in all generations divided by the computed number of original parent cells present at the start of the experiments, and it is therefore a measure of the increase in cell number in the culture over the course of the experiment.
Quantification of secreted IgA isotype
Abs produced in cell cultures were detected by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well round-bottom plates polyvinyl plates (Costar) were coated with affinity-purified anti–mouse isotype-specific antibody (Southern Biotechnology) at a concentration of 2 μg/mL. After overnight incubation at 4°C, plates were blocked with 3% bovine serum albumin in PBS for 1 hour at room temperature. Culture supernatants or standards (100 μL; BD PharMingen) diluted in 3% bovine serum albumin–PBS were added to antibody-coated wells. After overnight incubation at 4°C, plates were washed with 0.05% Tween in PBS, and optimal concentration of horseradish peroxidase–conjugated goat anti–mouse antibody (Southern Biotechnology) was added (for IgA, 1:2000). Plates were incubated for an additional 1 hour at room temperature and then washed with PBS, and finally tetramethylbenzidine substrate (Sigma) was added for 20 minutes. Optical density at 405 nm was measured using a Titertek Multiskan ELISA reader (Labsystems).
Statistical analysis
The 2-tailed Student t test was used to determine the statistical significance of the data. A P value of less than .05 was considered statistically significant and indicated with an asterisk.
Results
MCs and B cells display tight spatial interactions in the T cell–rich area of secondary lymphoid organs
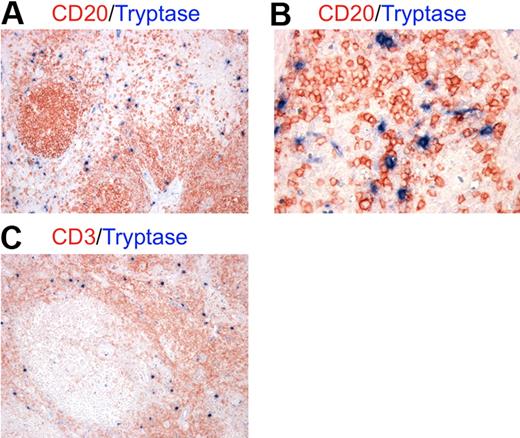
To investigate the actual occurrence of cellular interactions between MCs and B cells in an inflammatory background, we performed double immunohistochemical staining for MC tryptase and the CD20 B-cell marker on tissue sections from lymph nodes showing reactive follicular hyperplasia. We found numerous MCs scattered in the paracortical and medullary area of lymph nodes and surrounding follicles (Figure 1A). MCs intermingled with B cells and the 2 cell populations clearly showed signs of cell-to-cell interaction (Figure 1B). Because of the characteristic distribution of MCs observed, the above-mentioned interactions with B cells were likely to occur with recirculating naive B cells and activated post–germinal center B cells in a T cell–rich environment (Figure 1C).27
MCs localize in the paracortical and medullary area of lymph nodes in close contact with B cells. Double immunohistochemistry for mast cell tryptase and either CD20 (A-B) or CD3 (C) on lymph node samples with reactive follicular hyperplasia. (A-B) Mast cells (blue) are localized mainly in the parafollicular zone extending into the medullary area and showing tight spatial interactions with B cells (red). (C) In lymph nodes, MCs (blue) are likely to interact also with T cells (red) as the parafollicular area is rich in T lymphocytes. These pictures are illustrative of the interactions occurring between MCs and B cells in the context of T cell–rich areas of lymph nodes (STREPT-ABC and APAAP methods; original magnifications: panels A,C: ×100; panel B, ×200).
MCs localize in the paracortical and medullary area of lymph nodes in close contact with B cells. Double immunohistochemistry for mast cell tryptase and either CD20 (A-B) or CD3 (C) on lymph node samples with reactive follicular hyperplasia. (A-B) Mast cells (blue) are localized mainly in the parafollicular zone extending into the medullary area and showing tight spatial interactions with B cells (red). (C) In lymph nodes, MCs (blue) are likely to interact also with T cells (red) as the parafollicular area is rich in T lymphocytes. These pictures are illustrative of the interactions occurring between MCs and B cells in the context of T cell–rich areas of lymph nodes (STREPT-ABC and APAAP methods; original magnifications: panels A,C: ×100; panel B, ×200).
MCs enhance survival and proliferation of naive and activated B cells
To study whether MCs could influence activation and proliferation of B cells, we performed coculture assays using murine bone marrow–derived MCs and splenic B cells.
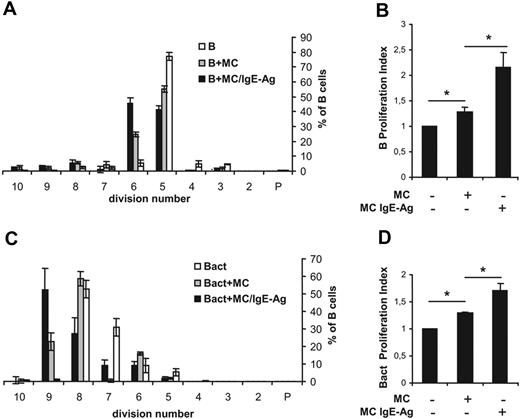
Proliferation assays were performed by labeling B cells with the fluorescent dye carboxyfluorescein succinimidyl ester (CFSE) and by coculturing with MCs. After 72 hours, cells were harvested and analyzed by flow cytometry. Only CD19+ cells in the morphologic gate were included in the analysis (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). As shown in Figure 2A, naive B cells (accounting for 6% of total events) in coculture with nonsensitized MCs progressed through generations faster than B cells alone, which were programmed to die in culture in the absence of appropriate signals. Indeed, the total number of B cells over the fifth generation was 9% (± 1.2%), 39% (± 2.4%), and 57% (± 3.1%) in control and in coculture with nonsensitized and with IgE-Ag–stimulated MCs, respectively. These data were confirmed by the cellular proliferation index, which is a measure of the increase in cell number in the culture over the course of the experiment.28 A significant increase in the B-cell proliferation index was observed between nonsensitized and IgE-Ag–activated MCs (Figure 2B). Moreover, proliferation of CD40- and IgM-activated B cells was substantially enhanced in the presence of activated MCs (Figure 2C-D). MCs induced B-cell proliferation, and staining with annexin V and propidium iodide demonstrated that MCs also promoted B-cell survival, as the percentage of viable B cells increased whereas that of necrotic cells was reduced in the coculture system (Table 1). Together, these data suggest that MCs support the survival and proliferation of primary B cells. No significant differences were observed in B-cell proliferation between cocultures using nonsensitized and IgE-sensitized MCs (not shown). Moreover, decreasing MC/B ratios (1:1, 1:2, and 1:4) were tested, but no significant differences were measured, suggesting that even low MC concentrations were effective in influencing B-cell survival and proliferation (not shown).
MCs promote proliferation of naive B cells and activated B cells. Naive (B) or activated (Bact) mouse B cells were labeled with CFSE and cocultured with nonsensitized mast cells (MCs) or IgE-sensitized MCs stimulated with the antigen DNP (MC/IgE-Ag). The statistical analyses of CFSE data from 3 independent experiments with naive B cells (A) or activated B cells (C) are expressed as percentages of cells in each generation and presented as means ± SD. P indicates the parent generation. Proliferation index of naive B cells (B) and activated B cells (D) is expressed as fold induction over B cells alone, where the proliferation index average ± SD of naive B and activated B cells alone was fixed to 1 in panels B and D, respectively. Results shown are the mean of at least 3 separate experiments from 3 different MC cultures. *P < .05.
MCs promote proliferation of naive B cells and activated B cells. Naive (B) or activated (Bact) mouse B cells were labeled with CFSE and cocultured with nonsensitized mast cells (MCs) or IgE-sensitized MCs stimulated with the antigen DNP (MC/IgE-Ag). The statistical analyses of CFSE data from 3 independent experiments with naive B cells (A) or activated B cells (C) are expressed as percentages of cells in each generation and presented as means ± SD. P indicates the parent generation. Proliferation index of naive B cells (B) and activated B cells (D) is expressed as fold induction over B cells alone, where the proliferation index average ± SD of naive B and activated B cells alone was fixed to 1 in panels B and D, respectively. Results shown are the mean of at least 3 separate experiments from 3 different MC cultures. *P < .05.
Percentages of viable, necrotic, and apoptotic B cells after 72 hours of coculture
| . | % Cells . | ||
|---|---|---|---|
| Viable . | Apoptotic . | Necrotic . | |
| B | 73.5 ± 2.1 | 6.5 ± 0.7 | 20.0 ± 1.4 |
| B + MC | 82 ± 1.4 | 12.5 ± 0.7 | 5.5 ± 0.7 |
| B + MC/IgE-Ag | 93.5 ± 0.8 | 3.5 ± 0.1 | 3.4 ± 0.6 |
| Bact | 87.1 ± 0.5 | 3.8 ± 0.2 | 9.0 ± 0.7 |
| Bact + MC | 96.7 ± 0.3 | 1.5 ± 0.1 | 1.7 ± 0.4 |
| Bact + MC/IgE-Ag | 94.5 ± 0.7 | 3 ± 0.1 | 2.5 ± 0.7 |
| . | % Cells . | ||
|---|---|---|---|
| Viable . | Apoptotic . | Necrotic . | |
| B | 73.5 ± 2.1 | 6.5 ± 0.7 | 20.0 ± 1.4 |
| B + MC | 82 ± 1.4 | 12.5 ± 0.7 | 5.5 ± 0.7 |
| B + MC/IgE-Ag | 93.5 ± 0.8 | 3.5 ± 0.1 | 3.4 ± 0.6 |
| Bact | 87.1 ± 0.5 | 3.8 ± 0.2 | 9.0 ± 0.7 |
| Bact + MC | 96.7 ± 0.3 | 1.5 ± 0.1 | 1.7 ± 0.4 |
| Bact + MC/IgE-Ag | 94.5 ± 0.7 | 3 ± 0.1 | 2.5 ± 0.7 |
MC-induced B-cell proliferation requires cell-cell contact and soluble factors: the role of mast cell–expressed IL-6 and CD40L
Both cell-cell proximity and soluble factors may contribute to B-cell proliferation enhancement by activated MCs. Thus, we investigated the importance of cell-cell proximity using B cells cocultured with MCs in the presence of a membrane filter to separate the 2 cell populations. As shown in Figure 3A and B, B-cell proliferation was partially reduced when MCs and B cells were separated. Indeed, 19% (± 1.3%) of naive B cells and 93% (± 0.9%) of activated B cells cocultured with activated MCs distributed over the fifth generation, compared with 8% (± 0.7%) and 84% (± 3.2%) of B cells cocultured with activated MCs in the presence of transwell membranes, supporting the relevance of cell-cell contact. These data were confirmed by the cellular proliferation index as detailed in Figure 3C and D.
The enhancement of B-cell proliferation by MCs requires cell-cell contact and soluble factors. Naive or activated B cells were labeled with CFSE and cocultured for 72 hours with activated MCs separated or not by a transwell membrane. The statistical analyses of CFSE data of B cells in contact (A) or separated (C) by a transwell (TW) are expressed as percentages of cells in each generation and presented as means ± SD of 3 separate experiments from 2 different MC cultures. Open and black histograms represent the percentage of naive (B) and activated (Bact) B cells in each generation, respectively; P indicates the parent generation. Results of proliferation index are expressed as fold induction over B cells alone, where the proliferation index average ± SD of naive B and activated B cells alone was fixed to 1 in panels B and D, respectively. *P < .05.
The enhancement of B-cell proliferation by MCs requires cell-cell contact and soluble factors. Naive or activated B cells were labeled with CFSE and cocultured for 72 hours with activated MCs separated or not by a transwell membrane. The statistical analyses of CFSE data of B cells in contact (A) or separated (C) by a transwell (TW) are expressed as percentages of cells in each generation and presented as means ± SD of 3 separate experiments from 2 different MC cultures. Open and black histograms represent the percentage of naive (B) and activated (Bact) B cells in each generation, respectively; P indicates the parent generation. Results of proliferation index are expressed as fold induction over B cells alone, where the proliferation index average ± SD of naive B and activated B cells alone was fixed to 1 in panels B and D, respectively. *P < .05.
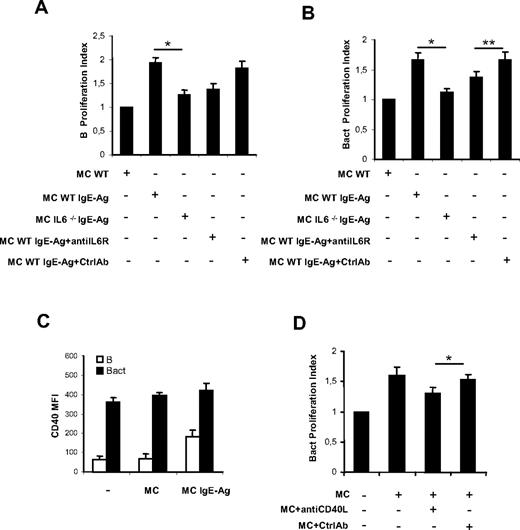
Activation with IgE-Ag stimulates MCs to express a specific set of cytokine genes including IL-4, IL-5, IL-6, IL-13, transforming growth factor-β (TGF-β), and tumor necrosis factor-α, which can regulate precise events of B-cell physiology.2 In particular, IL-6 is a key factor influencing B-cell proliferation, maturation, and survival that has been postulated to play a direct role in the maintenance of the proliferative spur in B- and plasma cell neoplasms through its interaction with IL-6R (CD126, CD130) expressed on neoplastic cells.29-33 On this basis, we decided to use MCs from wild-type (WT) and IL-6–deficient (IL-6−/−) mice to assess whether MC-derived IL-6 was required for MC-induced B-cell proliferation. In MC-naive B-cell (Figure 4A) or MC-activated B-cell (Figure 4B) cocultures, IL-6 derived from activated MCs proved to be a key cytokine implicated in B-cell proliferation, because IgE-Ag–stimulated IL-6−/− MCs did not enhance B-cell proliferation as efficiently as WT MCs. Moreover, the B-cell proliferative response to IgE-Ag–stimulated IL-6−/− MCs was partially reconstituted by the addition of recombinant murine IL-6 (data not shown), further supporting that MC-derived IL-6 strongly sustains B-cell proliferation. To confirm these data, we repeated coculture experiments using WT MCs with or without an anti–IL-6R blocking antibody. Blockade of IL-6R partially inhibited MC-induced proliferation of naive (Figure 4A) and activated (Figure 4B) B cells, producing a picture similar to that observed with IL-6−/− MCs.
MC-derived IL-6 and CD40-CD40L interactions are involved in B-cell proliferation up-regulation. Naive (B) or activated B cells (Bact) were labeled with CFSE and cocultured as indicated. Proliferation index of naive B (A) and activated B cells (B) cultured alone, with nonsensitized MCs (MC), with activated MCs (MC/IgE-Ag), and with activated IL-6−/− MCs and in the presence of anti–IL-6R or isotype control antibody (Ctrl) were analyzed by flow cytometry. Proliferation indexes are from 3 independent experiments with naive B cells and activated B cells, respectively. Proliferation indexes from naive B and activated B cells alone were fixed to 1. *P < .05. Date are the average ± SD (C) The mean fluorescence intensity of CD40 expression on naive B cells (B) and on activated B cells (Bact) at baseline and in coculture with nonsensitized MCs (MC) or activated MCs (MC/IgE-Ag) is shown. (D) Proliferation indexes of activated B cells alone, cocultured with activated MCs without and with blocking CD40L Ab or control Ab, are calculated. Proliferation index of activated B cells alone was fixed to 1. Results are from 3 independent experiments using at least 3 different cultures of MCs. *P < .05; ** P < .02.
MC-derived IL-6 and CD40-CD40L interactions are involved in B-cell proliferation up-regulation. Naive (B) or activated B cells (Bact) were labeled with CFSE and cocultured as indicated. Proliferation index of naive B (A) and activated B cells (B) cultured alone, with nonsensitized MCs (MC), with activated MCs (MC/IgE-Ag), and with activated IL-6−/− MCs and in the presence of anti–IL-6R or isotype control antibody (Ctrl) were analyzed by flow cytometry. Proliferation indexes are from 3 independent experiments with naive B cells and activated B cells, respectively. Proliferation indexes from naive B and activated B cells alone were fixed to 1. *P < .05. Date are the average ± SD (C) The mean fluorescence intensity of CD40 expression on naive B cells (B) and on activated B cells (Bact) at baseline and in coculture with nonsensitized MCs (MC) or activated MCs (MC/IgE-Ag) is shown. (D) Proliferation indexes of activated B cells alone, cocultured with activated MCs without and with blocking CD40L Ab or control Ab, are calculated. Proliferation index of activated B cells alone was fixed to 1. Results are from 3 independent experiments using at least 3 different cultures of MCs. *P < .05; ** P < .02.
Signaling through costimulatory complexes represents the basis of immune cell activation processes, including that of B cells. In the model of MC–B-cell interaction we investigated, MCs may act as accessory cells promoting B-cell activation through the expression of several costimulatory molecules. To clarify this issue, we examined MCs for the surface expression of B cell–modulating molecules by flow cytometry. We and others have demonstrated that nonsensitized MCs express several costimulatory molecules,5,6,33,34 including CD40L. In our model, CD40L expression on MCs was increased by IgE-Ag stimulation, but was not further enhanced by coculture with B cells (supplemental Figure 2). To assess whether MCs could promote or enhance the expression of CD40L-complementary surface molecule CD40 on B cells, CD40 expression on B cells cocultured with MCs was analyzed. We observed a weak constitutive surface expression of CD40 on naive B cells, which was significantly increased by coculture with IgE-Ag–activated MCs (Figure 4C). By contrast, only a slight increase of CD40 expression was observed on activated B cells in the presence of MCs (Figure 4C). Moreover, blockade of CD40L on MCs produced a partial, although significant, inhibition of MC-induced proliferation of activated B cells (Figure 4D and supplemental Figure 3), but did not influence naive B-cell proliferation (not shown). Thus, neither cell-cell contact alone nor the mere release of a soluble mediator was responsible for the whole effect of MCs on B-cell proliferation, but both of these mechanisms contributed to the final effect.
Plasma cell differentiation and IgA isotype secretion are the end products of MC–B-cell interactions
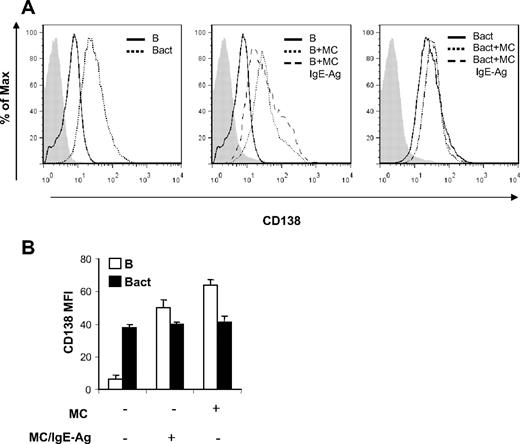
Because proliferation of B cells is regarded as a prerequisite for the progression toward Ig-secreting plasma cells,35 IL-6 is active in inducing B-cell differentiation,36 and CD40/CD40L axis has a major role in promoting isotype switching,37 we decided to investigate whether MCs were able to sustain differentiation of mature activated B cells into Ig-producing plasma cells. To this aim, we first analyzed the expression of the CD138 plasma cell marker on the surface of B cells upon coculture with MCs. As shown in Figure 5, CD138 expression on gated CD19+ cells was increased in naive B cells cocultured with MCs, independently of the MC activation status. Activation of B cells enhanced the expression level of CD138, which was not influenced by MC presence in the coculture, as indicated by unchanged MIF values (Figure 5B).
MCs induce increase of CD138 expression on naive B cells. Surface expression of CD138 on B cells was analyzed by flow cytometry after 72 hours of B-cell and MC cocultures in the same condition of Figure 2. (A) Histograms of cell numbers on the y-axis plotted against log fluorescence intensity of CD138 on x-axis. A representative histogram is shown here of 2 independent experiments. Filled histograms (left, gray) indicate isotype control antibody. (B) The mean fluorescence intensity (MFI) of CD138 expression on naive B cells (B) and on activated B cells (Bact) at baseline and in coculture with nonsensitized MCs (MC) or activated MCs (MC/IgE-Ag) is shown. Results shown are representative of at least 2 independent experiments.
MCs induce increase of CD138 expression on naive B cells. Surface expression of CD138 on B cells was analyzed by flow cytometry after 72 hours of B-cell and MC cocultures in the same condition of Figure 2. (A) Histograms of cell numbers on the y-axis plotted against log fluorescence intensity of CD138 on x-axis. A representative histogram is shown here of 2 independent experiments. Filled histograms (left, gray) indicate isotype control antibody. (B) The mean fluorescence intensity (MFI) of CD138 expression on naive B cells (B) and on activated B cells (Bact) at baseline and in coculture with nonsensitized MCs (MC) or activated MCs (MC/IgE-Ag) is shown. Results shown are representative of at least 2 independent experiments.
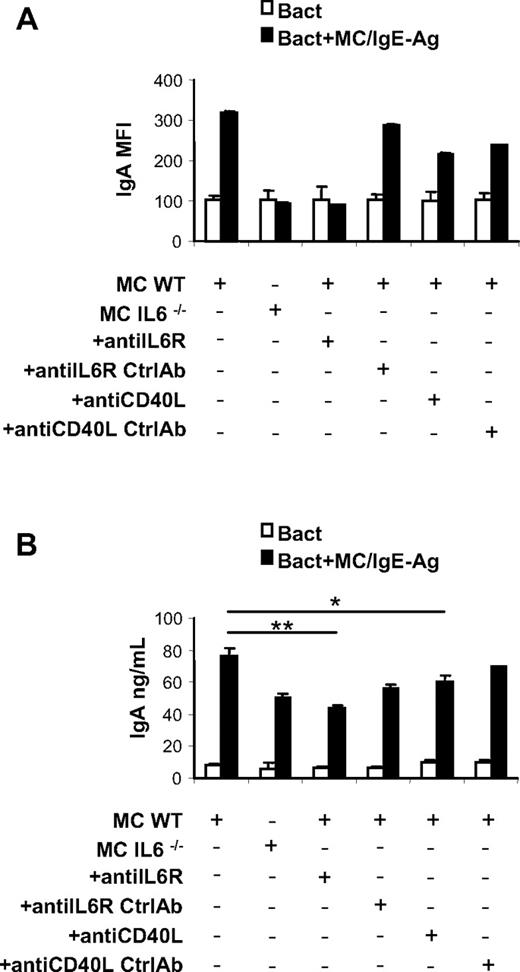
Subsequently, the expression of immunoglobulin molecules was evaluated on naive and activated B cells in the presence of MCs. No significant differences in IgG, IgM, and IgD surface expression were detected between activated B cells alone or in coculture with unstimulated and activated MCs (data not shown), whereas only a slight increase in surface IgE expression was observed on B cells activated in the presence of IgE-Ag–stimulated MCs (supplemental Figure 4A). Instead, IgA expression was selectively increased on activated B cells cocultured with IgE-Ag–activated MCs, suggesting that in the presence of MCs B cells successfully underwent isotype switching (Figure 6A and supplemental Figure 4B). The increase of IgA surface expression on B cells induced by coculture with MCs was prevented using IL-6–deficient MCs and blocking IL-6R or CD40/CD40L interaction (Figure 6A and supplemental Figure 4B).
MC-derived IL-6 and CD40L-CD40 signaling induces IgA surface expression and secretion by activated B cells. Analysis of surface IgA by activated B cells in presence of cultured activated MCs. (A) MIFs of IgA surface expression on activated B cells cultured alone or with activated WT MCs and with IL-6−/− MCs and in the presence of anti–IL-6R or anti-CD40L antibody are measured by flow cytometry. IgA surface expression on B cells alone with anti–IL-6R and of anti-CD40L isotype control antibody was also analyzed by flow cytometry (Ctrl). (B) Secreted IgA by activated B cells cultured alone, with activated WT MCs and with IL-6−/− MCs and in the presence of anti–IL-6R or control antibody (Ctrl), and in the presence of anti-CD40L antibody or control antibody (Ctrl), was detected by ELISA. The results are the average ± SD of values from 3 separate experiments using at least 3 different cultures of MCs. *P < .05; **P < .001.
MC-derived IL-6 and CD40L-CD40 signaling induces IgA surface expression and secretion by activated B cells. Analysis of surface IgA by activated B cells in presence of cultured activated MCs. (A) MIFs of IgA surface expression on activated B cells cultured alone or with activated WT MCs and with IL-6−/− MCs and in the presence of anti–IL-6R or anti-CD40L antibody are measured by flow cytometry. IgA surface expression on B cells alone with anti–IL-6R and of anti-CD40L isotype control antibody was also analyzed by flow cytometry (Ctrl). (B) Secreted IgA by activated B cells cultured alone, with activated WT MCs and with IL-6−/− MCs and in the presence of anti–IL-6R or control antibody (Ctrl), and in the presence of anti-CD40L antibody or control antibody (Ctrl), was detected by ELISA. The results are the average ± SD of values from 3 separate experiments using at least 3 different cultures of MCs. *P < .05; **P < .001.
Because activated B cells are a potential source of IL-6, we wanted to rule out the possibility of a contribution of endogenous IL-6 to the activated B-cell plasmacytic differentiation we observed in coculture experiments. At the same time, we studied the production of 2 other key promoters of B-cell plasmacytic differentiation, namely IL-5 and TGF-β.38,39 IL-6, IL-5, and TGF-β levels were assessed in the supernatants of MC–B-cell cocultures. In contrast to a previous report,40 B-cell activation through anti-CD40 and anti-IgM Ab cross-linking did not result in IL-6 production (supplemental Figure 5A), and the only source of IL-6 in the coculture proved to be sensitized MCs. IL-5 and TGF-β levels (supplemental Figure 5B-C) were exclusively detected upon MC activation, supporting the central role of MCs in the B-cell differentiation toward IgA-producing plasma cells.
Finally, to further confirm the actual differentiation of B cells into Ig-secreting plasma cells, the supernatants of MC–B-cell cocultures were harvested and analyzed for IgA content by ELISA. Activated B cells cocultured with activated MCs secreted considerable amounts of IgA, and this production was significantly limited by IL-6R or CD40L blockade (Figure 6B).
In vivo evidence of concomitant accumulation of MCs and IgA-secreting plasma cells at sites of tissue inflammation
MCs are known to accumulate at sites of inflammation in the colonic mucosa of patients with inflammatory bowel disease.41 However, their role in the pathogenesis of these disorders, as well as their exact contribution to the composition of the inflammatory infiltrate, is largely obscure.
We assessed the distribution of MCs in bioptic samples of inflammatory bowel disease (ulcerative colitis, Crohn disease, nonspecific colitis) patients and found that MCs preferentially accumulated in the lamina propria, where they colocalized with CD20+ B cells (Figure 7A) and associated with abundant IgA secretion (Figure 7B).
Colocalization between MCs and IgA-secreted plasma cell in inflamed mucosal tissue. (A-B) In the gut mucosa of patients with inflammatory bowel disease, MCs (A-B blue signal) accumulate at sites of inflammation where they colocalize with CD20-expressing B cells (A red signal) and IgA-secreting plasma cells (B, red signal). (C-D) In lymph nodes with reactive follicular hyperplasia, in which IgG class switch is prominent, the distribution of IgG-expressing plasma cells (C red signal) appears to be unrelated to that of MCs (C-D blue signal). By contrast, the few IgA-expressing plasma cells (D red signal) localize mainly in close proximity of MCs and even show signs of cell-cell contact (inset). (STREPT-ABC and APAAP methods; original magnifications: panels A-B, ×200; panels B-C, ×100; inset, ×400.)
Colocalization between MCs and IgA-secreted plasma cell in inflamed mucosal tissue. (A-B) In the gut mucosa of patients with inflammatory bowel disease, MCs (A-B blue signal) accumulate at sites of inflammation where they colocalize with CD20-expressing B cells (A red signal) and IgA-secreting plasma cells (B, red signal). (C-D) In lymph nodes with reactive follicular hyperplasia, in which IgG class switch is prominent, the distribution of IgG-expressing plasma cells (C red signal) appears to be unrelated to that of MCs (C-D blue signal). By contrast, the few IgA-expressing plasma cells (D red signal) localize mainly in close proximity of MCs and even show signs of cell-cell contact (inset). (STREPT-ABC and APAAP methods; original magnifications: panels A-B, ×200; panels B-C, ×100; inset, ×400.)
IgA class switch is a characteristic feature of lymphocytes populating the mucosa-associated lymphoid tissue,42 and its occurrence in peripheral lymph nodes is a rare event. Nevertheless, lymph nodes with reactive follicular hyperplasia commonly show low numbers of IgA-secreting plasma cells scattered among more abundant IgG plasma cells (Figure 7C). Interestingly, we found that these few IgA-expressing plasma cells selectively occurred in areas populated by MCs, where the 2 cell populations displayed tight spatial interactions (Figure 7D inset).
Taken together these pictures are suggestive of an interplay between MCs, B cells, and IgA-secreting plasma cells that takes place in vivo in an inflammatory background.
Discussion
The major compelling element strengthening the need for a deep understanding of MC–B-cell interactions is the long-standing evidence of the association between MCs and B-cell lymphomatous aggregates, which was originally described by Lennert's group in 1977 (Satodate et al).43 Since then, many other reports have focused on the abundance of MCs at sites of leukemic/lymphomatous infiltration, especially in mature indolent B-cell neoplasms such as chronic lymphocytic leukemia and lymphoplasmacytic lymphoma (Waldenström macroglobulinemia).11-16 In these conditions, MCs can be found surrounding neoplastic lymphoid aggregates (supplemental Figure 6A) or even intermingling with neoplastic cells (supplemental Figure 6B), suggesting an active participation of MCs to the composition of the lymphoma-associated microenvironment. In this regard, a direct contribution of neoplastic cells to the recruitment of MCs at sites of infiltration via CCL5 synthesis has been demonstrated in Hodgkin lymphoma (HL).44 The hypothesis that MCs have an influence on neoplastic B cells is corroborated by retrospective studies reporting the prognostic significance of MCs in B-cell lymphoproliferative disorders.15,45 The majority of these reports put into evidence a detrimental role of MCs in the prognosis of HL disease and B-cell non-Hodgkin lymphoma lymphomas, but contrasting evidence of the association of MCs with a favorable prognosis in some specific subtypes of B-cell non-Hodgkin lymphoma (ie, diffuse large B-cell lymphoma) has also been reported.12 Altogether, these data provided an overview on the complexity of the role of MCs in B-cell lymphoproliferative diseases, as MCs may be able to directly interact with neoplastic B cells, influencing their growth and survival, or to shape the tumor-associated stromal and inflammatory microenvironment. In keeping with the first hypothesis are data demonstrating that MCs can activate Hodgkin lymphoma Reed-Sternberg cells through CD30L/CD30 interaction, probably leading to an increased proliferation of Hodgkin lymphoma Reed-Sternberg cells.15 Moreover, in lymphoplasmacytic lymphoma, MCs have been reported to support neoplastic cell proliferation and survival through CD40/CD40L or CD27/CD70 interactions.13,16 On the other hand, MCs as well-known sources of proinflammatory and proangiogenic mediators have been described in association with an inflammatory cell–rich background and increased angiogenesis in HL.46
Finally, data on the possible link between MCs and B lymphocytes come also from the reported spatial association between the neoplastic MC aggregates of indolent systemic mastocytosis and surrounding B-cell lymphoid aggregates, as well as from few detailed reports on the concurrence of systemic mastocytosis and chronic lymphocytic leukemia that allow for speculation on the role of MCs in B-cell recruitment, activation, and proliferation.14
In the present study, we have uncovered a specific role of MCs in B-cell growth and differentiation. We reported that mouse bone marrow–derived MCs can induce both survival and proliferation of primary naive B cells and proliferation and further differentiation of activated B cells into IgA-secreting plasma cells.
B-cell proliferation was reduced when MCs and B cells were separated by the transwell membranes or when MCs were IL-6 deficient, suggesting that soluble factors and cell-cell contact are important in this event.
Interestingly, analyzing the immunophenotype of MCs and B cells in the coculture, we reported evidence that MCs can influence the surface expression of CD40 on naive B cells, suggesting that MCs activated by IgE and Ag are fully capable of enhancing B-cell activation. Indeed, it is known that B-cell activation results in enhanced CD40 expression47,48 and that the interaction of this molecule with its receptor on MCs results in costimulatory signals leading to the productive survival, activation, and expansion of B cells. Moreover, we demonstrated that the interaction of CD40 on B-cell surface and CD40L on MCs is important for the proliferation of B cells and for their differentiation into plasma cells.
We showed for the first time that activated MCs are able to induce IgA surface expression on activated B cells and IgA secretion. Hence, we suggested a new role of MCs in the induction of humoral immune responses, which is likely to occur through the release of cytokines, such as IL-5, IL-6, TGF-β, and others. Indeed, it is known that IL-6 is an efficient cofactor of IL-5 in IgA synthesis36 and TGF-β is a costimulator for IgA production.49 Accordingly, our findings might contribute to clarify the mechanisms underlying defective IgA-mediated humoral immune responses to specific pathogens (eg, Giardia lamblia) that can be observed in Kitw/wv mice lacking mast cells.50
Taken together our data significantly expand the paradigm of MCs as key players in the orchestration of the immune response, as they add a valuable piece to the mosaic of functions that innate immune cells endorse in adaptive immunity. Moreover, they cast new light on the dynamics possibly underlying MC involvement in B-cell homeostasis and in B-cell lymphoproliferative disorders. In this setting, we suggest that MCs could have a share in directly sustaining the expansion of the B-cell clone through IL-6 production and cell-cell interaction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Elena Betto and Luca Danelli for helpful suggestions.
This work was supported by grants from the Italian Ministry of Health, AIRC (Associazione Italiana Ricerca sul Cancro), Ministero dell'Istruzione, Università e Ricerca (PRIN 2005), Agenzia Spaziale Italiana (Progetto OSMA), and LR.11 del Friuli Venezia Giulia.
Authorship
Contribution: S.M. and B.F. equally contributed to discussion of experimental design and data analysis, and wrote the paper; S.P. performed analysis of CFSE proliferation; B.F. set up the in vitro MC–B-cell cocultures; G.G. performed flow cytometric analyses and discussed the results; C.T. performed histopathologic and immunophenotypical analyses; S.P. helped with experiments; and C.T. and C.P. directed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio Tripodo, Department of Human Pathology, University of Palermo, Via del Vespro, 129-90127 Palermo, Italy; e-mail: tripodo@unipa.it.
References
Author notes
S.M. and B.F. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal