Cardiac complications in 110 patients (mean age, 32.5 ± 11.4 years) with thalassemia intermedia (TI) were studied. Sixty-seven (60.9%) of them had not been transfused or were minimally transfused (group A). The rest had started transfusions after the age of 5 years (mean, 15.1 ± 10.1 years), initially on demand and later more frequently (group B). Overall mean hemoglobin and ferritin levels were 9.1 ± 1.1 g/dL and 1657 ± 1477 ng/mL, respectively. Seventy-six healthy controls were also studied. The investigation included thorough history taking, clinical examination, electrocardiography, chest radiograph, and full resting echocardiography. Of 110 patients, 6 (5.4%) had congestive heart failure (CHF), and 9 (8.1%) had a history of acute pericarditis. Echocardiography showed pericardial thickening, with or without effusion, in 34.5% of the patients. Valvular involvement included leaflet thickening (48.1%), endocardial calcification (20.9%), and left-sided valve regurgitation (aortic, 15.4%; mitral, 47.2%). All patients had normal left ventricular contractility (fractional shortening, 0.43 ± 0.05), and high cardiac output (CO; 9.34 ± 2.28 L/min). Pulmonary hypertension (PHT), defined as Doppler peak systolic tricuspid gradient greater than 30 mm Hg, developed in 65 patients (59.1%). PHT correlated positively with age and CO and did not differ significantly between groups. Cardiac catheterization in the 6 patients with CHF revealed severe PHT, increased pulmonary resistance (PVR), and normal capillary wedge pressure. It was concluded that in patients with TI, the heart is primarily affected by PHT, which is the leading cause of CHF. High CO resulting from chronic tissue hypoxia and increased PVR are the main contributing factors. Doppler tricuspid gradient measurement should be considered, in addition to other factors, when determining the value of transfusion therapy for patients with TI.
Introduction
β-Thalassemia is an inherited hemoglobin disorder caused by impaired synthesis of the β-globin chain and resulting in chronic hemolytic anemia.1 Depending on clinical severity, 2 forms—thalassemia major (TM) and thalassemia intermedia (TI)—are distinguished.1 TM is rapidly fatal unless adequate transfusions, in conjunction with intensive iron chelation therapy, are started early enough.1 In contrast, TI is generally characterized by a mild clinical picture, better prognosis, and better chance for survival, and patients require therapeutic intervention only later in life, if at all.1
Cardiac complications are a main feature of the clinical spectrum of β-thalassemia. They are the leading cause of death2 and have been well documented only in TM. The prominent finding in this condition is left ventricle (LV) dysfunction,3 which is attributed mainly to iron overload and leads gradually to cardiac failure and cardiogenic death.2 3 Although TM and TI share common basic pathophysiological mechanisms, cardiac involvement may be different in the latter because these patients live longer and generally have low hemoglobin levels and lower iron loads.
Pulmonary hypertension and right ventricle failure have been reported in 7 patients with TI.4 The purpose of the present study was to determine the form and severity of cardiac abnormalities in a large group of patients with TI, thereby extending and substantiating systematically the results of that original case report.
Patients, materials, and methods
Study population
In total, 880 patients with β-thalassemia who were treated at 3 major Thalassemia Units in Athens were followed up through our Cardiac Outpatient Clinic. All patients with the diagnosis of TI, established on the basis of conventional clinical and hematologic criteria, were considered for analysis. Accordingly, 123 patients were initially enrolled. We excluded 12 patients because of inadequate echocardiographic quality and one because of an atrial septal defect. The remaining 110 patients, 57 (51.8%) men and 53 (48.2%) women, aged 32.5 ± 11.4 years, were separated in 2 groups. Group A consisted of 67 patients (60.9%; mean age, 34.0 ± 13.0 years) who had never or had rarely undergone transfusion (no more than 50 U blood totally). Group B consisted of 43 patients (39.1%; mean age, 30.7 ± 9.0 years) who started undergoing occasional transfusions at a mean age of 15.1 ± 10.1 years. Transfusions became gradually more frequent thereafter, reaching an average of 32.2 ± 6.4 U for each of the 2 years preceding the study. Mean total number of units per patient in this group was 294 ± 161. In the last 2 years, the combined mean hemoglobin level for both groups was 9.1 ± 1.1 g/dL. Mean serum ferritin concentration during the last 10 years was 1657 ± 1477 ng/mL, with a mean peak level of 2477 ± 2011 ng/mL at an average patient age of 23.4 ± 9.4 years. Iron chelation therapy, with subcutaneous infusion of 40 mg/kg per day deferoxamine, was administrated to 53 patients, including all patients in group B and 10 patients in group A, whose serum ferritin levels exceeded 1000 ng/mL in the course of disease. Iron chelation was discontinued when ferritin levels fell lower than these levels. Splenectomy had been performed in 61 (55.5%) patients (mean age, 20.5 ± 15.2 years) and often resulted in significantly increased platelet counts (mean, 658 × 103/μL ± 222 × 103/μL vs 243 × 103/μL ± 98 × 103/μL in patients who did not undergo splenectomy).
Cardiac investigation was performed as part of the routine follow-up. In patients who underwent transfusion therapy, it was scheduled for the day before transfusion. The standard evaluation consisted of a thorough medical history and physical examination, electrocardiography, chest radiographs, and echocardiography. Patients with congestive heart failure (CHF) also underwent right cardiac catheterization 5 days after cardioactive medication was discontinued.
Seventy-six age- and sex-matched control subjects were randomly selected; they did not smoke and had no evidence of anemia or of liver, respiratory, or cardiovascular disease. Although patients with TM were initially considered as control subjects, they were not chosen because our patients with TM could not be age-matched with patients with TI.
Echocardiography
Complete M-mode, 2-dimensional, and Doppler (pulsed-wave, continuous-wave, and color) echocardiography was performed at rest, using an Acuson 128 Computed Sonography System (Mountain View, CA) with 2.5- to 3.5-MHz transducers. All echo-Doppler studies were carried out by the same cardiologist, and the tracings were interpreted by 2 independent cardiologists who were unaware of the patient data. The echo study addressed pericardial and valvular condition, cardiac dimensions, LV mass (LVMass), LV systolic and diastolic function, and hemodynamics of pulmonary circulation.
Pericardial thickening was considered present if pericardial echoes consisted of at least 2 separate lines and were more than 4 mm thick,5 whereas pericardial effusion was evaluated visually using 2-dimensional echocardiography.6 Valvular leaflet thickening was defined as dense echo reflection (thicker than 4 mm for the mitral and tricuspid valves and 3 mm for the aortic and pulmonary valves), and endocardial calcification was defined as a brighter echo density, with or without acoustic shadowing, at minimal gain settings. Valvular regurgitation was graded semiquantitatively by color Doppler,7,8 while mitral valve prolapse (MVP) was diagnosed using the criteria of Levine et al.9
Cardiac dimensions were measured according to the recommendations of the American Society of Echocardiography (ASE) by M-mode,10 and 2-dimensional echo was used wherever M-mode measurements were considered unreliable.11 LVMass was calculated using the ASE cube formula as corrected by Devereux et al.12 LVMass Index was also calculated by dividing LVMass by body surface area (BSA).
Left ventricle volume and, subsequently, LV ejection fraction, stroke volume (SV), and cardiac output (CO), were estimated by the method of discs according to ASE recommendations and using apical 2- and 4-chamber views.13 Stroke index and cardiac index were calculated as SV/BSA and CO/BSA, respectively. Diastolic LV function was evaluated by Doppler parameters, including early (E) and late (A) diastolic peak flow velocities, E/A ratio, deceleration time of E wave (DT),14 and LV isovolumic relaxation time (IVRT).15
Assessment of pulmonary artery pressure and pulmonary vascular resistance (PVR) was provided by Doppler recording of the systolic pulmonary flow and the measurement of pulmonary acceleration time (PAT) and PAT-to-right ventricular ejection time ratio (PAT/RVET).16 In addition, tricuspid valve continuous-wave Doppler tracing was obtained in patients with tricuspid regurgitation to measure peak systolic right ventricular to right atrial pressure gradient (TG). This is considered the most reliable noninvasive method of estimating pulmonary artery pressure.17 Measurements were obtained at maximal velocity jet; TG was calculated according to the simplified Bernoulli equation.17 Apical 4-chamber, parasternal short-axis, and parasternal long-axis views were used while the subject suspended breathing at the end of a normal expiration, and 3 cardiac cycles were averaged.
Statistical analysis
Statistical analysis was performed using the SPSS 7.5 statistical software package. Continuous variables were expressed as mean ± SD. P < .05 was considered statistically significant. Student t and χ2 tests were used to compare variables between patients and controls or between patient groups. Bivariate correlation and multivariate regression analyses were used to investigate for potential relations between variables.
Results
Congestive heart failure developed in 6 (5.4%) patients aged 41.6 ± 11.6 years. Nine (8.1%) patients had at least one episode of acute pericarditis at a mean age of 25.7 ± 14.4 years. None had a history of rheumatic fever. Thirty-two (29%) patients had hepatomegaly greater than 3 cm below the right costal border. All patients who did not undergo splenectomy had splenomegaly. Cardiac auscultation revealed a systolic murmur over the cardiac base or apex in most (61.8%) patients; midsystolic click and fourth heart sound were found in 7 (6.3%) and 5 (4.5%) patients, respectively. Gallop rhythm was present in 4 (3.6%) patients, all of whom had CHF. All patients were normotensive, with mean systolic and diastolic blood pressure levels of 120 ± 15 and 80 ± 7 mm Hg, respectively.
Electrocardiography
According to more recent criteria,18 left and right ventricular hypertrophy were present in 15 (13.6%) and 8 (7.2%) patients, respectively, and 7 (6.3%) had biventricular hypertrophy. In addition, 7 (6.3%) patients had right QRS axis deviation, 9 (8.1%) had right bundle branch block, and 1 had complete (third-degree) atrioventricular block. Premature atrial contraction (PAC) was observed in 18 (16.3%) patients, and atrial fibrillation was observed in 7 (6.3%) patients aged 45.8 ± 12.3 years.
Chest radiography
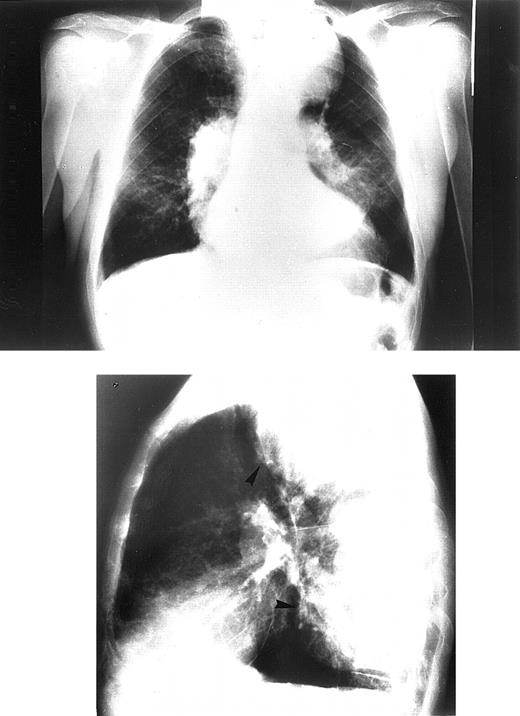
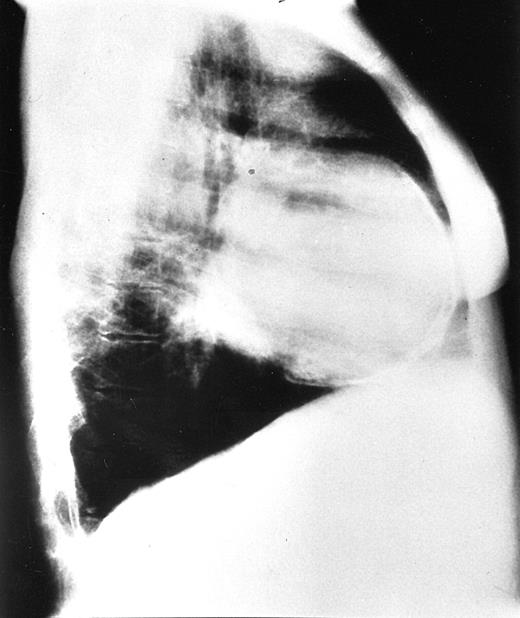
Chest radiographs revealed increased cardiothoracic index levels in 54 (49%) patients. Eighteen (16.3%) had prominent pulmonary arteries. Signs of obvious bone marrow expansion were present in 25 (22.7%) patients and included intrathoracic extramedullary hemopoietic masses in 21 (19%) patients and widening of ribs in 10 (9%) patients (Figure 1). Pericardial calcification was found in a 42-year-old patient with a history of pericarditis but no history of tuberculosis and negative findings on the Mantoux test (Figure 2).
Chest radiograph of the oldest patient with congestive heart failure.
Frontal view (upper panel). Lateral view (lower panel). Patient shows signs of pulmonary hypertension combined with large intrathoracic extramedullary hemopoietic masses.
Chest radiograph of the oldest patient with congestive heart failure.
Frontal view (upper panel). Lateral view (lower panel). Patient shows signs of pulmonary hypertension combined with large intrathoracic extramedullary hemopoietic masses.
Chest radiograph of a 42-year-old patient.
Lateral view shows pericardial calcification and enlargement of ribs.
Chest radiograph of a 42-year-old patient.
Lateral view shows pericardial calcification and enlargement of ribs.
Echocardiography
The occurrence of pericardial and valvular abnormalities was significantly higher in patients than in controls. Pericardial findings consisted of thickening in 38 patients (34.5% vs 9.2% in controls;P < .001), 16 of whom also displayed small or moderate effusion with no acute symptoms. Of the patients with pericardial abnormalities, only 6 had histories of pericarditis. Valvular leaflet thickening was found in 53 patients (48.1% vs 14.4% in controls;P < .001), whereas endocardial calcification of variable extent, located at mitral annulus and leaflets, papillary muscles, and aortic valve, was present in 23 patients (20.9% vs 1.3% in controls;P < .001). Three patients with intense endocardial calcification involving the aortic valve had peak systolic aortic gradients of 23, 25, and 38 mm Hg, respectively, in continuous-wave Doppler measurement; a fourth patient had third-degree atrioventricular block, and a pacemaker was implanted. Regurgitation was encountered at all valvular sites. Left-sided valvular regurgitation, usually of mild or moderate severity, was significantly higher in patients than in controls (aortic valve, 15.4% vs 2.6%; P < .01; mitral valve, 47.2% vs 15.7%; P < .001). Mitral valve prolapse was also more frequent in the patients (5.4%) than in controls (3.9%), but this difference was not statistically significant.
CO and cardiac index (CI) were significantly higher in patients than in controls (CO, 9.34 ± 2.28 L/min vs 6.39 ± 1.44 L/min;P < .001; CI, 5.45 ± 0.33 L/min · m2 vs 3.82 ± 0.80 L/min · m2;P < .001), and so were the indexes of cardiac dimensions and LVMass (P < .001). LV fraction shortening and ejection fraction were normal in all patients, ranging above 0.30 and 0.55, respectively, even in patients with CHF. Regarding the LV diastolic function indices, E and A were significantly higher in patients (P < .001) and were compatible with high output state; E-to-A ratio, DT, and IVRT did not differ statistically between patients and controls. Individual patient analyses revealed that 5 patients without CHF had impaired LV relaxation pattern, expressed as E-to-A ratio less than 1, and prolonged deceleration time.
The presence of pulmonary hypertension (PHT) in the patient group was indicated by Doppler indices of pulmonary artery flow and by peak systolic tricuspid pressure gradient, measured in all patients. PAT and PAT/RVET were significantly lower in patients than in controls (111 ± 22 msec vs 127 ± 27 msec, P < .001, and 0.37 ± 0.06 vs 0.42 ± 0.06, P < .05, respectively). Seventy-one (64.5%) patients had PAT values lower than 120 msec, indicating increased pulmonary artery pressure.16Accordingly, total PVR was also significantly higher in patients with TI (451 ± 294 dyn/sec · cm−5 vs 245 ± 93 dyn/sec · cm−5; P < .001). Mean TG in the patient group was 33.15 ± 14.06 mm Hg. Reported TG in healthy persons with tricuspid regurgitation is 19.3 ± 4.0 mm Hg.19 TG values higher than 30 mm Hg, characterized definitively as PHT,19 were found in 65 (59.1%) patients, and TG values higher than 50 mm Hg were found in 8 (7.3%) patients (Figure 3).
Tricuspid gradient in patient group.
(left) Correlation of tricuspid gradient with age in patients with TI. Solid triangles represent patients with CHF. (right) Distribution of peak systolic tricuspid gradient in patient group.
Tricuspid gradient in patient group.
(left) Correlation of tricuspid gradient with age in patients with TI. Solid triangles represent patients with CHF. (right) Distribution of peak systolic tricuspid gradient in patient group.
In multivariate regression analysis for the entire sample, TG correlated positively with age (P < .001; Figure 3) and cardiac output (P < .001). Total PVR also correlated with age (P < .001). Although TG was significantly higher in patients who underwent splenectomy (P < .05), this relationship became weaker (P > .05) in multivariate analysis because of the confounding effect of age. TG and total PVR failed to show any correlation with left atrial and LV dimensions, hemoglobin level, transfusion therapy, total number of blood units received, serum ferritin level, peak ferritin level, or history of smoking.
Patients who had not received transfusions or who had received minimal transfusions (group A) had significantly lower hemoglobin concentrations than those who had received transfusions (group B) (8.8 ± 1.2 g/dL vs 9.3 ± 1.0 g/dL, P < .05, during the 2-year period preceding the study). Accordingly, CO levels were significantly higher in group A (10.01 ± 2.31 L/min vs 8.61 ± 2.02 L/min; P = .001). Patients in group A had higher pulmonary artery pressure levels (TG, 33.63 ± 15.78 mm Hg vs 32.59 ± 11.89 mm Hg), and they were older (34.0 ± 13.0 years vs 30.7 ± 9.0 years), but neither of these differences was statistically significant (P > .05). Serum ferritin concentrations were significantly higher in group B (2088 ± 1386 ng/mL vs 1144 ± 1431 ng/mL in group A; P < .01). Mean peak ferritin levels were 1526 ± 578 ng/mL in group A and 3900 ± 1662 ng/mL in group B (P < .001). Mean patient ages were 27.1 ± 10.4 and 20.3 ± 7.3 years, respectively; (P < .05).
Cardiac catheterization
Right cardiac catheterization (Table1) was performed in the 6 patients with CHF; none of them had been previously studied. The presence of PHT and high output state was confirmed. In addition, PVR was increased in all 6 patients, whereas capillary wedge pressure proved normal. Arterial blood gas determinations revealed variable degrees of hypoxemia, but there was no right ventricular oxygen step-up.
Right cardiac catheterization data in 6 patients with congestive heart failure
| . | Patient . | |||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | |
| Age (y) | 34 | 35 | 37 | 39 | 40 | 65 |
| Age at transfusion initiation (y) | 14 | 20 | — | 18 | 10 | — |
| Total blood units | 210 | 434 | — | 25 | 42 | — |
| Serum ferritin (ng/dL) | 1540 | 1654 | 1246 | 912 | 944 | 752 |
| Peak serum ferritin (ng/dL) | 3500 | 3872 | 2012 | 1432 | 1756 | 1233 |
| Age at peak serum ferritin (y) | 18 | 22 | 30 | 26 | 29 | 27 |
| RAP (mm Hg) | 12 | 10 | 15 | 12 | 12 | 17 |
| RVP (mm Hg) S/D | 120/16 | 70/13 | 115/19 | 85/15 | 83/14 | 112/20 |
| PAP (mm Hg) S/D/M | 120/45/65 | 70/31/44 | 115/54/69 | 85/37/48 | 83/29/39 | 112/48/63 |
| PCWP (mm Hg) M | 10 | 8 | Unsuccessful | 12 | 9 | 9 |
| CO (L/min) | 7.2 | 9.1 | 10.5 | 9.5 | 9.4 | 8.2 |
| CI (L/min · m2) | 4.35 | 5.98 | 6.72 | 5.24 | 6.06 | 5.06 |
| PVR (dyn/sec · cm−5) | 611 | 307 | — | 252 | 238 | 538 |
| TPR (dyn/sec · cm−5) | 720 | 386 | 525 | 353 | 306 | 614 |
| pH | 7.36 | 7.43 | 7.39 | 7.41 | 7.41 | 7.37 |
| PO2 | 58.3 | 66 | 64.2 | 70 | 69 | 59.2 |
| PCO2 | 41.7 | 37.1 | 39.3 | 38.1 | 38.3 | 41.3 |
| HCO3 | 23.6 | 23.4 | 24.1 | 22.9 | 23.3 | 23.1 |
| . | Patient . | |||||
|---|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | |
| Age (y) | 34 | 35 | 37 | 39 | 40 | 65 |
| Age at transfusion initiation (y) | 14 | 20 | — | 18 | 10 | — |
| Total blood units | 210 | 434 | — | 25 | 42 | — |
| Serum ferritin (ng/dL) | 1540 | 1654 | 1246 | 912 | 944 | 752 |
| Peak serum ferritin (ng/dL) | 3500 | 3872 | 2012 | 1432 | 1756 | 1233 |
| Age at peak serum ferritin (y) | 18 | 22 | 30 | 26 | 29 | 27 |
| RAP (mm Hg) | 12 | 10 | 15 | 12 | 12 | 17 |
| RVP (mm Hg) S/D | 120/16 | 70/13 | 115/19 | 85/15 | 83/14 | 112/20 |
| PAP (mm Hg) S/D/M | 120/45/65 | 70/31/44 | 115/54/69 | 85/37/48 | 83/29/39 | 112/48/63 |
| PCWP (mm Hg) M | 10 | 8 | Unsuccessful | 12 | 9 | 9 |
| CO (L/min) | 7.2 | 9.1 | 10.5 | 9.5 | 9.4 | 8.2 |
| CI (L/min · m2) | 4.35 | 5.98 | 6.72 | 5.24 | 6.06 | 5.06 |
| PVR (dyn/sec · cm−5) | 611 | 307 | — | 252 | 238 | 538 |
| TPR (dyn/sec · cm−5) | 720 | 386 | 525 | 353 | 306 | 614 |
| pH | 7.36 | 7.43 | 7.39 | 7.41 | 7.41 | 7.37 |
| PO2 | 58.3 | 66 | 64.2 | 70 | 69 | 59.2 |
| PCO2 | 41.7 | 37.1 | 39.3 | 38.1 | 38.3 | 41.3 |
| HCO3 | 23.6 | 23.4 | 24.1 | 22.9 | 23.3 | 23.1 |
RAP indicates right atrial pressure; RVP, right ventricular pressure; S, systolic; D, diastolic; PAP, pulmonary artery pressure; M, mean; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; TPR, total pulmonary resistance.
Discussion
Thalassemia intermedia is a heterogeneous disease, in terms of both clinical manifestations and underlying molecular defects. Several different genotypes—homozygous, heterozygous, and compound heterozygous—have been considered responsible.20 In contrast to patients with TM, patients with TI have less severe anemia and do not require transfusions during at least the first few years of life. Furthermore, even when transfusions begin, they are usually not very frequent. Therefore, the clinical picture of TI is dominated by the multiple long-term effects of chronic anemia and tissue hypoxia and their compensatory reactions, including enhanced erythropoiesis and increased iron absorption. The former is expressed as bone marrow expansion and extramedullary hemopoiesis, leading in turn to bone deformities and to spleen and liver enlargement.
Little is known about cardiac involvement in TI. Our sample of 110 patients, none of whom had a transfusion before the age of 5 or was subjected to a hypertransfusion regimen, showed the above characteristic findings of chronic anemia. Radiographic signs of increased erythropoiesis were frequent and splenomegaly was universal, necessitating splenectomy in most. Cardiac manifestations included rhythm disorders, pericardial and valvular abnormalities, and cardiomegaly. The most prominent findings, however, reflected in electrocardiogram (ECG), radiographs, and echo abnormalities, were PHT and high CO. Six patients with clinical signs of CHF also had as a cause PHT that was confirmed by catheterization.
Pericardial and valvular disorders
Pericarditis, a well-known cardiac complication of TM,21 was also present in 8.1% of our patients with TI, occurring at a mean patient age of 25.7 ± 14.4 years. Chronic evolutionary pericardial changes were furthermore detected by echocardiography, even in patients without histories of pericarditis. These changes included small-to- moderate pericardial effusion and pericardial thickening. However, the limitations of echocardiography to evaluate this particular disorder must be kept in mind.22Pericardial calcification, not yet reported in thalassemia, was also noted, probably attributed to the longer lifespans of TI patients. The pathogenesis of pericarditis in these patients was unclear; a likely cause was increased susceptibility to viral infection given the anemia, iron overload, and splenectomy levels in this group. Hemodynamic effects of pericardial involvement in β-thalassemia could be confounded with those of myocardial restriction because of iron overload, but this is not as heavy in TI as it is in TM.
Valvular lesions are also hard to characterize and to grade with precision by means of echocardiography. Leaflet thickening and endocardial calcification, followed often by valvular regurgitation and occasionally by moderate aortic stenosis, were nevertheless pronounced in our sample. These modifications were probably caused by the volume overload and the resultant cardiac dilatation. All of them, on the other hand, have been related in the literature to pseudoxanthoma elasticum (PXE).23 A diffuse elastic tissue disorder that resembles PXE has been described in sickling syndromes and β-thalassemia, particularly in TI patients older than 30.24-27 PXE has furthermore been related to MVP,28 a high occurrence of which has also been reported in β-thalassemia29 and sickle cell disease,30 though it is questioned in the latter.31 We did not find a high incidence of MVP in our patients with TI (5.4%); we used, however, the most recent diagnostic criteria for MVP.9 These criteria are more stringent than those used in previous studies, in which a prevalence ranging widely between 5% and 35% are reported in healthy controls.32
Pulmonary hypertension
Pulmonary hypertension, reported previously in a small sample of 7 patients with TI,4 was the main cardiac finding in the current systematic study. PHT develops as patients grow older, leads to right ventricular deterioration, and is the main cause of CHF. Present in groups A and B in the absence of regular transfusions, PHT was probably related primarily to the consequences of TI rather than to transfusion-associated events such as iron overload. Tissue hypoxia, inevitable in patients who undergo delayed or infrequent blood transfusions, seems to play the central role in the development of PHT in TI. Hypoxia-induced compensatory reactions, which accumulate gradually with age, finally lead to high CO and increased PVR, both characteristic of the patients described here.
Cardiac output
The degree of pulmonary hypertension, expressed by increased TG, correlated strongly with CO levels. The high output state in TI results not only from chronic anemia but also from the usual presence of hemoglobin F, which impairs oxygen delivery and thereby enhances tissue hypoxia. This, in turn, increases intramedullary and extramedullary erythropoiesis, leading to shunt development that augments gradually with age and raises CO substantially.33 Thus, for a given level of pulmonary vascular resistance, any CO elevation aggravates PHT.
Pulmonary vascular resistance
The increased PVR found in TI has a multifactorial origin. Besides the mechanical damage of pulmonary vascular endothelium caused by the high CO, increased PVR may also result from lung injury caused by recurrent respiratory tract infections, chest wall deformities, and extramedullary intrapulmonary hemopoietic masses and by age-related diffuse elastic tissue disorders found in TI.24 In this study, TG and PVR failed to show any correlation with serum ferritin concentration or transfusion therapy. This negative result, however, is not conclusive. Serum ferritin levels, despite the differences between groups, stayed universally low, both because transfusions had been delayed and were not as frequent and because chelation therapy was started on time, as soon as ferritin levels started rising. Nevertheless, with advancing age, even moderate levels of iron load may play a role, resulting in interstitial pulmonary fibrosis34 and increased PVR.
Particularly in patients with TI, acquired defects of erythrocyte membrane phospholipids have been noticed,35 leading to hypercoagulability that improved after the initiation of hypertransfusions.36 This hypercoagulable state is clinically manifested by a wide spectrum of thromboembolic events.37 On autopsy, extensive thromboembolic lesions were found in the pulmonary arterioles of patients with thalassemia who underwent splenectomy,38 which emphasizes the consequences of splenectomy on pulmonary vessels. Pulmonary involvement and respiratory dysfunction with subsequent hypoxemia—especially after splenectomy—has been documented in thalassemia and positively correlated with age,39 40 as was PHT in our study group. The role of splenectomy for PHT development in our study group was obscured by the confounding effect of age.
Left ventricular function
It is important to stress that LV function, as estimated by echo-Doppler findings and catheterization data, was normal in our TI sample. This is in contrast to findings in TM, in which LV dysfunction is the main cardiac finding,3 and to findings of reports attributing PHT in hyperkinetic states to LV inability to cope with volume overload.41 Ejection fraction was normal in all patients. There was no pseudonormalization because no correlation was found between Doppler indices of pulmonary artery pressure and left cardiac size, whereas the catheterization measurement showed a normal capillary wedge pressure in patients with CHF. Of course, under certain conditions such as physical exercise, febrile status, or significant anemia aggravation, transient LV dysfunction may be followed by left atrial pressure elevation.
Therapeutic considerations
In TM, timely and intensive transfusion–iron chelation therapy aims at pretransfusional hemoglobin concentrations of 10 g/dL or more, thereby minimizing hypoxia. This therapeutic strategy, however, has been routinely adopted only in recent years and is not always followed even today. Thus, many patients with thalassemia, especially older ones, have been exposed to the multiple effects of chronic anemia, which, consistent with our analysis, may explain the reported development of PHT in some patients with TM.42
Therapeutic strategies in TI, on the other hand, are still a matter of considerable controversy. Several different criteria concerning severe potential complications, are used to decide the initiation of transfusion-chelation therapy.1 We believe that Doppler-determined pulmonary arterial pressure might be added to this list of criteria. In patients with severe PHT, in addition to the standard cardioactive medications, therapy should be aimed at keeping hemoglobin concentrations close to normal. Besides preventing hypoxia-related complications, transfusions also improve coagulation defects.36 In patients with thrombocytosis and a large number of circulating erythroblasts after splenectomy, the administration of hydroxyurea might be helpful. Hydroxyurea has been used in TI to enhance hemoglobin F synthesis,43 whereas an additional benefit in patients with PHT would be the restriction of thrombocytosis and the reduction of circulating myeloid elements. The use of antiplatelet agents has also been proposed for patients who have thalassemia with thrombocytosis and hypoxemia, but it could be dangerous for patients with PXE-like lesions, which predispose to hemorrhagic disorders. In fact, intracranial hemorrhages have been reported in patients with thalassemia who have such lesions.44 Finally, patients with TI after intensive hyper–transfusion-chelation therapy from an early age run a higher risk for cardiomyopathy and left-sided cardiac dysfunction and failure.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Athanasios Aessopos, First Department of Internal Medicine, University of Athens School of Medicine, Laiko General Hospital, 17 Aghiou Thoma St, Athens 115 27, Greece.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal