Abstract
Hydroxyurea (HU) enhances fetal hemoglobin (Hb) production. An increase in total Hb level has been repeatedly reported during HU treatment in patients with sickle cell disease and in several patients with β-thalassemia intermedia. Effects in patients with β-thalassemia major are controversial. We now report a marked elevation of total Hb levels with HU that permitted regular transfusions to be stopped in 7 children with transfusion-dependent β-thalassemia. The median follow-up was 19 ± 3 months (range, 13-21 months). We conclude that HU can eliminate transfusional needs in children with β-thalassemia major, which could be particularly useful in countries such as Algeria, where supplies of blood or chelating agents are limited.
Introduction
Hydroxyurea (HU) promotes fetal hemoglobin (HbF) production via a reactivation of γ-genes as a result of molecular mechanisms that are not yet elucidated. The clinical benefit induced by this compound in patients affected with sickle cell disease has been repeatedly demonstrated.1,2 A significant benefit could also be expected in patients with β-thalassemia, because the imbalance in globin chains could be ameliorated by the newly synthesized γ-chains being able to neutralize the excess α-chains, which could partially correct ineffective erythropoiesis. Clinical and hematologic improvements have been reported in patients with thalassemia intermedia,3-8 but responses in patients with thalassemia major are controversial.5,9 We have followed 7 children with transfusion-dependent β-thalassemia, 6 of them with severe transfusional complications, and have treated them with hydroxyurea in the hope that this drug could reduce transfusional needs.
Study design
Seven children were included, 3 pairs of siblings and 1 single child. Their main clinical and biologic characteristics are given in Table 1. As blood supplies are limited in Algeria, the targeted post-transfusional hemoglobin level is only 70.0 to 90.0 g/L (7-9 g/dL). Six patients had major transfusional complications: transfusion-induced anaphylactic reactions (patient 1); severe chills and fever (patients 2, 6); post-transfusional hemochromatosis (ferritin level, 3500 μg/L [3500 ng/mL]) with cutaneous, hepatic, and gonadic manifestations (patient 3); multiple red cell allo-immunization (patient 4); and lack of venous access (patient 5). Patient 1 also had painful retroperitoneal masses, and scintigraphic and tomodensitometric studies attributed these masses to extramedullary hematopoiesis. The parents of child 1 requested HU treatment for their other affected child (patient 7). The protocol was approved by the Hôpital Franz Fanon internal review board, and all families gave their informed consent.
Main clinical and biologic characteristics of patient population
Patient . | Age, y/sex . | Genotype . | Xmn polymorphism . | Initial Hb, g/dL . | Phenotype . | Age at 1st transfusion, mo . | EC/y . | Splenectomy . |
|---|---|---|---|---|---|---|---|---|
| 1 | 12/M | Codon 6(-A)/codon 39 (C>T) | +/- | 6.7 | Intermediate | 30 | 5 | Total |
| 2 | 12/F | Codon 6(-A)/IVS1-nt1 10G>A | +/- | 6.4 | Intermediate | 48 | 3 | No |
| 3 | 16/M | Homozygous IVS1-nt1 10G>A | -/- | 4.9 | Major | 9 | 15 | Partial |
| 4 | 12/F | Homozygous codon 6(-A) | +/+ | 4.2 | Major | 13 | 15 | No |
| 5 | 8/F | Homozygous IVS1-nt1 10G>A | -/- | 5.9 | Major | 15 | 18 | No |
| 6 | 4/M | Homozygous codon 6(-A) | +/+ | 3.9 | Major | 13 | 9 | No |
| 7 | 6/M | Codon 6(-A)/codon 39(C>T) | +/- | 3.6 | Major | 16 | 13 | Total |
Patient . | Age, y/sex . | Genotype . | Xmn polymorphism . | Initial Hb, g/dL . | Phenotype . | Age at 1st transfusion, mo . | EC/y . | Splenectomy . |
|---|---|---|---|---|---|---|---|---|
| 1 | 12/M | Codon 6(-A)/codon 39 (C>T) | +/- | 6.7 | Intermediate | 30 | 5 | Total |
| 2 | 12/F | Codon 6(-A)/IVS1-nt1 10G>A | +/- | 6.4 | Intermediate | 48 | 3 | No |
| 3 | 16/M | Homozygous IVS1-nt1 10G>A | -/- | 4.9 | Major | 9 | 15 | Partial |
| 4 | 12/F | Homozygous codon 6(-A) | +/+ | 4.2 | Major | 13 | 15 | No |
| 5 | 8/F | Homozygous IVS1-nt1 10G>A | -/- | 5.9 | Major | 15 | 18 | No |
| 6 | 4/M | Homozygous codon 6(-A) | +/+ | 3.9 | Major | 13 | 9 | No |
| 7 | 6/M | Codon 6(-A)/codon 39(C>T) | +/- | 3.6 | Major | 16 | 13 | Total |
EC indicates erythrocyte concentrate—packed red blood cells.
Mean HU dose was 18.35 ± 2.1 mg/kg per day (range, 15-20 mg/kg), given every day. Dosages were maximal at the start of treatment and were raised according to the children's weight increase. HU treatment was begun at a mean of 34 ± 16 days (range, 15-65 days) after the last transfusion. Each month we determined complete blood counts (using the Sysmex 2000 counter [TOA Medical Electronics, Kobe, Japan]) and biochemical parameters, including blood urea nitrogen (BUN), creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase levels. HbF levels were calculated with the alkali denaturation technique (modified Betke method).
Results and discussion
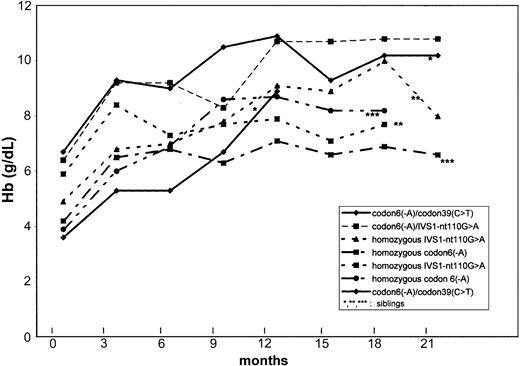
The median follow-up was 19 months (range, 13-21 months) in October 2002. Total Hb increased in all patients, as indicated in Figure 1.
Hb levels under HU. Changes in hemoglobin (Hb) levels with hydroxyurea treatment in 7 transfusion-dependent β-thalassemic children.
Hb levels under HU. Changes in hemoglobin (Hb) levels with hydroxyurea treatment in 7 transfusion-dependent β-thalassemic children.
In all patients, total Hb increased in the first month after beginning HU. Comparing initial and last values, mean Hb levels rose from 65.0 to 105.0 g/L (6.5-10.5 g/dL) in the 2 children with thalassemia intermedia, and from 45.0 ± 9.0 to 79.0 ± 8.0 g/L (4.5 ± 0.9 to 7.9 ± 0.8 g/dL) in the 5 children with thalassemia major. This increase permitted transfusions to be stopped in patients 1, 2, 3, 4, and 6. Patient 5 needed 2 erythrocyte concentrates after Hb fell to 47.0 g/L (4.7 g/dL) during a pulmonary infection. Patient 7 underwent splenectomy for hypersplenism 6 months after the beginning of HU and received 2 units of blood before splenectomy and 1 unit perioperatively. None of these patients have received further transfusions.
Mean corpuscular volume increased from 72 ± 3.5 to 87.4 ± 5.4 fL. Mean HbF increased from 90.9% ± 12.8% to 97.7% ± 2.1%. Mean circulating erythroblasts/100 leukocytes decreased from 149 400 ± 194 000 to 13 400 ± 12 300 (P = .01). All children reported that they felt better and were more active. Median spleen size decreased in children without splenectomy from 6 to 3 cm. In addition, retroperitoneal masses observed in patient 1 regressed.
Clinical and hematologic safety was good. Increasing the HU dose to 25 mg/kg per day in patient 4 induced transient leukopenia (3.8 × 109/L) and thrombopenia (89 × 109/L), which resolved when the dose was decreased to 16.5 mg/kg per day. Two patients noted nausea at the beginning of treatment, which resolved spontaneously.
In conclusion, our series agrees with previous studies reporting clinical and hematologic improvement with HU and regression of extramedullary hematopoietic masses in patients with β-thalassemia intermedia.7,10 HU has already been successfully used in an irregularly transfused patient with β-thalassemia major.11 This report is, to our knowledge, the first one showing sustained discontinuation of transfusions after beginning treatment with HU in 5 children affected with severe β-thalassemia major. It is possible that the effect of HU on γ-globin expression is associated with the type of thalassemic mutation or the Gγ –158C>T polymorphism (Xmn I). It is notable that our patients had similar increases in Hb level whatever their genotypes (β0 or β+ mutations, Xmn I polymorphism). In addition, the increase in total Hb level in our patients is probably not completely explained by the sole increase in HbF from 91% to 98%. A reduction in ineffective erythropoiesis is strongly suggested by the marked decrease in the number of circulating erythroblasts. β-Thalassemia is a very heterogeneous group of diseases related to multiple mutations, and our observation cannot be generalized prematurely to all β-thalassemic syndromes. Since these initial promising results, 7 more children with transfusion-dependent β-thalassemia have been treated with HU. After only some months of follow-up, transfusions have been stopped in 2 patients, spaced out in 2 patients (1 every 2 months instead of 1 every month), and continued in 3 patients. Moreover, the posttreatment Hb level (79.0 ± 8.0 g/L [7.9 ± 0.8 g/dL]) we observed in our children affected with thalassemia major is in fact within the target range defined in Algeria, but it is under the generally recommended post-transfusional Hb level in countries with sufficient blood supplies. However, we think it is important to emphasize that in some patients affected with severe β-thalassemia, HU raises total Hb levels while being well tolerated, at least in short- and-medium-term use, and may represent a useful alternative to erythrocyte transfusions.
Prepublished online as Blood First Edition Paper, April 17, 2003; DOI 10.1182/blood-2003-01-0117.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal