Abstract
Despite recent advances in understanding the pathophysiologic mechanisms behind the thalassemia intermedia (TI) phenotype, data on the effects of treatment are deficient. To provide such data, we evaluated 584 TI patients for the associations between patient and disease characteristics, treatment received, and the rate of complications. The most common disease-related complications were osteoporosis, extramedullary hematopoeisis (EMH), hypogonadism, and cholelithiasis, followed by thrombosis, pulmonary hypertension (PHT), abnormal liver function, and leg ulcers. Hypothyroidism, heart failure, and diabetes mellitus were less frequently observed. On multivariate analysis, older age and splenectomy were independently associated with an increased risk of most disease-related complications. Transfusion therapy was protective for thrombosis, EMH, PHT, heart failure, cholelithiasis, and leg ulcers. However, transfusion therapy was associated with an increased risk of endocrinopathy. Iron chelation therapy was in turn protective for endocrinopathy and PHT. Hydroxyurea treatment was associated with an increased risk of hypogonadism yet was protective for EMH, PHT, leg ulcers, hypothyroidism, and osteoporosis. Attention should be paid to the impact of age on complications in TI, and the beneficial role of splenectomy deserves revisiting. This study provides evidence that calls for prospective evaluation of the roles of transfusion, iron chelation, and hydroxyurea therapy in TI patients.
Introduction
Knowledge of the molecular basis of thalassemia intermedia (TI) has progressed significantly in the last decade, including an increased understanding of the genetic mutations that lead to the associated phenotypes.1,2 It is now established that such clinical phenotypes lie in severity between those of thalassemia minor (clinically silent, mildly hypochromic, and microcytic anemia) and transfusion-dependent thalassemia major (TM), although there is substantial clinical overlap between the 3 conditions.3 Three main factors are responsible for the clinical sequelae of TI: ineffective erythropoiesis, chronic anemia, and iron overload.3 The degree of ineffective erythropoiesis is the primary determinant of the development of anemia, whereas peripheral hemolysis of mature red blood cells (RBCs) remains secondary.4 Although the first is mainly associated with skeletal complications attributed to compensatory extramedullary hematopoiesis (EMH),5 the latter has been linked to more severe complications, such as pulmonary hypertension (PHT),6,7 with secondary heart failure (HF), and thromboembolic phenomena.8 Moreover, chronic anemia leads to an increase in gastrointestinal iron absorption,9 resulting in iron overload, which in turn can cause several serious complications, including HF and endocrine abnormalities, such as diabetes mellitus, hypothyroidism, osteoporosis, and hypogonadism.3,10,11 There are several options that may be available for managing patients with TI, including splenectomy, transfusion therapy, iron chelation therapy, and modulation of fetal hemoglobin (HbF) production.3,5,12-15 However, despite the availability of several treatment options, these modalities have rarely been evaluated in TI patients, and the lack of clear guidelines still presents a significant clinical challenge.5
We herein present the largest overview to date on the current status of TI patients, in 6 comprehensive care centers, by assessing the rate of disease-associated complications in relation to currently practiced treatment options.
Methods
This was a retrospective review of the medical charts of all TI patients currently registered at 6 comprehensive care centers in Lebanon, Italy, Iran, Egypt, United Arab Emirates, and Oman. Institutional review boards at each center approved the study protocol. All patients were diagnosed with TI based on criteria previously described.16,17 Data included demographics (age and sex), splenectomy status, mean hemoglobin (Hb), alanine transaminase, and steady-state serum ferritin levels of all available laboratory records (mean, 18.6 ± 8.2, 8.1 ± 6.2, and 13.5 ± 6.6 readings, over 10.6 ± 4.2, 5.1 ± 3.2, and 8.5 ± 3.6 years, respectively), and type of treatment received (RBC transfusion, iron chelation, and hydroxyurea). The main indications for splenectomy were growth retardation or poor health; leukopenia; thrombocytopenia; increased transfusion demand; or symptomatic splenomegaly. For transfusion status, data were categorized as follows: regular transfusion (patients on regular-interval transfusion protocols [once every 1-3 months for a pretransfusion Hb of ≥ 90 g/L] initiated mainly for failure to thrive in childhood; bone deformities; progressive splenic enlargement; persistent worsening anemia; or development of complications during the course of the disease); occasional transfusion (patients who required incidental transfusions for transient severe anemia secondary to infections, surgery, or pregnancy); and never transfused. Iron chelation therapy had to be administered for at least one year or else the patient was considered nonchelated. Complications were defined according to Table 1.18-23 The prevalence of other elements (family history of cardiovascular or endocrine disease, acquired or inherited thrombophilia, anticoagulant or antiplatelet use for reasons other than overt thrombosis, malignancy, orthopedic surgery, hepatitis C or B virus infection) in the patients' medical history that could modify the rate of complications was low; and hence, these parameters were not included in further analysis.
Clinical definitions required to confirm identified complications
| Complication . | Definition . |
|---|---|
| EMH | Radiologic evidence of extramedullary hematopoietic foci with or without symptoms |
| PHT | Systolic pulmonary artery pressure > 35 mm Hg, which corresponds to a tricuspid regurgitant velocity on Doppler echocardiography of > 2.8 m/s18 plus exertional dyspnea without evidence of left heart disease |
| HF | Modified Framingham criteria19 |
| Thrombosis | Compression ultrasonography, contrast venography, or angiography evidence of thrombus |
| Cholelithiasis | Ultrasonographic evidence of gallbladder stones |
| Abnormal liver function | ALT > 50 U/L |
| Leg ulcers | Ischemic or necrotic skin lesion on the lower extremity by general visual inspection |
| DM | Fasting blood sugar ≥ 126 mg/dL, or 2-hour postprandial blood sugar ≥ 200 mg/dL, or symptoms of hyperglycemia and a casual (random) plasma glucose ≥ 200 mg/dL20 |
| Hypothyroidism | TSH > 4.7 μU/L and a free T4 < 0.8 ng/dL21 |
| Osteoporosis | Bone densitometry T score: 2.5 SD22 |
| Hypogonadism | Females: > 13 y, not yet Tanner B2 (ie, prepubertal breast development) or > 14 y requiring estrogen replacement therapy or > 15 y with primary amenorrhea; males: > 14 y, not yet Tanner G2 (ie, prepubertal genital development) or on androgen replacement therapy or > 17 y, not yet Tanner G4 (ie, midpubertal genital development)23 |
| Complication . | Definition . |
|---|---|
| EMH | Radiologic evidence of extramedullary hematopoietic foci with or without symptoms |
| PHT | Systolic pulmonary artery pressure > 35 mm Hg, which corresponds to a tricuspid regurgitant velocity on Doppler echocardiography of > 2.8 m/s18 plus exertional dyspnea without evidence of left heart disease |
| HF | Modified Framingham criteria19 |
| Thrombosis | Compression ultrasonography, contrast venography, or angiography evidence of thrombus |
| Cholelithiasis | Ultrasonographic evidence of gallbladder stones |
| Abnormal liver function | ALT > 50 U/L |
| Leg ulcers | Ischemic or necrotic skin lesion on the lower extremity by general visual inspection |
| DM | Fasting blood sugar ≥ 126 mg/dL, or 2-hour postprandial blood sugar ≥ 200 mg/dL, or symptoms of hyperglycemia and a casual (random) plasma glucose ≥ 200 mg/dL20 |
| Hypothyroidism | TSH > 4.7 μU/L and a free T4 < 0.8 ng/dL21 |
| Osteoporosis | Bone densitometry T score: 2.5 SD22 |
| Hypogonadism | Females: > 13 y, not yet Tanner B2 (ie, prepubertal breast development) or > 14 y requiring estrogen replacement therapy or > 15 y with primary amenorrhea; males: > 14 y, not yet Tanner G2 (ie, prepubertal genital development) or on androgen replacement therapy or > 17 y, not yet Tanner G4 (ie, midpubertal genital development)23 |
ALT indicates alanine transaminase; DM, diabetes mellitus; TSH, thyroid stimulating hormone; and T4, thyroxine.
Statistical analysis
Descriptive statistics are expressed as percentages or means. For each complication, a univariate analysis was done to determine the effect of study parameters (age, sex, serum ferritin level, Hb level, splenectomy, transfusion, hydroxyurea, and iron chelation therapy) using χ2 and Fisher exact test. Multivariate logistic regression analysis was done for each complication as a dependent variable to determine the independent effect of study parameters; where all variables with a P value less than .1 (on univariate analysis) were entered into the model. In the multivariate model age was divided into 2 groups (≤ 35 and > 35 years) and transfusion status was defined as transfused versus never transfused to preserve sample size. Differences in the mean number of complications between different treatment modalities were evaluated using the independent samples t test. All P values are 2-sided with the level of significance set at less than .05.
Results
Patient, disease, and treatment characteristics
A total of 584 TI patients were identified in the 5 participating centers. The mean age was 25.44 plus or minus 13.86 years (range, 2-76 years) with a male-to-female ratio of 291:293. A total of 325 (55.7%) patients were splenectomized. The mean Hb, alanine transaminase, and steady-state serum ferritin levels of the whole study group were 89 plus or minus 14.9 g/L (range, 49-140 g/L), 31.68 plus or minus 32.42 IU/L (range, 6-148 IU/L), and 967.5 plus or minus 853.9 μg/L (17-10 793 μg/L), respectively. The most common disease-related complications were osteoporosis, EMH, hypogonadism, and cholelithiasis, followed by thrombosis, PHT, abnormal liver function, and leg ulcers. Hypothyroidism, HF, and diabetes mellitus were less frequently observed (Table 2). The types of treatment received are summarized in Table 2.
Patient and disease characteristics of the study population
| Parameter . | Frequency, no. (%) . |
|---|---|
| Age, y | |
| Less than 18 | 172 (29.5) |
| 18-35 | 288 (49.3) |
| More than 35 | 124 (21.2) |
| Male:female | 291 (49.8):293 (50.2) |
| Splenectomized | 325 (55.7) |
| Serum ferritin, μg/L | |
| Less than 1000 | 376 (64.4) |
| 1000-2500 | 179 (30.6) |
| More than 2500 | 29 (5) |
| Treatment | |
| Hydroxyurea | 202 (34.6) |
| Occasional transfusion | 143 (24.5) |
| Regular transfusion | 302 (51.7) |
| Iron chelation | 336 (47.5) |
| Complications | |
| Osteoporosis | 134 (22.9) |
| EMH | 124 (21.2) |
| Hypogonadism | 101 (17.3) |
| Cholelithiasis | 100 (17.1) |
| Thrombosis | 82 (14) |
| PHT | 64 (11) |
| Abnormal liver function | 57 (9.8) |
| Leg ulcers | 46 (7.9) |
| Hypothyroidisim | 33 (5.7) |
| HF | 25 (4.3) |
| Diabetes mellitus | 10 (1.7) |
| Parameter . | Frequency, no. (%) . |
|---|---|
| Age, y | |
| Less than 18 | 172 (29.5) |
| 18-35 | 288 (49.3) |
| More than 35 | 124 (21.2) |
| Male:female | 291 (49.8):293 (50.2) |
| Splenectomized | 325 (55.7) |
| Serum ferritin, μg/L | |
| Less than 1000 | 376 (64.4) |
| 1000-2500 | 179 (30.6) |
| More than 2500 | 29 (5) |
| Treatment | |
| Hydroxyurea | 202 (34.6) |
| Occasional transfusion | 143 (24.5) |
| Regular transfusion | 302 (51.7) |
| Iron chelation | 336 (47.5) |
| Complications | |
| Osteoporosis | 134 (22.9) |
| EMH | 124 (21.2) |
| Hypogonadism | 101 (17.3) |
| Cholelithiasis | 100 (17.1) |
| Thrombosis | 82 (14) |
| PHT | 64 (11) |
| Abnormal liver function | 57 (9.8) |
| Leg ulcers | 46 (7.9) |
| Hypothyroidisim | 33 (5.7) |
| HF | 25 (4.3) |
| Diabetes mellitus | 10 (1.7) |
Determinants of complication rate
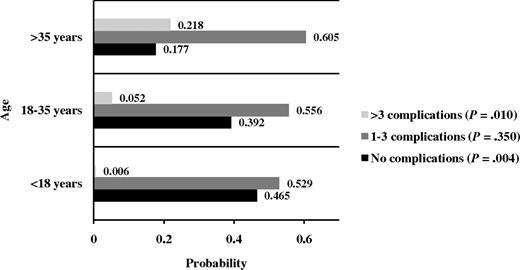
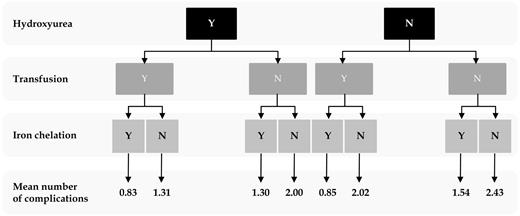
Results of univariate analysis are summarized in Table 3. On multivariate analysis (Table 4), older age and splenectomy were independently associated with an increased risk of most disease-related complications; splenectomy was protective only against the development of EMH. The probability of having 0, 1 to 3, and more than 3 complications at different age intervals is summarized in Figure 1. Female sex was associated with an increased risk of osteoporosis, hypogonadism, and cholelithiasis. Although a mean Hb more than or equal to 90 g/L was only associated with a reduced risk of thrombotic events, transfusion therapy was protective for thrombosis, EMH, PHT, HF, cholelithiasis, and leg ulcers. However, transfusion therapy was associated with an increased risk of endocrinopathy (hypothyroidism, osteoporosis, and hypogonadism). Whereas a mean serum ferritin level of more than 1000 μg/L was only independently associated with an increased risk of hypogonadism, iron chelathion therapy was protective for a multitude of other complications (hypogonadism, PHT, cholelithiasis, and osteoporosis). Hydroxyurea treatment was associated with an increased risk of hypogonadism yet was protective for EMH, PHT, leg ulcers, hypothyroidism, and osteoporosis. The mean number of complications for different management schemes are summarized in Figure 2. The lowest mean number of complications (0.827) reflected patients who received the 3 treatment modalities, whereas the highest mean number of complications (2.43) reflected patients who received no treatment; the difference between both means was statistically significant (t test; P < .001). The difference remained statistically significant after controlling for the effects of age and splenectomy (P = .03).
Univariate analysis for determinants of complication rate
| . | EMH . | PHT . | HF . | Thrombosis . | Cholelithiasis . | Abnormal liver function . | Leg ulcers . | DM . | Hypothyroidism . | Osteoporosis . | Hypogonadism . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | |||||||||||
| Less than 18 (n = 172) | 36 | 2.3 | 1.7 | 4.1 | 2.3 | 6.4 | 2.9 | 0 | 0.6 | 2.3 | 14 |
| 18-35 (n = 288) | 12.8 | 11.5 | 5.2 | 13.9 | 17 | 11.5 | 6.2 | 1.4 | 6.2 | 20.5 | 21.5 |
| More than 35 (n = 124) | 20.2 | 21.8 | 5.6 | 28.2 | 37.9 | 10.5 | 18.5 | 4.8 | 11.3 | 57.3 | 12.1 |
| P | < .001* | < .001* | .145 | < .001* | < .001* | .199 | < .001* | 0.005* | < .001* | < .001* | .026* |
| Sex | |||||||||||
| Male (n = 291) | 21 | 9.6 | 5.2 | 11 | 12.4 | 10 | 7.2 | 1 | 5.8 | 17.2 | 10.7 |
| Female (n = 293) | 21.5 | 12.3 | 3.4 | 17.1 | 21.8 | 9.6 | 8.5 | 2.4 | 5.5 | 28.7 | 23.9 |
| P | .873 | .303 | .299 | .035* | .002* | .868 | .555 | .206 | .842 | .001* | < .001* |
| Ferritin, μg/L | |||||||||||
| Less than 1000 (n = 376) | 21.8 | 9.8 | 4.5 | 10.1 | 17 | 8 | 6.1 | 2.1 | 5.1 | 18.1 | 13.3 |
| 1000 or more (n = 208) | 20.2 | 13 | 3.8 | 21.2 | 17.3 | 13 | 11.1 | 1 | 6.7 | 31.7 | 24.5 |
| P | .059 | .245 | .700 | < .001* | .930 | .051 | .034* | .298 | .400 | < .001* | < .001* |
| Hemoglobin, g/L | |||||||||||
| Less than 90 (n = 282) | 21.6 | 11 | 4.6 | 19.5 | 16.7 | 11.7 | 9.6 | 1.8 | 4.6 | 24.1 | 18.1 |
| 90 or more (n = 302) | 20.9 | 10.9 | 4 | 8.9 | 17.5 | 7.9 | 6.3 | 1.7 | 6.6 | 21.9 | 16.6 |
| P | .820 | .980 | .704 | < .001* | .777 | .127 | .141 | .913 | .293 | .516 | .625 |
| Splenectomy | |||||||||||
| No (n = 259) | 26.3 | 3.9 | 1.9 | 3.5 | 5.0 | 10 | 2.7 | 0.4 | 1.5 | 9.3 | 13.9 |
| Yes (n = 325) | 17.2 | 16.6 | 6.2 | 22.5 | 26.8 | 9.5 | 12 | 2.8 | 8.9 | 33.8 | 20 |
| P | .008* | < .001* | .012* | < .001* | < .001* | .840 | < .001* | .027* | < .001* | < .001* | .053 |
| Transfusion | |||||||||||
| None (n = 139) | 60.4 | 20.1 | 14.4 | 26.6 | 27.3 | 7.2 | 13.7 | 0.7 | 0 | 15.1 | 2.2 |
| Occasional (n = 143) | 19.6 | 14.3 | 0 | 18.2 | 19.6 | 14.7 | 13.3 | 1.4 | 9.8 | 44.1 | 18.2 |
| Regular (n = 302) | 4 | 5.3 | 1.7 | 6.3 | 11.3 | 8.6 | 2.6 | 2.3 | 6.3 | 16.6 | 23.8 |
| P | < .001* | < .001* | < .001* | < .001* | < .001* | .066 | < .001* | .459 | 0.001* | < .001* | < .001* |
| Hydroxyurea | |||||||||||
| No (n = 382) | 23.8 | 13.9 | 3.1 | 17 | 20.9 | 9.4 | 11.5 | 2.6 | 8.4 | 34.6 | 11.5 |
| Yes (n= 202) | 16.3 | 5.4 | 6.4 | 8.4 | 9.9 | 10.4 | 1 | 0 | 0.5 | 1 | 28.2 |
| P | .035* | .002* | .061 | .004* | .001* | .707 | < .001* | .020* | < .001* | < .001* | < .001* |
| Iron chelation | |||||||||||
| No (n = 248) | 19.4 | 16.1 | 6.5 | 17.7 | 28.2 | 10.5 | 10.9 | 3.2 | 8.1 | 32.7 | 12.9 |
| Yes (n = 336) | 22.6 | 7.1 | 2.7 | 11.3 | 8.9 | 9.2 | 5.7 | 0.6 | 3.9 | 15.8 | 20.5 |
| P | .340 | .001* | .026* | .027* | < .001* | .613 | .020* | .015* | .030* | < .001* | .016* |
| . | EMH . | PHT . | HF . | Thrombosis . | Cholelithiasis . | Abnormal liver function . | Leg ulcers . | DM . | Hypothyroidism . | Osteoporosis . | Hypogonadism . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | |||||||||||
| Less than 18 (n = 172) | 36 | 2.3 | 1.7 | 4.1 | 2.3 | 6.4 | 2.9 | 0 | 0.6 | 2.3 | 14 |
| 18-35 (n = 288) | 12.8 | 11.5 | 5.2 | 13.9 | 17 | 11.5 | 6.2 | 1.4 | 6.2 | 20.5 | 21.5 |
| More than 35 (n = 124) | 20.2 | 21.8 | 5.6 | 28.2 | 37.9 | 10.5 | 18.5 | 4.8 | 11.3 | 57.3 | 12.1 |
| P | < .001* | < .001* | .145 | < .001* | < .001* | .199 | < .001* | 0.005* | < .001* | < .001* | .026* |
| Sex | |||||||||||
| Male (n = 291) | 21 | 9.6 | 5.2 | 11 | 12.4 | 10 | 7.2 | 1 | 5.8 | 17.2 | 10.7 |
| Female (n = 293) | 21.5 | 12.3 | 3.4 | 17.1 | 21.8 | 9.6 | 8.5 | 2.4 | 5.5 | 28.7 | 23.9 |
| P | .873 | .303 | .299 | .035* | .002* | .868 | .555 | .206 | .842 | .001* | < .001* |
| Ferritin, μg/L | |||||||||||
| Less than 1000 (n = 376) | 21.8 | 9.8 | 4.5 | 10.1 | 17 | 8 | 6.1 | 2.1 | 5.1 | 18.1 | 13.3 |
| 1000 or more (n = 208) | 20.2 | 13 | 3.8 | 21.2 | 17.3 | 13 | 11.1 | 1 | 6.7 | 31.7 | 24.5 |
| P | .059 | .245 | .700 | < .001* | .930 | .051 | .034* | .298 | .400 | < .001* | < .001* |
| Hemoglobin, g/L | |||||||||||
| Less than 90 (n = 282) | 21.6 | 11 | 4.6 | 19.5 | 16.7 | 11.7 | 9.6 | 1.8 | 4.6 | 24.1 | 18.1 |
| 90 or more (n = 302) | 20.9 | 10.9 | 4 | 8.9 | 17.5 | 7.9 | 6.3 | 1.7 | 6.6 | 21.9 | 16.6 |
| P | .820 | .980 | .704 | < .001* | .777 | .127 | .141 | .913 | .293 | .516 | .625 |
| Splenectomy | |||||||||||
| No (n = 259) | 26.3 | 3.9 | 1.9 | 3.5 | 5.0 | 10 | 2.7 | 0.4 | 1.5 | 9.3 | 13.9 |
| Yes (n = 325) | 17.2 | 16.6 | 6.2 | 22.5 | 26.8 | 9.5 | 12 | 2.8 | 8.9 | 33.8 | 20 |
| P | .008* | < .001* | .012* | < .001* | < .001* | .840 | < .001* | .027* | < .001* | < .001* | .053 |
| Transfusion | |||||||||||
| None (n = 139) | 60.4 | 20.1 | 14.4 | 26.6 | 27.3 | 7.2 | 13.7 | 0.7 | 0 | 15.1 | 2.2 |
| Occasional (n = 143) | 19.6 | 14.3 | 0 | 18.2 | 19.6 | 14.7 | 13.3 | 1.4 | 9.8 | 44.1 | 18.2 |
| Regular (n = 302) | 4 | 5.3 | 1.7 | 6.3 | 11.3 | 8.6 | 2.6 | 2.3 | 6.3 | 16.6 | 23.8 |
| P | < .001* | < .001* | < .001* | < .001* | < .001* | .066 | < .001* | .459 | 0.001* | < .001* | < .001* |
| Hydroxyurea | |||||||||||
| No (n = 382) | 23.8 | 13.9 | 3.1 | 17 | 20.9 | 9.4 | 11.5 | 2.6 | 8.4 | 34.6 | 11.5 |
| Yes (n= 202) | 16.3 | 5.4 | 6.4 | 8.4 | 9.9 | 10.4 | 1 | 0 | 0.5 | 1 | 28.2 |
| P | .035* | .002* | .061 | .004* | .001* | .707 | < .001* | .020* | < .001* | < .001* | < .001* |
| Iron chelation | |||||||||||
| No (n = 248) | 19.4 | 16.1 | 6.5 | 17.7 | 28.2 | 10.5 | 10.9 | 3.2 | 8.1 | 32.7 | 12.9 |
| Yes (n = 336) | 22.6 | 7.1 | 2.7 | 11.3 | 8.9 | 9.2 | 5.7 | 0.6 | 3.9 | 15.8 | 20.5 |
| P | .340 | .001* | .026* | .027* | < .001* | .613 | .020* | .015* | .030* | < .001* | .016* |
All data except P values are presented as percentages. DM indicates diabetes mellitus.
Statistically significant.
Multivariate analysis for determinants of complication rate
| Complication/parameter . | RR . | 95% CI . | P . |
|---|---|---|---|
| EMH | |||
| Age > 35 y | 0.85 | 0.46-1.58 | .610 |
| Ferritin ≥ 1000 μg/L | 0.85 | 0.51-1.44 | .548 |
| Splenectomy | 0.44 | 0.26-0.73 | .001* |
| Transfusion | 0.06 | 0.03-0.09 | < .001* |
| Hydroxyurea | 0.52 | 0.30-0.91 | .022* |
| PHT | |||
| Age > 35 y | 2.59 | 1.08-6.19 | .032* |
| Splenectomy | 4.11 | 1.99-8.47 | < .001* |
| Transfusion | 0.33 | 0.18-0.58 | < .001* |
| Hydroxyurea | 0.42 | 0.20-0.90 | .025* |
| Iron chelation | 0.53 | 0.29-0.95 | .032* |
| HF | |||
| Splenectomy | 2.88 | 0.99-8.32 | .051 |
| Transfusion | 0.06 | 0.02-0.17 | < .001* |
| Hydroxyurea | 1.84 | 0.98-3.47 | .057 |
| Iron chelation | 0.45 | 0.18-1.12 | .086 |
| Thrombosis | |||
| Age > 35 y | 2.60 | 1.39-4.87 | .003* |
| Female | 1.27 | 0.74-2.19 | .387 |
| Hb ≥ 90 g/L | 0.41 | 0.23-0.71 | .001* |
| Ferritin ≥ 1000 μg/L | 1.86 | 1.09-3.16 | .023* |
| Splenectomy | 6.59 | 3.09-14.05 | < .001* |
| Transfusion | 0.28 | 0.16-0.48 | < .001* |
| Hydroxyurea | 0.56 | 0.28-1.10 | .090 |
| Iron chelation | 0.97 | 0.56-1.68 | .912 |
| Cholelithiasis | |||
| Age > 35 y | 2.76 | 1.56-4.87 | < .001* |
| Female | 1.96 | 1.18-3.25 | .010* |
| Splenectomy | 5.19 | 2.72-9.90 | < .001* |
| Transfusion | 0.36 | 0.21-0.62 | < .001* |
| Hydroxyurea | 0.55 | 0.29-1.02 | .058 |
| Iron chelation | 0.30 | 0.18-0.51 | < .001* |
| Abnormal liver function | |||
| Ferritin ≥ 1000 μg/L | 1.74 | 1.00-3.02 | .049* |
| Transfusion | 1.56 | 0.76-3.17 | .224 |
| Leg ulcers | |||
| Age > 35 y | 2.09 | 1.05-4.16 | .036* |
| Ferritin ≥ 1000 μg/L | 1.29 | 0.67-2.47 | .449 |
| Splenectomy | 3.98 | 1.68-9.39 | .002* |
| Transfusion | 0.39 | 0.20-0.76 | .006* |
| Hydroxyurea | 0.10 | 0.02-0.43 | .002* |
| Iron chelation | 0.68 | 0.35-1.34 | .269 |
| DM | |||
| Age > 35 y | 2.00 | 0.53-7.62 | .309 |
| Splenectomy | 5.79 | 0.71-47.21 | .101 |
| Hydroxyurea | 0.24 | 0.03-2.20 | .208 |
| Iron chelation | 0.40 | 0.10-1.62 | .197 |
| Hypothyroidism | |||
| Age > 35 y | 1.01 | 0.46-2.23 | .984 |
| Splenectomy | 6.04 | 2.03-17.92 | .001* |
| Transfusion | 13.3 | 1.78-100.00 | .012 |
| Hydroxyurea | 0.05 | 0.01-0.45 | .003* |
| Iron chelation | 0.49 | 0.22-1.07 | .073 |
| Osteoporosis | |||
| Age > 35 y | 3.51 | 2.06-5.99 | < .001* |
| Female | 1.97 | 1.19-3.27 | .009* |
| Ferritin ≥ 1000 μg/L | 1.60 | 0.96-2.68 | .072 |
| Splenectomy | 4.73 | 2.72-8.24 | < .001* |
| Transfusion | 3.10 | 1.64-5.85 | < .001* |
| Hydroxyurea | 0.02 | 0.01-0.09 | < .001* |
| Iron chelation | 0.40 | 0.24-0.68 | .001* |
| Hypogonadism | |||
| Age > 35 y | 1.05 | 0.51-2.15 | .900 |
| Female | 2.98 | 1.79-4.96 | < .001* |
| Ferritin ≥ 1000 μg/L | 2.63 | 1.59-4.36 | < .001* |
| Splenectomy | 1.65 | 0.97-2.77 | .056 |
| Transfusion | 16.13 | 4.85-52.63 | < .001* |
| Hydroxyurea | 4.32 | 2.49-7.49 | < .001* |
| Iron chelation | 2.51 | 1.48-4.26 | .001* |
| Complication/parameter . | RR . | 95% CI . | P . |
|---|---|---|---|
| EMH | |||
| Age > 35 y | 0.85 | 0.46-1.58 | .610 |
| Ferritin ≥ 1000 μg/L | 0.85 | 0.51-1.44 | .548 |
| Splenectomy | 0.44 | 0.26-0.73 | .001* |
| Transfusion | 0.06 | 0.03-0.09 | < .001* |
| Hydroxyurea | 0.52 | 0.30-0.91 | .022* |
| PHT | |||
| Age > 35 y | 2.59 | 1.08-6.19 | .032* |
| Splenectomy | 4.11 | 1.99-8.47 | < .001* |
| Transfusion | 0.33 | 0.18-0.58 | < .001* |
| Hydroxyurea | 0.42 | 0.20-0.90 | .025* |
| Iron chelation | 0.53 | 0.29-0.95 | .032* |
| HF | |||
| Splenectomy | 2.88 | 0.99-8.32 | .051 |
| Transfusion | 0.06 | 0.02-0.17 | < .001* |
| Hydroxyurea | 1.84 | 0.98-3.47 | .057 |
| Iron chelation | 0.45 | 0.18-1.12 | .086 |
| Thrombosis | |||
| Age > 35 y | 2.60 | 1.39-4.87 | .003* |
| Female | 1.27 | 0.74-2.19 | .387 |
| Hb ≥ 90 g/L | 0.41 | 0.23-0.71 | .001* |
| Ferritin ≥ 1000 μg/L | 1.86 | 1.09-3.16 | .023* |
| Splenectomy | 6.59 | 3.09-14.05 | < .001* |
| Transfusion | 0.28 | 0.16-0.48 | < .001* |
| Hydroxyurea | 0.56 | 0.28-1.10 | .090 |
| Iron chelation | 0.97 | 0.56-1.68 | .912 |
| Cholelithiasis | |||
| Age > 35 y | 2.76 | 1.56-4.87 | < .001* |
| Female | 1.96 | 1.18-3.25 | .010* |
| Splenectomy | 5.19 | 2.72-9.90 | < .001* |
| Transfusion | 0.36 | 0.21-0.62 | < .001* |
| Hydroxyurea | 0.55 | 0.29-1.02 | .058 |
| Iron chelation | 0.30 | 0.18-0.51 | < .001* |
| Abnormal liver function | |||
| Ferritin ≥ 1000 μg/L | 1.74 | 1.00-3.02 | .049* |
| Transfusion | 1.56 | 0.76-3.17 | .224 |
| Leg ulcers | |||
| Age > 35 y | 2.09 | 1.05-4.16 | .036* |
| Ferritin ≥ 1000 μg/L | 1.29 | 0.67-2.47 | .449 |
| Splenectomy | 3.98 | 1.68-9.39 | .002* |
| Transfusion | 0.39 | 0.20-0.76 | .006* |
| Hydroxyurea | 0.10 | 0.02-0.43 | .002* |
| Iron chelation | 0.68 | 0.35-1.34 | .269 |
| DM | |||
| Age > 35 y | 2.00 | 0.53-7.62 | .309 |
| Splenectomy | 5.79 | 0.71-47.21 | .101 |
| Hydroxyurea | 0.24 | 0.03-2.20 | .208 |
| Iron chelation | 0.40 | 0.10-1.62 | .197 |
| Hypothyroidism | |||
| Age > 35 y | 1.01 | 0.46-2.23 | .984 |
| Splenectomy | 6.04 | 2.03-17.92 | .001* |
| Transfusion | 13.3 | 1.78-100.00 | .012 |
| Hydroxyurea | 0.05 | 0.01-0.45 | .003* |
| Iron chelation | 0.49 | 0.22-1.07 | .073 |
| Osteoporosis | |||
| Age > 35 y | 3.51 | 2.06-5.99 | < .001* |
| Female | 1.97 | 1.19-3.27 | .009* |
| Ferritin ≥ 1000 μg/L | 1.60 | 0.96-2.68 | .072 |
| Splenectomy | 4.73 | 2.72-8.24 | < .001* |
| Transfusion | 3.10 | 1.64-5.85 | < .001* |
| Hydroxyurea | 0.02 | 0.01-0.09 | < .001* |
| Iron chelation | 0.40 | 0.24-0.68 | .001* |
| Hypogonadism | |||
| Age > 35 y | 1.05 | 0.51-2.15 | .900 |
| Female | 2.98 | 1.79-4.96 | < .001* |
| Ferritin ≥ 1000 μg/L | 2.63 | 1.59-4.36 | < .001* |
| Splenectomy | 1.65 | 0.97-2.77 | .056 |
| Transfusion | 16.13 | 4.85-52.63 | < .001* |
| Hydroxyurea | 4.32 | 2.49-7.49 | < .001* |
| Iron chelation | 2.51 | 1.48-4.26 | .001* |
RR indicates adjusted relative risk; CI, confidence interval; and DM, diabetes mellitus.
Statistically significant.
Probability of acquiring disease-related complications at different age intervals.
Probability of acquiring disease-related complications at different age intervals.
Discussion
Our study confirms previously reported complication rates in TI and further highlights the high prevalence of those specific complications that are thought to be more frequent in TI compared with TM: thrombosis, PHT, EMH, leg ulcers, and cholelithiasis.3,10,24
In our group, splenectomized patients had significantly higher rates than nonsplenectomized patients for almost all complications. Few clinical observations have suggested that splenectomy in TI can contribute to an increased susceptibility to thrombosis.24,25 The development of these complications has been ascribed to the presence of high platelet counts and aggregation after splenectomy26,27 and/or to increased number of RBCs with negatively charged membranes that carry thrombogenic potential.28 In splenectomized TI patients, thrombin generation is significantly higher than in control subjects and patients who had not undergone splenectomy.25 A study by Atichartakarn et al also noted that splenectomized thalassemia patients have a high frequency of PHT, mostly attributed to chronic thromboembolic disease.29 Our study confirms these findings and recognizes additional complications that have never been associated with splenectomy. The higher incidence of iron overload-related complications in splenectomized patients suggests that the intact spleen may be a reservoir of excess iron and may have a possible scavenging effect on iron-free fractions, including non–transferrin-bound iron.30 As per expert opinion and this report, the current indications for splenectomy in TI include growth retardation or poor health, leukopenia, thrombocytopenia, increased transfusion demand, or symptomatic splenomegaly.5 However, our data call for a review of splenectomy as a procedure of choice, especially with its potential role in increasing TI-related complications and the inherent risk of infection associated with the procedure, even for persons without hematologic disorders.31
In patients with TM, a remarkable improvement in life expectancy and prevention of morbidity have been achieved in recent decades.32 This may be attributed to several key factors, including improved methods of blood transfusion, better understanding of iron toxicity, and a continuous improvement in iron chelation therapy.32 TI has, however, been largely regarded as a mild to moderate disease with limited complications, and the prevailing approach has been avoidance of early blood transfusions and the concomitant requirement for chelation therapy. Consequently, unlike TM, evaluation of the role of transfusion and iron chelation therapy in the management of TI and prevention of its complications has been limited. In this study, it seems that patients who were placed on transfusion regimens had fewer complications relevant to chronic anemia, ineffective erythropoiesis, and hemolysis (mainly EMH, PHT, and thrombosis) while having a higher rate of iron overload-related endocrinopathy. Unlike TM, in which cardiac siderosis is the predominant cause of morbidity and mortality,33 in TI increased thrombosis and PHT dominate the clinical picture.24,34 These dismal complications observed with increasing frequency in untreated TI patients suggest that earlier intervention may benefit TI patients.35 Although earlier introduction of blood transfusions will increase the rate of iron accumulation, effective methods of iron chelation are now available, and the benefits of transfusion therapy may greatly outweigh the cost and inconvenience of iron chelation therapy. This was also supported in our study in which patients who receive iron chelation therapy had fewer iron overload complications.
Thus, although current recommendations suggest initiating transfusion therapy after complications have manifested, it may be worthwhile considering earlier initiation as a preventive approach that will also help alleviate the increased risk of alloimmunization with delayed initiation of transfusion.36 The initiation of chelation therapy in TI patients depends primarily on the extent of iron overload and rate of endogenous iron accumulation; but, as with other aspects of the management of TI, clear guidelines are not available.5 In light of our recommendation of early initiation of transfusion therapy, iron chelation therapy would follow the same recommendations as with patients with TM.5
Increasing the synthesis of HbF can help alleviate anemia and ineffective erythropoeisis and therefore improve the clinical status of patients with TI.37 Production of HbF is reactivated during recovery from marrow suppression after treatment with cytotoxic drugs; therefore, it is postulated that these agents may alter the pattern of erythropoiesis and increase the expression of γ-chain genes. Several cytotoxic agents with this effect have been identified, including cytosine arabinoside and hydroxyurea.38-40 Our data support the encouraging results of a single report, evaluating 6 years of hydroxyurea therapy in transfusion-dependent patients with TI.12
Few studies outlined the effect of age in TI patients on the risk of iron overload and thrombosis.34,41 Our study further expands on these findings and attributes a high rate of most disease-related complications to age. This brings further attention to our aging TI patient population. It directly calls for earlier intervention to prevent serious long-term sequelae and fortifies the notion that complications substantially increase as thalassemia patients enter adulthood.42
Our study carries the limitation of being retrospective in nature without a clear identification of the onset and chronology of complications with respect to treatment options received. Another limitation of our study is the use of serum ferritin instead of liver R2 magnetic resonance imaging for the assessment of iron overload, as studies have shown that serum ferritin may underestimate iron burden in TI,41 and the use of echocardiography instead of cardiac catheterization for the diagnosis of PHT, which may increase the rate of false-positive findings. However, our patients were mainly screened for PHT after presenting with exertional dyspnea with no evidence of left heart disease. Moreover, echocardiography is still the modality of choice used in many studies on thalassemia and sickle cell anemia for financial/practical reasons and relying on reports of good relationship between Doppler estimates and invasive measurements of pulmonary arterial pressure at baseline and after treatment, despite the variable echocardiographic cutoff values used to label patients with PHT.7,10,34,43
Despite several available treatment options, there are currently no clear guidelines for managing patients with TI. Current practice follows recommendations extracted from expert opinion, small series, or studies that were not necessarily designed to investigate the role of various interventions. Our study confirms some of these recommendations yet challenges others through novel findings from a large cohort of patients. This should hopefully bridge the gap between evidence and practice by calling for prospective clinical trials that evaluate the efficacy, safety, and cost-effectiveness of these therapies. Such studies are expected to evaluate the optimal timing; dose and duration of transfusion, iron chelation, or hyrdoxyurea theapy; and the added advantage of multimodal therapy. Moreover, our study turns the attention toward an increasing complication rate in the elderly TI population and questions the role of splenectomy in this patient population. Until solid evidence-based guidelines are available, a system-centered risk stratification model that individualizes patient treatment should be entertained.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.T.T., K.M.M., and M.D.C. were responsible for conception and design, data analysis and interpretation, and manuscript writing; K.M.M. performed statistical analysis; M.K., A.E.-B., K.B., S.D., and M.-S.E.S. gave administrative support and helped in provision of study material or patients; A.-H.E.-C. and M.R.F. helped in collection and assembly of data; and all authors gave final approval of the manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ali T. Taher, Department of Internal Medicine, American University of Beirut Medical Center, PO Box 11-0236, Riad El Solh 1107 2020, Beirut, Lebanon; e-mail: ataher@aub.edu.lb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal