Abstract
The role of reduced-intensity conditioning (RIC) regimens in pediatric cancer treatment is unclear. To define the efficacy of a busulfan/fludarabine/antithymocyte globulin RIC regimen in pediatric patients ineligible for myeloablative transplantation, we completed a trial at 23 institutions in the Pediatric Blood and Marrow Transplant Consortium. Forty-seven patients with hematologic malignancies were enrolled. Sustained engraftment occurred in 98%, 89%, and 90%, and full donor chimerism was achieved in 88%, 76%, and 78% of evaluable related bone marrow/peripheral blood stem cells (BM/PBSCs), unrelated BM/PBSCs, and unrelated cord blood recipients. With a median follow-up of 24 months (range, 11-53 months), 2-year event-free survival, overall survival (OS), transplantation-related mortality, and relapse were 40%, 45%, 11%, and 43%, respectively. Univariate analysis revealed an inferior outcome when patients had undergone previous total body irradiation (TBI)–containing myeloablative transplantation (2-year OS, 23% vs 63% vs 52%, previous TBI transplantation vs no TBI transplantation vs no transplantation, P = .02) and when patients not previously treated with TBI had detectable disease at the time of the RIC procedure (2-year OS, 0% vs 63%, detectable vs nondetectable disease, P = .01). Favorable outcomes can be achieved with RIC approaches in pediatric patients in remission who are ineligible for myeloablative transplantation. This study was registered at www.clinicaltrials.gov as #NCT00795132.
Introduction
Over the past decade, reduced-intensity conditioning (RIC) regimens have become a well-established approach in adult patients, offering curative allogeneic hematopoietic stem cell therapy to older persons and patients with comorbidities, rendering them otherwise ineligible for myeloablative procedures.1-3 Because pediatric patients generally tolerate more intensive transplantation approaches, myeloablative regimens have continued to be the preferred approach in all but the highest-risk persons. In addition, although most RIC regimens use peripheral blood stem cells (PBSCs), pediatric centers have preferred umbilical cord blood (CB) and bone marrow (BM) to PBSCs because of the lack of demonstration of a survival advantage with PBSCs in pediatric recipients4 and a hesitancy to collect PBSCs from minor donors.5,6 Data regarding the safety and efficacy of RIC approaches to treat hematologic malignancies in pediatric patients are limited to single institution studies, and the role of this approach in pediatric cancer has yet to be defined.7-10
With these issues in mind, Pediatric Blood and Marrow Transplantation Consortium (PBMTC) investigators developed a modified busulfan/fludarabine/antithymocyte globulin (ATG) approach that allowed the use of all available stem cell sources (BM, PBSCs, and CB) from related or unrelated donors. Strict eligibility criteria were established that defined subgroups at approximately 50% or greater risk for transplantation-related mortality (TRM) and/or with a history of previous myeloablative transplantation. In addition to previous transplantation, inclusion criteria included patients with significant organ dysfunction, active fungal infection, or those receiving unrelated donor transplantation in more than or equal to CR3. The study, conducted at 23 PBMTC centers in the United States, Canada, and Australia, showed that, in a multi-institutional setting, high rates of engraftment, low TRM, and encouraging rates of survival can be achieved despite the very-high-risk population entering the trial.
Methods
Patients
Between November 2003 and September 2007, a total of 47 pediatric patients at centers in the United States, Canada, and Australia were enrolled in the PBMTC cooperative trial ONC0313. The trial was approved by the local institutional review board or ethics committee at each institution and monitored centrally by the PBMTC Data Safety Monitoring Committee. Informed consent was obtained from the guardians and assent or consent from patients, if applicable, in accordance with the Declaration of Helsinki.
Patients were required to be 21 years of age or younger with hematologic malignancies treatable with allogeneic hematopoietic cell transplantation (acute and chronic leukemias, myelodysplasia [MDS], or lymphomas). Only patients at significant risk for TRM with standard myeloablative approaches were enrolled. Patients were defined as being at significant risk by (1) the presence of organ system dysfunction or severe systemic infections known to increase the risk of TRM with standard myeloablative transplantation regimens, (2) a history of previous myeloablative allogeneic or autologous transplantation, (3) undergoing unrelated donor transplantation in a third or higher complete remission (CR), or (4) a combination of toxicities that put the child at high risk (> 50%) of TRM with myeloablative transplantation.
Definitions of qualifying organ system dysfunction were as follows: (1) pulmonary: carbon monoxide lung diffusion capacity (DLCO), forced expiratory volume in 1 second (FEV1), or forced vital capacity (FVC) less than 60% but not less than 30% predicted. Patients too young for pulmonary function tests with suspected pulmonary toxicity were assessed by a consulting pulmonologist. If the pulmonologist judged the child to have moderate to severe pulmonary disease, they qualified for inclusion. (2) renal: creatinine clearance less than 60 but not less than 30 mL/m per 1.73 m2 or requiring dialysis; (3) hepatic: transaminases more than 4 times normal but not more than 10 times normal or total bilirubin more than 2.0 mg/dL but not more than 3.0 mg/dL or evidence of synthetic dysfunction with an international normalized ratio more than 2.0; and (4) cardiac: ejection fraction less than 50% but not less than 30%.
Patents with severe systemic fungal, bacterial, or other opportunistic infections (eg, atypical mycobacterium) that responded after a minimum of 2 weeks of therapy, but were persistent at the time of trial entry (eg, multiple pulmonary nodules that were shrinking but still visible), were eligible to enroll in the study. Progressive infections despite therapy were not allowed, and viral infections did not qualify patients for the study.
Previous myeloablative transplantation as an entry criterion was derived from reports of excessive toxicity with second myeloablative allogeneic regimens11-13 (TRM approaching or exceeding 50%). The choice of allowing eligibility of patients receiving unrelated donor (URD) transplantation in third or higher remission was derived from published reports describing 50% to 60% TRM in children undergoing URD transplantation for acute lymphoblastic leukemia (ALL) in CR3.14,15 Patients entering by criterion number 4, a combination of toxicities leading to an expected risk of TRM more than 50%, required consultation between the local principal investigator and the study chair. Only patients with recognized, significant toxicities reported to increase TRM not included in criteria 1 to 3 (delineated earlier in “Patients”) were allowed enrollment. The small number of patients entering by this criterion were children with Down syndrome plus additional toxicities or patients who had very significant toxicity before transplantation and had recovered with borderline function in multiple organs.
Patients with ALL were required to be in morphologic remission (< 5% blasts), whereas patients with acute myelogenous leukemia (AML) could have M1 or M2 marrows. Patients with juvenile myelomonocytic leukemia (JMML) and MDS were required to have less than 5% blasts, and those with chronic myelogenous leukemia had to be in first chronic phase, accelerated phase, or subsequent chronic phase with less than 5% blasts. Patients with non-Hodgkin lymphoma or Hodgkin lymphoma were required to have responsive disease with no persistent masses more than 5 cm.
Treatment protocol
Related and unrelated BM and PBSC donors were allowed if they were fully matched or had no more that a single antigen mismatch at human leukocyte antigen (HLA) A or B. (DRB1 antigen or allele mismatches were not allowed.) CB units had to be at least a 4 of 6 match at HLA A, B, and DRB1 with high resolution typing of the DRB1 allele. Minimum prethaw CB cell dose was 3 × 107 total nucleated cells per kilogram of recipient body weight. Multiple CB infusions were not allowed.
The preparative regimen consisted of a single “targeting” dose of intravenous busulfan (0.8 mg/kg) given on day −7 for related recipients and −10 URD and cord recipients (CB). This was followed by 7 more busulfan doses given every 6 hours starting on days −3 and −2 (days −6 and −5 URD, CB). The interval between the targeting and subsequent busulfan doses gave time for calculation of busulfan pharmacokinetics, and doses 2 to 8 of busulfan were adjusted to obtain a target area under the curve of 900 to 1100 μM/min. Patients also received fludarabine 30 mg/m2 on days −7 through −2 (days −10 through −5 URD, CB) for a total dose of 180 mg/m2. Related donor BM and PBSC recipients received thymoglobulin (rabbit ATG) at a dose of 2.5 mg/kg as a single dose on day −1, whereas unrelated BM, PBSC, and CB recipients received a total of 4 doses of thymoglobulin (rabbit ATG) at 2.5 mg/kg daily on days −4 through −1 for a total dose of 10 mg/kg.
Graft-versus-host disease (GVHD) prophylaxis consisted of cyclosporine starting on day −3 at a dose of 2.5 mg/kg intravenously every 12 hours or 3 mg/kg per oral dose every 12 hours with a suggested target trough level of 250 to 350 ng/mL. Mycophenylate mofetil was started on day 0 approximately 4 to 6 hours after stem cell infusion at a dose of 15 mg/kg intravenously or orally twice a day. In the absence of GVHD, mycophenylate mofetil was stopped at day 30 in recipients of matched sibling grafts and umbilical CB but was tapered off in unrelated and mismatched BM/PBSC recipients between days 40 and 96. Cyclosporine was tapered in matched sibling graft recipients starting at day 42 over 2 months, whereas cyclosporine tapers for mismatched and URDs, including unrelated CB recipients, occurred between days 100 and 180.
Supportive care measures, such as use of growth factors or infection prophylaxis, were according to institutional practice. Guidelines for donor lymphocyte infusion (DLI) for patients with persistent or progressive disease were included in the protocol but not mandated.
Statistical methods
Neutrophil engraftment was defined as achievement of an absolute neutrophil count of more than or equal to 500 neutrophils/mm3 sustained for 3 consecutive laboratory measurements on different days. Platelet engraftment was defined as achievement of a platelet count recovery of more than or equal to 20 000 platelets/mm3 sustained for 3 consecutive laboratory measurements on different days with no platelet transfusions in the previous 7 days. A severity grade for acute GVHD was calculated according to the reported stages of skin, liver, and intestinal involvement using the Glucksberg grading system.16 Full donor chimerism was defined as more than 95% donor measured by variable number tandem repeats (VNTR) or fluorescence in situ hybridization (FISH) from whole blood and sorted T-cell samples of BM or peripheral blood. Treatment-related mortality was defined as death in continuous complete remission. Death from any cause was considered an event for overall survival (OS). Events for event-free survival (EFS) included rejection (donor chimerism < 5%), relapse, or death in remission. Very early relapse occurred in 2 patients and death occurred in 1 patient before engraftment on days 18, 18, and 23. These patients were not included in the engraftment analysis.
Disease status at transplantation for acute leukemias was defined as CR for blast counts less than 5% by morphology and partial remission (PR) for AML patients with M2 marrows. Both JMML patients received chemotherapy before transplantation and were in CR based on current Center for International Blood and Marrow Transplant Research pretransplantation reporting criteria (CR indicates normalization of WBC and organomegaly, Center for International Blood and Marrow Transplant Research Form 2015 [JMML], Version 1.0 (4-4), July 2007). Hodgkin and non-Hodgkin lymphoma patients were judged to be in CR when no visible residual disease was present and PR if tumors decreased in size more than or equal to 50%. MDS patients included one patient with refractory anemia by French-American-British (FAB) definition and 6 others with secondary MDS. The chronic myelogenous leukemia patient was in a chronic phase after relapsing after a first allogeneic transplantation. A subset of patients (n = 26) had more detailed response data available, allowing a closer look for signs of persistent disease at the time of transplantation. Detectable disease was defined as the presence of any measurable disease by local flow cytometry (a clone consistent with earlier disease required), FISH positivity for known tumor markers, positive cytogenetics, or positive gallium or positron emission tomography (PET) scans.
Univariate probabilities of EFS and OS were calculated using the Kaplan-Meier estimator; the log-rank test was used for univariate comparisons of survival.17 Probabilities of chronic GVHD, relapse, and TRM were calculated using the cumulative incidence function estimator with a subsequent transplantation as a censoring event.18,19 For chronic GVHD, death without an event was a competing risk. For TRM, relapse was the competing risk; for relapse, TRM was the competing risk. Cox proportional hazards regression and associated Wald tests were used for univariate and multivariate estimates of relative risk. SPSS version 14.0 statistical software was used for the Kaplan-Meier analysis, and R version 2.8.0 (R Foundation for Statistical Computing) was used for the competing risks analysis of relapse, TRM, and chronic GVHD as well as the Cox proportional hazards analysis.
Results
The primary objective of the study was to assess engraftment with a reduced intensity approach in very-high-risk pediatric patients receiving a variety of stem cell sources. The large majority of patients enrolled on the trial had ALL or AML/MDS, with a small number enrolled with other myeloid leukemias and lymphoma (Table 1). The most common qualifying toxicity was a history of a previous myeloablative transplantation (60% of patients), with 2 patients who had received 2 previous myeloablative transplantations: one patient had autologous followed by allogeneic transplantation for secondary MDS, and the second patient had 2 allogeneic transplantations (one non–total body irradiation [TBI] followed by a TBI regimen) for JMML. Seventy-seven percent of the previous myeloablative transplantations were allogeneic. Thirteen patients had organ toxicity or invasive fungal infections. Six patients qualified based on judgment by the local principal investigator and the study chair of excessive risk to the patient using myeloablative approaches. This group included 4 patients with Down syndrome (3 receiving URD grafts), all of whom had experienced significant toxicity with pretransplantation chemotherapy (one with recent renal failure requiring dialysis). The other 2 patients included a Ewing sarcoma patient with secondary AML who experienced severe pulmonary toxicity with induction therapy and a patient with ALL who had profound neurotoxicity and poor functional status. Only 2 patients qualified solely because they received an URD graft and were CR3+ (18 additional patients with other qualifying toxicities received URD grafts and were CR3+). Besides the 18 CR3+ patients with other qualifying toxicities, 3 patients had 2 and one had 3 qualifying toxicities (Table 1).
Patient and transplantation characteristics
| Characteristic . | Value . |
|---|---|
| No. enrolled | 47 |
| Median age, y (range) | 11 (2-20) |
| Sex, male/female | 25/22 |
| Diagnoses, no. of recipients | |
| ALL | |
| CR2 | 4 |
| CR3 | 12 |
| Secondary | 1 |
| AML | |
| CR2 | 7 |
| CR3 | 3 |
| PR2+ | 2 |
| Secondary | 3 |
| MDS | |
| RA | 1 |
| Secondary | 6* |
| JMML | |
| CR2 | 1 |
| CR3 | 1 |
| MLL | |
| CR3 | 1 |
| CML | |
| CP relapse after a myeloablative allogeneic transplantation | 1 |
| Hodgkin lymphoma | |
| CR3 | 3 |
| PR3 | 1* |
| Non-Hodgkin lymphoma | |
| PR3 | 1 |
| Stem cell source (HLA matching) | |
| Related donor bone marrow | 8 (2 patients, 7/8) |
| Related donor peripheral blood stem cells | 8 (all patients, 8/8) |
| Unrelated donor bone marrow | 10 (1 patient, 7/8; 1 patient, 6/8) |
| Unrelated donor peripheral blood stem cells | 9 (2 patients, 7/8; 1 patient, 6/8) |
| Unrelated donor cord blood | 12 (6 patients, 5/6; 6 patients, 4/6) |
| Qualifying toxicities | |
| Previous myeloablative allogeneic BMT | |
| TBI regimen | 16 |
| Non-TBI regimen | 7 |
| Previous myeloablative autologous BMT | |
| Non-TBI regimen | 7 |
| Significant organ toxicity | |
| Cardiac | 4 |
| Pulmonary | 2 |
| Renal | 3 |
| Liver | 1 |
| Infection | |
| Invasive fungal | 3 |
| Recipient CR3+ receiving unrelated donor BMT | |
| Primary qualifying toxicity | 2 |
| CR3+ unrelated donor with other qualifying toxicity | 18 |
| Other factors placing recipient at high risk of TRM | |
| Down syndrome | 4 |
| Combination of toxicities | 2 |
| Characteristic . | Value . |
|---|---|
| No. enrolled | 47 |
| Median age, y (range) | 11 (2-20) |
| Sex, male/female | 25/22 |
| Diagnoses, no. of recipients | |
| ALL | |
| CR2 | 4 |
| CR3 | 12 |
| Secondary | 1 |
| AML | |
| CR2 | 7 |
| CR3 | 3 |
| PR2+ | 2 |
| Secondary | 3 |
| MDS | |
| RA | 1 |
| Secondary | 6* |
| JMML | |
| CR2 | 1 |
| CR3 | 1 |
| MLL | |
| CR3 | 1 |
| CML | |
| CP relapse after a myeloablative allogeneic transplantation | 1 |
| Hodgkin lymphoma | |
| CR3 | 3 |
| PR3 | 1* |
| Non-Hodgkin lymphoma | |
| PR3 | 1 |
| Stem cell source (HLA matching) | |
| Related donor bone marrow | 8 (2 patients, 7/8) |
| Related donor peripheral blood stem cells | 8 (all patients, 8/8) |
| Unrelated donor bone marrow | 10 (1 patient, 7/8; 1 patient, 6/8) |
| Unrelated donor peripheral blood stem cells | 9 (2 patients, 7/8; 1 patient, 6/8) |
| Unrelated donor cord blood | 12 (6 patients, 5/6; 6 patients, 4/6) |
| Qualifying toxicities | |
| Previous myeloablative allogeneic BMT | |
| TBI regimen | 16 |
| Non-TBI regimen | 7 |
| Previous myeloablative autologous BMT | |
| Non-TBI regimen | 7 |
| Significant organ toxicity | |
| Cardiac | 4 |
| Pulmonary | 2 |
| Renal | 3 |
| Liver | 1 |
| Infection | |
| Invasive fungal | 3 |
| Recipient CR3+ receiving unrelated donor BMT | |
| Primary qualifying toxicity | 2 |
| CR3+ unrelated donor with other qualifying toxicity | 18 |
| Other factors placing recipient at high risk of TRM | |
| Down syndrome | 4 |
| Combination of toxicities | 2 |
One patient with Hodgkin lymphoma in PR3 also had secondary MDS.
Engraftment/chimerism
Patients were grouped into related donor BM or PBSC, unrelated donor BM or PBSC, and unrelated CB cohorts to assess for engraftment endpoints (Table 2). Median time to neutrophil engraftment was similar for all stem cell sources, varying from 19 to 24 days. Platelet engraftment was slower in the CB cohort, as expected (median of 43 days to engraftment > 20 000/μL), whereas the median in the related donor cohort was zero, as the majority of related donor recipients did not require platelet transfusions (platelets remained > 20 000/μL). Four patients rejected their grafts; one was a mismatched related BM recipient, 2 received URD marrow (one 10/10 HLA match, a second 8/10, and the fourth a 4/6 cord match). Three patients experienced early relapse or death before engraftment. Excluding these 3 patients, neutrophil engraftment occurred in 94%, 89%, and 90% in the related BM/PBSC cohort, the URD BM/PBSC cohort, and the CB cohort, respectively. Of note, no rejections occurred in recipients of PBSC grafts.
Neutrophil/platelet engraftment and chimerism
| . | Related donor . | Unrelated donor . | CB . | ||
|---|---|---|---|---|---|
| BM . | PBSCs . | BM . | PBSCs . | ||
| Median time to neutrophil engraftment, d (range) | 24 (5-33) | 20 (14-26) | 19 (12-27) | 18 (11-28) | 20 (13-42) |
| Median time to platelet engraftment, d (range) | 0 (0-22) | 0 (0-17) | 19 (0-40) | 18 (11-71) | 43 (0-251) |
| Outcome/condition (no. of patients) | |||||
| Rejection | 1 | 0 | 2 | 0 | 1 |
| Death/relapse before engraftment | 0 | 0 | 1 | 0 | 2 |
| Partial chimerism | 1 | 0 | 1 | 2 | 1 |
| Full chimerism | 6 | 8 | 6 | 7 | 7 |
| Chimerism data missing | 0 | 0 | 0 | 0 | 1* |
| . | Related donor . | Unrelated donor . | CB . | ||
|---|---|---|---|---|---|
| BM . | PBSCs . | BM . | PBSCs . | ||
| Median time to neutrophil engraftment, d (range) | 24 (5-33) | 20 (14-26) | 19 (12-27) | 18 (11-28) | 20 (13-42) |
| Median time to platelet engraftment, d (range) | 0 (0-22) | 0 (0-17) | 19 (0-40) | 18 (11-71) | 43 (0-251) |
| Outcome/condition (no. of patients) | |||||
| Rejection | 1 | 0 | 2 | 0 | 1 |
| Death/relapse before engraftment | 0 | 0 | 1 | 0 | 2 |
| Partial chimerism | 1 | 0 | 1 | 2 | 1 |
| Full chimerism | 6 | 8 | 6 | 7 | 7 |
| Chimerism data missing | 0 | 0 | 0 | 0 | 1* |
One cord blood patient with AML in PR3 engrafted but relapsed at day 81. Chimerism data were not available.
Excluding the 3 early relapse/TRM patients, 88%, 76%, and 78% of related, URD, and CB recipients achieved full donor chimerism. All related donor PBSC recipients achieved full donor chimerism by day 100. The 2 URD PBSC recipients who did not achieve rapid full donor chimerism had MDS; one did not achieve full chimerism because of relapse/progression noted by day 70, and the second rapidly achieved full myeloid chimerism, with slowly improving partial T-cell donor chimerism.
Toxicities, GVHD, and relapse
Acute GVHD occurred in 29% of evaluable patients, almost all of that being grades 1 or 2 (Table 3). More acute GVHD occurred in unrelated recipients, at a rate of 38%. Chronic GVHD occurred in 26% of evaluable patients with a trend toward more GVHD in PBSC recipients; no chronic GVHD occurred in CB recipients. The cumulative incidence of chronic GVHD and extensive chronic GVHD at 2 years was 19% (95% confidence interval [CI], 7%-31%) and 12% (95% CI, 2%-22%), respectively. TRM was infrequent (13% overall, cumulative incidence, 11% at 2 years; 95% CI, 2%-20%), with no TRM in recipients of related donors. Two of 6 patients with a history of previous autologous transplantation and 4 of 22 patients with a history of previous allogeneic transplantation experienced TRM, with no TRM occurring in patients without a previous history of transplantation. Relapse was the most common cause of failure of therapy, occurring in 45% of patients (cumulative incidence, 43% at 2 years; 95% CI, 28%–58%). Relapse occurred more frequently in related donor recipients compared with URD recipients. This was balanced by TRM in URD recipients, and outcome was indistinguishable between the related, URD, and CB cohorts (see section on analysis of EFS and OS).
Transplantation-related toxicities/relapse
| Toxicity . | Related donor . | Unrelated donor . | CB . | Overall, no. (%) . | ||
|---|---|---|---|---|---|---|
| BM . | PBSCs . | BM . | PBSCs . | |||
| N | 8 | 8 | 10 | 9 | 12 | 47 |
| Acute GVHD | ||||||
| No. evaluable | 7 | 8 | 8 | 9 | 9 | 41 |
| No. with acute GVHD, grades 1 or 2 | 1 | 1 | 2 | 5 | 2 | 11 (27) |
| No. with acute GVHD, grades 3 or 4 | 0 | 0 | 1 | 0 | 0 | 1 (2) |
| No. with no acute GVHD | 6 | 7 | 5 | 4 | 7 | 29 (71) |
| Chronic GVHD | ||||||
| No. evaluable | 5 | 7 | 6 | 7 | 6 | 31 |
| No. with chronic GVHD | 2 | 3 | 1 | 2 | 0 | 8 (26) |
| No. with no chronic GVHD | 3 | 4 | 5 | 5 | 6 | 23 (74) |
| Other events | ||||||
| No. (%) with transplantation-related mortality | 0 | 0 | 3 (30) | 2 (22) | 1 (8) | 6 (13) |
| No. (%) with relapse | 4 (50) | 5 (63) | 3 (30) | 4 (44) | 5 (42) | 21 (45) |
| Toxicity . | Related donor . | Unrelated donor . | CB . | Overall, no. (%) . | ||
|---|---|---|---|---|---|---|
| BM . | PBSCs . | BM . | PBSCs . | |||
| N | 8 | 8 | 10 | 9 | 12 | 47 |
| Acute GVHD | ||||||
| No. evaluable | 7 | 8 | 8 | 9 | 9 | 41 |
| No. with acute GVHD, grades 1 or 2 | 1 | 1 | 2 | 5 | 2 | 11 (27) |
| No. with acute GVHD, grades 3 or 4 | 0 | 0 | 1 | 0 | 0 | 1 (2) |
| No. with no acute GVHD | 6 | 7 | 5 | 4 | 7 | 29 (71) |
| Chronic GVHD | ||||||
| No. evaluable | 5 | 7 | 6 | 7 | 6 | 31 |
| No. with chronic GVHD | 2 | 3 | 1 | 2 | 0 | 8 (26) |
| No. with no chronic GVHD | 3 | 4 | 5 | 5 | 6 | 23 (74) |
| Other events | ||||||
| No. (%) with transplantation-related mortality | 0 | 0 | 3 (30) | 2 (22) | 1 (8) | 6 (13) |
| No. (%) with relapse | 4 (50) | 5 (63) | 3 (30) | 4 (44) | 5 (42) | 21 (45) |
EFS and OS
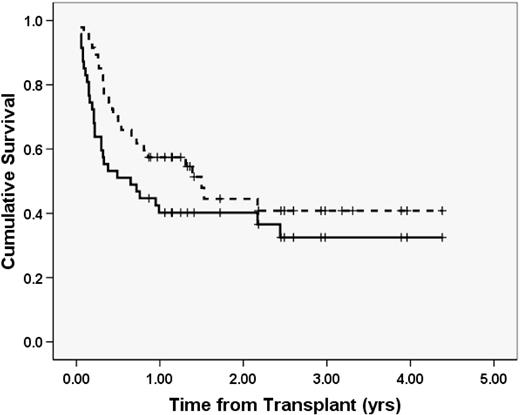
With a median follow-up of 24 months (range, 11-53 months), the Kaplan-Meier probability of 2-year EFS and OS of the cohort was 40.2% (SE 7.2%) and 44.5% (SE 8.2%), respectively (Figure 1). Although most patients lived only a few months after relapse or rejection, some patients are long-term survivors after salvage therapy. One patient with ALL received chemotherapy and DLI for low chimerism/relapse and is alive more than a year out from DLI and 3 years from transplantation. Another patient with ALL who rejected their initial mismatched related donor BM graft underwent a second reduced intensity regimen using the same donor and is alive after moderate chronic GVHD more than 3 years out from transplantation.
EFS and OS. The solid line represents EFS; dashed line, OS. Events included relapse, rejection, and death in remission. N = 47.
EFS and OS. The solid line represents EFS; dashed line, OS. Events included relapse, rejection, and death in remission. N = 47.
Univariate and multivariate analysis of EFS and OS
Univariate analysis of EFS and OS showed no effect of related versus URD stem cell sources or BM versus PBSC versus CB (Table 4). In addition, differences in outcome were not seen by disease (ALL vs AML vs other), previous transplantation with an autologous versus an allogeneic approach versus no previous transplantation, age, cytomegalovirus status, the presence of GVHD, length of time to relapse after first transplantation, or whether a different donor was used when this regimen was given as a second transplant. Of those with a history of previous myeloablative transplantations, only 5 of 25 with data available received a second graft from the same donor used previously. There were insufficient numbers of patients to address whether the use of a different second donor was advantageous and OS rates of those receiving the same or different donors were similar.
Univariate analysis of 2-year event-free and overall survival
| Variable . | Percentage event-free survival (SE) . | P (EFS) . | Percentage overall survival (SE) . | P (OS) . |
|---|---|---|---|---|
| Cohort | 40.2 (7.2) | 44.5 (8.0) | ||
| Stem cell source | ||||
| Related donor | 42.2 (12.7) | 50.1 (14.1) | ||
| Unrelated donor | 36.8 (11.1) | .909 | 39.5 (12.0) | .582 |
| CB | 41.7 (14.2) | 43.8 (16.5) | ||
| Disease | ||||
| ALL | 35.3 (11.6) | 36.8 (12.5) | ||
| AML | 41.7 (10.1) | .963 | 54.2 (10.2) | .986 |
| Other | 50 (20.4) | 50.0 (20.4) | ||
| History of previous BMT | ||||
| No BMT | 46.8 (11.6) | 52.1 (13) | ||
| Previous autologous BMT | 50 (20.4) | .539 | 62.5 (21.3) | .104 |
| Previous allogeneic BMT | 31.8 (9.9) | 32.7 (11.1) | ||
| No BMT | 46.8 (11.6) | 52.1 (13) | ||
| TBI-based BMT | 25 (10.8) | .232 | 23.4 (11) | .02 |
| Non–TBI-based BMT | 50.0 (14.4) | 62.5 (15.5) | ||
| Presence of acute or chronic GVHD | ||||
| GVHD | 50.0 (12.5) | .231 | 50.0 (14.8) | .380 |
| No GVHD | 39.6 (9.9) | 39.6 (10.5) | ||
| Chimerism by day 100 | ||||
| Full chimerism | 49.7 (8.6) | .016 | 49 (9.3) | .954 |
| Partial chimerism | 22.2 (14) | 44 (21) | ||
| Presence of detectable disease | ||||
| History of TBI | ||||
| Detectable disease | 20.0 (12.6) | .087 | 0 | .06 |
| No detectable disease | 50.0 (12.5) | 61.1 (12.6) | ||
| No previous TBI | ||||
| Detectable disease | 16.7 (15.2) | .038 | 0 | .010 |
| No detectable disease | 61.5 (13.5) | 62.7 (15.5) | ||
| Recipient CMV status | ||||
| CMV+ | 39.1 (10.2) | .908 | 44.7 (11.3) | .611 |
| CMV− | 40.0 (10.6) | 40.5 (11.5) | ||
| Recipient age, y | ||||
| > 10 | 44.0 (9.9) | .694 | 43.6 (10.9) | .885 |
| < 11 | 35.8 (10.3) | 45.6 (11.7) | ||
| Time to relapse after BMT 1 | ||||
| Less than 6 months | 33.3 (27.2) | 33.3 (27.2) | ||
| 6-12 months | 42.9 (18.7) | .988 | 42.9 (18.7) | .872 |
| More than 12 months | 33.3 (11.1) | 40.0 (13.0) | ||
| Donors for BMT 2 | ||||
| Same donor | 20.0 (17.9) | 40.0 (21.9) | ||
| Different donor | 38.5 (13.5) | .653 | 38.5 (13.5) | .377 |
| Auto/allo | 57.1 (18.7) | 68.6 (13.2) |
| Variable . | Percentage event-free survival (SE) . | P (EFS) . | Percentage overall survival (SE) . | P (OS) . |
|---|---|---|---|---|
| Cohort | 40.2 (7.2) | 44.5 (8.0) | ||
| Stem cell source | ||||
| Related donor | 42.2 (12.7) | 50.1 (14.1) | ||
| Unrelated donor | 36.8 (11.1) | .909 | 39.5 (12.0) | .582 |
| CB | 41.7 (14.2) | 43.8 (16.5) | ||
| Disease | ||||
| ALL | 35.3 (11.6) | 36.8 (12.5) | ||
| AML | 41.7 (10.1) | .963 | 54.2 (10.2) | .986 |
| Other | 50 (20.4) | 50.0 (20.4) | ||
| History of previous BMT | ||||
| No BMT | 46.8 (11.6) | 52.1 (13) | ||
| Previous autologous BMT | 50 (20.4) | .539 | 62.5 (21.3) | .104 |
| Previous allogeneic BMT | 31.8 (9.9) | 32.7 (11.1) | ||
| No BMT | 46.8 (11.6) | 52.1 (13) | ||
| TBI-based BMT | 25 (10.8) | .232 | 23.4 (11) | .02 |
| Non–TBI-based BMT | 50.0 (14.4) | 62.5 (15.5) | ||
| Presence of acute or chronic GVHD | ||||
| GVHD | 50.0 (12.5) | .231 | 50.0 (14.8) | .380 |
| No GVHD | 39.6 (9.9) | 39.6 (10.5) | ||
| Chimerism by day 100 | ||||
| Full chimerism | 49.7 (8.6) | .016 | 49 (9.3) | .954 |
| Partial chimerism | 22.2 (14) | 44 (21) | ||
| Presence of detectable disease | ||||
| History of TBI | ||||
| Detectable disease | 20.0 (12.6) | .087 | 0 | .06 |
| No detectable disease | 50.0 (12.5) | 61.1 (12.6) | ||
| No previous TBI | ||||
| Detectable disease | 16.7 (15.2) | .038 | 0 | .010 |
| No detectable disease | 61.5 (13.5) | 62.7 (15.5) | ||
| Recipient CMV status | ||||
| CMV+ | 39.1 (10.2) | .908 | 44.7 (11.3) | .611 |
| CMV− | 40.0 (10.6) | 40.5 (11.5) | ||
| Recipient age, y | ||||
| > 10 | 44.0 (9.9) | .694 | 43.6 (10.9) | .885 |
| < 11 | 35.8 (10.3) | 45.6 (11.7) | ||
| Time to relapse after BMT 1 | ||||
| Less than 6 months | 33.3 (27.2) | 33.3 (27.2) | ||
| 6-12 months | 42.9 (18.7) | .988 | 42.9 (18.7) | .872 |
| More than 12 months | 33.3 (11.1) | 40.0 (13.0) | ||
| Donors for BMT 2 | ||||
| Same donor | 20.0 (17.9) | 40.0 (21.9) | ||
| Different donor | 38.5 (13.5) | .653 | 38.5 (13.5) | .377 |
| Auto/allo | 57.1 (18.7) | 68.6 (13.2) |
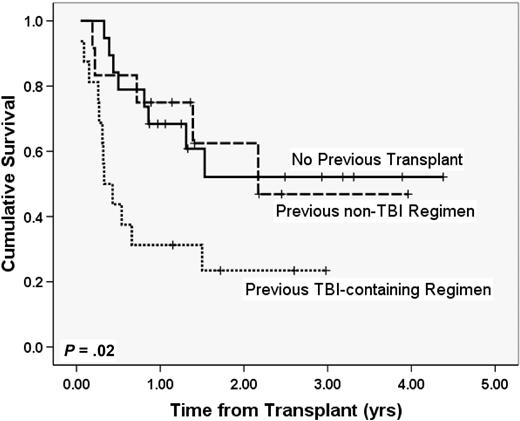
Two characteristics did have an impact on survival. Whereas patients who had previously undergone a non–TBI-containing myeloablative transplantation had outcomes identical to those who had not undergone a previous transplantation, those who had a history of a previous TBI-containing myeloablative transplantation did significantly worse (Figure 2, 2-year OS 52% no previous bone marrow transplantation [BMT] vs 63% with previous non-TBI myeloabative transplantation, P = .97; 2-year OS 52% no previous BMT vs 23% with history of a TBI-based transplantation, P = .02). In addition, although the large majority of enrolled patients (43 of 47 patients) met traditional criteria for CR at transplantation (2 AML patients had between 5% and 10% blasts, 2 lymphoma patients had small but measurable disease), the presence of any measurable disease was associated with a poor outcome. Of the 26 patients with detailed information about remission (institutional flow cytometry, cytogenetics, FISH, PET, and gallium scans), 61% (SE 12.6%) of patients with no measurable disease at transplantation are alive at 2 years, whereas only 2 of 10 who had measurable disease at transplantation are alive with 1.2 and 1.3 years of follow-up and the 2-year Kaplan-Meier probability of survival in the group is 0% (P = .06).
OS with and without a history of previous myeloablative transplantation. Patients had either no history of previous myeloablative transplantation (n = 19), previous myeloablative transplantation with a non-TBI regimen (n = 12), or previous myeloablative transplantation with a fractionated TBI-based regimen (n = 16).
OS with and without a history of previous myeloablative transplantation. Patients had either no history of previous myeloablative transplantation (n = 19), previous myeloablative transplantation with a non-TBI regimen (n = 12), or previous myeloablative transplantation with a fractionated TBI-based regimen (n = 16).
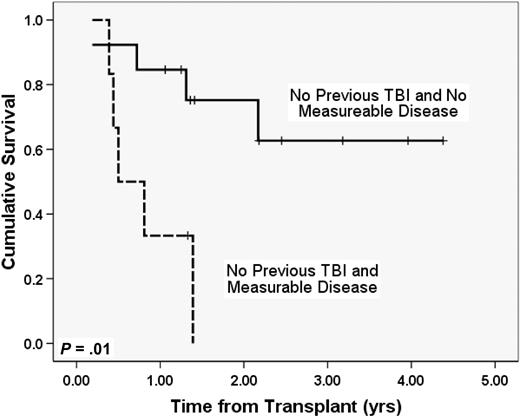
We analyzed whether the presence of measurable disease at transplantation impacted outcomes of patients with a history of previous TBI-based myeloablative regimens, previous non-TBI regimens, or no history of previous BMT. Seven patients previously treated with TBI-containing myeloablative regimens had detailed remission information (3 negative for measurable disease, 4 with disease). Only one patient in the previous TBI group was alive (measurable disease present at BMT, follow-up 1.2 years). Two-year Kaplan-Meier probability of survival of patients treated with non-TBI regimens or no previous BMT who had no measureable disease was 75% (SE 13%, n = 13), whereas only a single patient in the non-TBI, non-BMT group who had measurable disease was alive with a follow-up of 1.3 years (n = 6, Figure 3, P = .01).
OS in patients without previous TBI by the presence or absence of measurable disease. Whereas almost all patients were in morphologic CR at transplantation, further data for “measurable disease” (flow cytometry, FISH, cytogenetics, or PET positivity) was available for 26 patients. These curves compare survival in patients with no measurable disease who had not previously received TBI (n = 13) to similar patients with measurable disease at transplantation (n = 6).
OS in patients without previous TBI by the presence or absence of measurable disease. Whereas almost all patients were in morphologic CR at transplantation, further data for “measurable disease” (flow cytometry, FISH, cytogenetics, or PET positivity) was available for 26 patients. These curves compare survival in patients with no measurable disease who had not previously received TBI (n = 13) to similar patients with measurable disease at transplantation (n = 6).
We performed a Cox proportional hazards regression analysis, including variables known to influence survival outcomes (stem cell source, disease, GVHD, detectable disease, and previous BMT). This analysis confirmed that a history of a previous TBI-containing myeloablative transplantation independently predicted a poorer outcome (P = .003).
Discussion
With more than a decade having passed since the introduction of reduced intensity regimens, the role of these approaches in older or high-risk adult patients is becoming better understood. Older patients and patients with significant comorbidities clearly benefit from the approach.20,21 Whereas relapse rates after reduced intensity approaches may be higher, reductions in TRM have generally compensated for this, and several studies have shown outcomes comparable with myeloablative approaches.22,23 Trials published assessing this approach in pediatrics have been performed largely at single institutions and have focused mainly on nonmalignant disorders,24-34 although groups in Chicago, New York, Mexico, and Germany have published small studies looking at recipients with malignancies.35-39
The outcomes presented herein represent the first large cooperative group study of a reduced intensity regimen in pediatric patients with hematologic malignancies. The goal of the study was to test a regimen with enough immunoablation to ensure adequate engraftment given the many stem cell sources that pediatric programs use. Secondary goals were to allow a measure of disease control while avoiding TRM in very high-risk patients. These goals were both accomplished, with high rates of engraftment and very low TRM. Rejection only occurred in mismatched or URD BM or CB recipients, and no rejection occurred in fully matched related BM or related or unrelated PBSC donors.
The large majority of patients qualifying for enrollment on this trial had undergone previous myeloablative transplantation. Published studies looking at second myeloablative transplantation outcomes have shown high rates of transplantation-related toxicities and mortality.11,12,40-43 TRM has ranged from 45% to 51%, and relapse rates vary from 26% to 59%. Whereas it is clear that both children and adults who undergo a first allogeneic transplantation are at very high risk for TRM with a second myeloablative procedure, the data are less clear that a previous autologous transplantation significantly increases TRM for second myeloablative transplantations in pediatrics.11,13,40 Survival in second allogeneic myeloablative transplantation studies ranges from 25% to 32%, with survival of selected populations (younger patients in remission with late relapse after first transplantation) approaching 50%. Consistent factors improving prognosis across studies include younger age (< 16-18 years in most studies, < 10 years in one study), late relapse after first transplantation (> 6-12 months), and the attainment of remission before second transplantation. TBI as part of the second transplantation has been shown to be beneficial in several studies, but when TBI was used for the first transplantation, outcomes of a second myeloablative transplantation have been very poor, with increased TRM (high risk of severe veno-occlusive disease), high rates of relapse, and poor survival (disease-free survival, 14%).12 Several studies of reduced intensity and nonmyeloablative regimens used as a second transplantation for adult patients relapsed after myeloablative procedures have shown decreased TRM, which has resulted in improved outcome in these patients.44-46
This study confirms many of these earlier observations and expands on them. We did not detect a difference in outcome based on time from previous transplantation, but only 3 patients enrolled had relapsed within 6 months of their previous transplantation (median time to relapse from previous BMT, 446 days; range, 124-2047 days). Because all of the patients enrolled in this trial were young, we did not detect a difference in outcomes based on age. In light of the fact that outcomes in adult studies using reduced intensity regimens for second allogeneic transplantations have improved, it may be that lower TRM with reduced intensity approaches could eliminate or modify age as a significant variable in predicting outcome after second allogeneic procedures.
Two key poor prognostic factors in this study are a history of the use of TBI for a first myeloablative transplantation procedure and the presence of any detectable disease at transplantation. Patients previously treated with TBI on this study had attained a remission and their TRM was no different from other patients. It may be that disease relapsed after TBI is either more resistant and/or the minimal residual disease (MRD) status of these patients was higher at the time of transplantation. Although prognosis in these patients was poor compared with other patients, 2-year EFS was measurable at 23.4% (SE 11.1%), so a small portion of even these “highest” risk patients achieved long-term survival.
Because of the broad inclusion criteria regarding diseases, this study did not look at a molecular minimal residual disease MRD marker. Several recent studies using myeloablative regimens have shown the importance of low or absent MRD at the time of transplantation to outcome.47-51 We showed in this study that, even if patients are in a pathologic remission, low levels of disease detectable by flow cytometry, FISH, or cytogenetics have prognostic significance for survival using this reduced intensity regimen. Further studies looking at more sensitive methods of MRD detection before reduced intensity regimens may be able to better identify patients at high risk for failure with this approach.
Our analysis identified a group of patients with a 2-year OS of 75% (patients with no history of previous TBI-containing myeloablative transplantation who had no measurable disease [local flow cytometry–, FISH-, and PET-negative] at transplantation). Because the patients in this group did so well despite being in second or third remission and at high risk for relapse, this raises the possibility that survival using this approach may be comparable with outcomes of myeloablative procedures in a subgroup of patients. Our data suggest that prospective trials randomizing this or other RIC approaches with myeloablative regimens would be most appropriately targeted at patients who are either MRD negative or, at a minimum, have no measurable disease as described in this study.
In conclusion, the use of this busulfan/fludarabine/ATG regimen in children at high risk for transplantation toxicity results in low rates of TRM, high rates of engraftment with all stem cell sources, and reasonable rates of survival. The approach works well for patients unable to undergo a first myeloablative regimen because of comorbidities and offers a distinct advantage of low TRM to patients relapsing after a first myeloablative regimen who obtain a second remission. Because outcomes after treatment with DLIs for relapse after myleoablative transplantation are generally poor in pediatric patients,52 a second reduced intensity transplantation may be a more attractive alternative for patients achieving remission. The main cause of failure of this approach is relapse, and future studies focusing on decreasing relapse by lessening MRD before transplantation with novel therapies, treating with agents at transplantation that reduce disease burden but do not increase toxicity, or using novel chemotherapeutic or immunologic approaches after transplantation may help cure more of these very-high-risk patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the research assistants and principal investigators at each institution for their diligence and attentiveness to detail.
This work was supported in part by an unrestricted educational grant from Otsuka Pharmaceutical Development & Commercialization, Inc, the manufacturer of intravenous Busulfex. Because of the nature of the grant, Otsuka Pharmaceutical had no input into study design, implementation, data analysis, or publication.
Authorship
Contribution: M.A.P. had primary responsibility for study design, data analysis, data interpretation, and manuscript writing, and responsibility for the entire paper as an accurate and verifiable report; K.M.B. had responsibility for study design, data file preparation, data analysis, interpretation of data, and manuscript writing; D.W. participated in study design, patient accrual, data analysis, interpretation of data, and manuscript writing; H.F., M.D., R.K.G., P.J.S., A.E.H., and M.G. participated in patient accrual, interpretation of data, and manuscript writing; S.A.G. and M.K. participated in study design, interpretation of data, and manuscript writing; and R.K. had responsibility for study design, patient accrual, data analysis, data interpretation, and manuscript writing, and responsibility for the entire paper as an accurate and verifiable report.
Conflict-of-interest disclosure: None of the authors has any other conflicts of interest to disclose.
A complete list of participating institutions and principal investigators is included in the supplemental Appendix (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Michael A. Pulsipher, University of Utah School of Medicine, Division of Hematology/Blood and Marrow Transplant, 30 North 1900 East, Room 5C402, Salt Lake City, UT 84132-2408; e-mail: michael.pulsipher@hsc.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal