Abstract
We show that lentiviral delivery of human γ-globin gene under β-globin regulatory control elements in hematopoietic stem cells (HSCs) results in sufficient postnatal fetal hemoglobin (HbF) expression to correct sickle cell anemia (SCA) in the Berkeley “humanized” sickle mouse. Upon de-escalating the amount of transduced HSCs in transplant recipients, using reduced-intensity conditioning and varying gene transfer efficiency and vector copy number, we assessed critical parameters needed for correction. A systematic quantification of functional and hematologic red blood cell (RBC) indices, organ pathology, and life span was used to determine the minimal amount of HbF, F cells, HbF/F-cell, and gene-modified HSCs required for correcting the sickle phenotype. We show that long-term amelioration of disease occurred (1) when HbF exceeded 10%, F cells constituted two-thirds of the circulating RBCs, and HbF/F cell was one-third of the total hemoglobin in sickle RBCs; and (2) when approximately 20% gene-modified HSCs repopulated the marrow. Moreover, we show a novel model using reduced-intensity conditioning to determine genetically corrected HSC threshold that corrects a hematopoietic disease. These studies provide a strong preclinical model for what it would take to genetically correct SCA and are a foundation for the use of this vector in a human clinical trial.
Introduction
Sickle cell anemia (SCA) results from a point mutation in the β-globin gene (βS), resulting in sickle hemoglobin (HbS). HbS polymerizes upon deoxygenation resulting in sickle-shaped RBCs that occlude microvasculature. Patients with SCA have intermittent acute vascular occlusions and cumulative organ damage, reducing the life span to 42 to 58.5 years.1,2 Besides sickling, excessive hemolysis and a state of chronic inflammation exist. SCA patients account for approximately 75 000 hospitalizations per year, resulting in an estimated annual expenditure of $475 million dollars in the United States alone.3 Worldwide, SCA is second only to thalassemia in incidence of monogenic disorders, with more than 200 000 children born annually in Africa.4
Current therapies include supportive care for episodic sickling, chronic transfusions with iron chelation, and hydroxyurea to induce fetal hemoglobin (HbF). These therapies impact disease morbidity, but their effectiveness is variable and dependent on compliance to an indefinite treatment regimen. A matched allogeneic hematopoietic stem cell (HSC) transplantation is curative, but restricted by the availability of matched related donors5 and has potential serious complications.6 A meta-analysis of 187 SCA transplantations shows 6% to 7% conditioning-related peritransplantation mortality, 7% to 10% acute rejection, and 13% to 20% chronic graft-versus-host disease (GVHD) in recipients.7
Gene therapy of autologous HSCs followed by transplantation could result in a one-time cure, avoid adverse immunologic consequences, and not be limited by availability of donors; it may also not require myeloablative-conditioning regimens, and thereby have lower toxicity. The amount of HbF/anti–sickling globin required to correct SCA via a transgene is unknown. Recently, 2 groups have used HIV-1–based lentivirus vectors carrying recombinant/mutated β-globin genes designed to have antisickling properties to show correction of the disease in mouse models of SCA when high levels of the mutant β-globin are expressed.8,9
Expression of HbF postnatally can be therapeutic, as is evident by the protective effect of HbF in neonatal sickle RBCs and in patients with hereditary persistence of HbF and SCA. The proportion of genetically corrected HSCs, the amount of exogenously expressed HbF, and the proportion of F cells that will correct the pathophysiology are unknown. We have previously shown complete correction of human thalassemia major in vitro, and in xenografted mice in vivo, with a lentivirus vector carrying the β-globin gene and locus control region (LCR) elements.10 In this report, we modified this β-globin lentivirus vector to encode γ-globin exons and transduced murine sickle HSCs. We first characterized functional correction with a careful and detailed quantification of RBC sickling, half-life, and deformability, with sickle to normal transplantations and high HbF production to define parameters of correction. Next, using reduced-intensity conditioning and varying the percentage of transduced HSCs, we performed transplantations on sickle mice with significant organ damage and demonstrate the proportions of (1) genetically corrected HSCs, (2) HbF, and (3) F cells, and (4) percentage of HbF/F cell required for correction of the sickle RBC and amelioration of organ damage in SCA.
Methods
Detailed methodology is provided in supplemental methods (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Vector
We have shown that a β-γ-globin hybrid gene carrying lentivirus vector, I8Hβ/γW,11 expresses high γ-globin mRNA in erythroid cells expressing “adultlike” globins.12 All β-globin coding sequences were changed to γ-globin using site-directed mutagenesis and the γ-β-globin hybrid gene, and LCR elements were cloned in reverse orientation to the viral transcriptional unit to generate sGbG lentivirus vector.13 Virus was made with cotransfection of 293T cells.10,13
Murine HSC enrichment
Bone marrow from 6- to 20-week-old BERK sickle mice14 was harvested and lineage depleted with biotinylated CD5, CD8, B220, Mac-1, CD11b, Gr-1, and TER-119 antibodies and magnetic beads. The bead-free cells were stained with antibodies to Sca-1, c-kit. Cells that were 7-AAD−, lineage−, c-kit+ then Sca-1+ (LSK cells) were sorted on FACSVantage (BD Biosciences). All experiments using Berkeley transgenic sickle mice and C57/BL6 mice were performed according to protocols approved by the Cincinnati Children's Hospital Medical Center.
Gene transfer and bone marrow transplantation
Myeloablative transplantations were performed from BERK→C57Bl/6 mice because of ease of transplantation and ready availability of normal recipients (9.5 ± 0.6 weeks old) after 11.75 Gy radiation. Radiation control experiments showed that BERK mice receiving 8 to 9 Gy radiation survived without receiving LSK cells; and the lethal dose was lower than in C57Bl/6 mice. BERK mice receiving more than 10.5 Gy died when no LSK cells were given; those given LSK rescue survived long term. BERK mice are difficult to breed in large numbers at a given time, therefore 2 mice/radiation dose level were to determine the sublethal dose. All BERK recipients (12.9 ± 0.4 weeks old) received 3 peritransplantation RBC transfusions (days 1-7). Organ pathology in BERK recipients 1 year after transplantation was compared with 12-week-old BERK mice that did not undergo transplantation. The radiation was higher than classical reduced-intensity radiation dose of 4 Gy to allow a large degree of donor HSC chimerism. A range of MOI was used to vary the proportion of transduced donor HSCs in the graft. LSK cells were prestimulated overnight13 and transduced twice at an MOI of 30 for BERK→C57BL/6 transplants and MOI of 30 to 100 for BERK→BERK transplants for 22 to 24 hours; 10 000 to 24 000 LSK cells and untransduced LK cells were cotransplanted into recipient C57BL/6 or BERK mice.
Copy number analysis
Copy number analysis was done on genomic DNA by real-time polymerase chain reaction using primers and probes described previously.13
Hematologic analysis
Hematologic analysis was obtained on Hemavet 950FS (Drew Scientific) under mouse settings. Reticulocyte analysis was performed as follows: 0.1 μL blood and 200 μL BD Retic-COUNT Reagent were mixed (Becton Dickinson), incubated at room temperature for 30 minutes, and analyzed by fluorescence-activated cell sorting (FACS).
Hemoglobin analysis
Hemoglobin electrophoresis was performed on cellulose acetate plates, as described previously.13 Ion exchange high-performance liquid chromatography (HPLC) was performed with an Alliance 2690 HPLC machine (Waters) using a PolyCAT A column (item no. 3.54CT0510; Poly LC Inc).
Red blood cell functional analysis
Irreversibly sickled cells (ISCs) were enumerated by scoring 500 RBCs in consecutive fields. Graded deoxygenation was performed using tonometry. RBC deformability was determined using a laser-assisted optical rotational cell analyzer (LORCA; RR Mechatronics).
RBC half-life
Mice were injected with 3 mg Sulfo-NHS biotin (Sigma) in 300 μL PBS as 2 separate injections 1 hour apart; 2 to 5 μL blood was drawn at serial times, and stained with APC-Cy7–conjugated streptavidin.
Histology
Spleen, liver, bones, brain, and kidney were harvested and placed in 5 mL of 10% formalin. Paraffin blocks were sectioned and stained with hematoxylin and eosin.
Results
High HbF after gene therapy and myeloablative transplantation corrects SCA
The sGbG vector carries γ-globin exons and β-globin noncoding and regulatory regions (supplemental Figure 1). Based upon our previously studied sBG vector, which expresses high levels of human β-globin,13 sGbG-transduced LSK cells from Berkeley sickle (BERK) mice14,15 were transplanted into lethally irradiated (myeloablated) normal C57Bl/6J mice (termed GbG mice). Mock transductions on BERK LSK cells from the same bone marrow pool followed by transplantation resulted in mice with SCA (termed mock mice; supplemental Figure 2B). The majority of RBCs in GbG mice expressed HbF (supplemental Figure 2A-D). Only GbG mice with 100% donor (HbS+) RBCs, with no evidence of residual recipient murine hemoglobin by electrophoresis and HPLC (supplemental Figure 2B-C), were analyzed for hematologic, functional, and pathologic analysis. GbG mice with a small proportion of recipient murine RBCs (supplemental Figure 2B) were used only to assess HbF/vector copy and frequency of transduced HSCs.
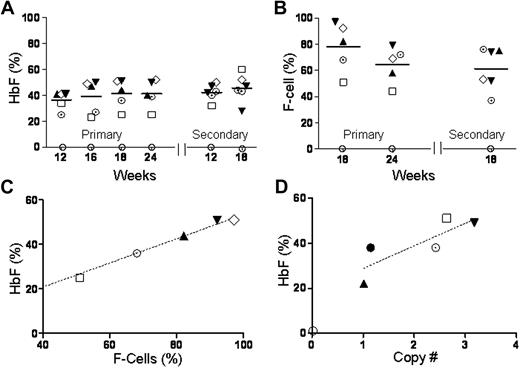
The percentage of HbF (HbF/HbS+HbF) in blood, quantified by FACS, was approximately 40% in primary mice followed for 6 months and in secondary recipients followed for 7.5 months (Figure 1A). Two-thirds of RBCs were F cells; their proportion was also stable in primary and secondary recipients (Figure 1B). The proportion of F cells and vector copies correlated with HbF (Figure 1C-D). Taken together, these data show significant HbF expression from the sGbG vector in the majority of RBC with stable long-term expression.
GbG mice that underwent transplantation after myeloablative conditioning have high HbF production that is stable and sustained in primary and secondary mice. GbG mice that were fully chimeric for donor RBCs were analyzed at different time points. The proportion of HbF (A) and F cells (B) in blood of individual mice, as determined by ion-exchange HPLC and FACS analysis, respectively, is shown at different time points after primary and secondary transplantations. (C) The amount of HbF in blood directly correlated with the proportion of F cells. (D) The amount of HbF produced was directly in proportion to the vector copy number in bone marrow. Each symbol represents one mouse (and consistently depicts the same particular mouse in all the panels).
GbG mice that underwent transplantation after myeloablative conditioning have high HbF production that is stable and sustained in primary and secondary mice. GbG mice that were fully chimeric for donor RBCs were analyzed at different time points. The proportion of HbF (A) and F cells (B) in blood of individual mice, as determined by ion-exchange HPLC and FACS analysis, respectively, is shown at different time points after primary and secondary transplantations. (C) The amount of HbF in blood directly correlated with the proportion of F cells. (D) The amount of HbF produced was directly in proportion to the vector copy number in bone marrow. Each symbol represents one mouse (and consistently depicts the same particular mouse in all the panels).
High levels of HbF result in sustained hematologic correction.
Table 1 shows improvement of hematologic parameters in GbG mice. The proportion of reticulocytes decreased from approximately 50% in mock mice to approximately 15% in GbG mice (P < .005; Figure 2A). There was correction of anemia by 12 weeks, which persisted throughout the posttransplantation period (Figure 2B-C).
Hematologic parameters of GbG mice that underwent transplantation after myeloablative conditioning
| Mouse type . | No. . | WBC, 103/μL . | RBC, 106/μL . | Hb, g/dL . | MCV, fL . | MCH, pg . | RDW, % . | Plt, 103/μL . | Reticulocytes, % . |
|---|---|---|---|---|---|---|---|---|---|
| BERK | 5 | 56.8 ± 5.4 | 5.3 ± 0.4 | 5.8 ± 0.5 | 48.2 ± 1.05 | 10.7 ± 0.5 | 35.3 ± 1.6 | 733 ± 80 | 60.8 ± 5.0 |
| GbG pri | 5 | 10.6 ± 3.1 | 9.4 ± 0.8 | 10.0 ± 0.8 | 40.7 ± 1.3 | 10.4 ± 0.6 | 27.6 ± 1.1 | 733 ± 82 | 15.8 ± 3.2 |
| Mock pri | 10 | 29.7 ± 1.4 | 5.8 ± 0.4 | 7.6 ± 0.7 | 48.5 ± 1.8 | 10.7 ± 0.2 | 32.0 ± 0.9 | 921 ± 50 | 40.0 ± 3.0 |
| P* | .001 | .007 | .03 | .001 | .9 | .009 | .06 | .006 | |
| GbG sec | 6 | 6.8 ± 1.4 | 8.9 ± 0.4 | 10.1 ± 0.5 | 40.5 ± 1.6 | 11.4 ± 0.5 | 28.3 ± 1.4 | 658 ± 33 | 13.8 ± 2.9 |
| Mock sec | 1 | 31.7 | 5.2 | 6.4 | 47.6 | 12.2 | 32.1 | 923 | 49 |
| Mouse type . | No. . | WBC, 103/μL . | RBC, 106/μL . | Hb, g/dL . | MCV, fL . | MCH, pg . | RDW, % . | Plt, 103/μL . | Reticulocytes, % . |
|---|---|---|---|---|---|---|---|---|---|
| BERK | 5 | 56.8 ± 5.4 | 5.3 ± 0.4 | 5.8 ± 0.5 | 48.2 ± 1.05 | 10.7 ± 0.5 | 35.3 ± 1.6 | 733 ± 80 | 60.8 ± 5.0 |
| GbG pri | 5 | 10.6 ± 3.1 | 9.4 ± 0.8 | 10.0 ± 0.8 | 40.7 ± 1.3 | 10.4 ± 0.6 | 27.6 ± 1.1 | 733 ± 82 | 15.8 ± 3.2 |
| Mock pri | 10 | 29.7 ± 1.4 | 5.8 ± 0.4 | 7.6 ± 0.7 | 48.5 ± 1.8 | 10.7 ± 0.2 | 32.0 ± 0.9 | 921 ± 50 | 40.0 ± 3.0 |
| P* | .001 | .007 | .03 | .001 | .9 | .009 | .06 | .006 | |
| GbG sec | 6 | 6.8 ± 1.4 | 8.9 ± 0.4 | 10.1 ± 0.5 | 40.5 ± 1.6 | 11.4 ± 0.5 | 28.3 ± 1.4 | 658 ± 33 | 13.8 ± 2.9 |
| Mock sec | 1 | 31.7 | 5.2 | 6.4 | 47.6 | 12.2 | 32.1 | 923 | 49 |
Hb indicates hemoglobin; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; RDW, red cell distribution width; Plt, platelets; pri, primary mice; and sec, secondary mice.
P values represent comparison of primary mock mice with the GbG group. Statistical comparisons of secondary mice were not made as only one secondary mock mouse was alive at the time of analysis.
GbG mice that underwent transplantation after myeloablative conditioning, which resulted in correction of hematologic parameters that correlated with the HbF expression. There was sustained reduction in reticulocytes (A), and increase in hematocrit (B) and RBC numbers (C) over time. (D) Leukocytosis decreased with normalization of WBC counts. Data shown represent mean (± SEM) values of GbG mice (n = 5; ●) and mice that underwent mock transplantation (n = 10; ○). ☆ represents mean values in BERK mice that were HSC donors for the GbG and mock transplantations. (E-G) Decrease in reticulocytes, and increased hematocrit and RBC numbers correlated with the proportion of F cells in individual mice. (H) WBC counts decreased but normalized when the F cells exceeded 60%. WBC counts, counted on an automated analyzer, were representative of circulating leukocytes, since only occasional nucleated RBCs were seen in peripheral smears. Each data point/symbol in panels E-H represents one GbG mouse and symbols for individual mice have been kept consistent, to trace individual mice in Figures 1 and 2. ☆ represents mean values in BERK mice that were HSC donors for the GbG and mock transplantations.
GbG mice that underwent transplantation after myeloablative conditioning, which resulted in correction of hematologic parameters that correlated with the HbF expression. There was sustained reduction in reticulocytes (A), and increase in hematocrit (B) and RBC numbers (C) over time. (D) Leukocytosis decreased with normalization of WBC counts. Data shown represent mean (± SEM) values of GbG mice (n = 5; ●) and mice that underwent mock transplantation (n = 10; ○). ☆ represents mean values in BERK mice that were HSC donors for the GbG and mock transplantations. (E-G) Decrease in reticulocytes, and increased hematocrit and RBC numbers correlated with the proportion of F cells in individual mice. (H) WBC counts decreased but normalized when the F cells exceeded 60%. WBC counts, counted on an automated analyzer, were representative of circulating leukocytes, since only occasional nucleated RBCs were seen in peripheral smears. Each data point/symbol in panels E-H represents one GbG mouse and symbols for individual mice have been kept consistent, to trace individual mice in Figures 1 and 2. ☆ represents mean values in BERK mice that were HSC donors for the GbG and mock transplantations.
High white blood cell (WBC) counts in humans with SCA and BERK mice reflect the baseline inflammation in this disease. WBC returned to normal levels in GbG mice (Figure 1D; Table 1). Notably, WBC counts were lower in the mock mice compared with BERK mice that did not undergo transplantation, likely because in the former, sickle HSCs were transplanted into a normal “noninflamed” C57/BL6 background. Indeed, 6 weeks after transplantation, WBC counts in mock group of mice were nearly normal, then gradually rose to high levels seen in SCA (Figure 2D). Overall, hematologic parameters showed marked improvement to near normal levels, and improvement was stable over a prolonged period in primary and secondary GbG mice. The degree of correction correlated with the proportion of F cells (Figure 2E-H) and HbF (data not shown).
High levels of HbF improve the functional parameters of RBCs in sickle mice. (1) Sickling: The irreversibly sickled cells (ISCs) were significantly reduced to 2.3% plus or minus 0.7% in GbG mice, compared with 12% plus or minus 0.8% in BERK controls and 10.2% plus or minus 0.3% in mock mice (Figure 3A-B). Deoxygenation of blood from a representative GbG mouse shows a dramatic reduction in sickling (Figure 3C). A systematic quantification showed a marked decrease in the proportion of sickle RBCs in GbG mice with increasing hypoxia (Figure 3D). (2) RBC membrane deformability: Normal RBCs deform readily at low shear stress (3 Pascals [Pa]), representative of shear stress in small vessels.16 Sickle RBCs have relatively rigid membranes with remarkably reduced deformability even at high shear stress (28 Pa; representative of shear stress in large vessels). There was markedly improved deformability of RBCs of GbG mice, although it did not achieve normal levels (Figure 3E). This may reflect the proportion of circulating sickle RBCs that did not contain HbF. (3) RBC survival: Survival of human sickle RBCs is an order of magnitude less than normal RBCs. We measured the time to 50% reduction (half-life) in GbG and mock/BERK sickle mice. The overall survival of the GbG RBCs was markedly improved, with the time to 50% reduction approximately 4 times longer in RBCs from GbG mice compared with BERK or mock mice (Figure 3F). (4) RBC hemolysis: RBC hemolysis detected by measuring lactate dehydrogenase (LDH) in blood was reduced from 2706 plus or minus 148 mg/dL in mock mice to 1286 plus or minus 345 mg/mL in GbG mice (n = 5; P < .004).
GbG mice that underwent transplantation after myeloablative conditioning, which resulted in correction of functional RBC parameters in primary and secondary mice. (A) Peripheral blood smears showing numerous irreversibly sickled cells (ISCs) in a mouse that underwent mock transplantation and a paucity of ISCs in a GbG mouse. (B) Quantification of ISCs in peripheral blood smears of BERK mice that did not undergo transplantation (n = 5), mock mice (n = 3), and GbG mice (n = 5). (*P < .05; **P < .01). (C) Deoxygenation of blood induces sickling of RBCs in a mock mouse; sickling is largely absent in a GbG mouse. (D) Quantification of sickle RBCs upon graded hypoxia (by tonometry) in the GbG mice (●), compared with mock mice (○). (E) RBC deformability by LORCA analysis in GbG, mock, and normal mice (C57, ⊗) analyzed at 18 weeks in primary transplant recipients. Similar data were seen in secondary recipients (data not shown). Flow at low (3 Pa) and high (28 Pa) shear stress is represented by shaded areas. (F) RBC half-life (determined by in vivo biotin labeling) in the GbG mice, mock/BERK mice, and normal mice after primary transplantations. Similar results were seen in secondary recipients (data not shown).
GbG mice that underwent transplantation after myeloablative conditioning, which resulted in correction of functional RBC parameters in primary and secondary mice. (A) Peripheral blood smears showing numerous irreversibly sickled cells (ISCs) in a mouse that underwent mock transplantation and a paucity of ISCs in a GbG mouse. (B) Quantification of ISCs in peripheral blood smears of BERK mice that did not undergo transplantation (n = 5), mock mice (n = 3), and GbG mice (n = 5). (*P < .05; **P < .01). (C) Deoxygenation of blood induces sickling of RBCs in a mock mouse; sickling is largely absent in a GbG mouse. (D) Quantification of sickle RBCs upon graded hypoxia (by tonometry) in the GbG mice (●), compared with mock mice (○). (E) RBC deformability by LORCA analysis in GbG, mock, and normal mice (C57, ⊗) analyzed at 18 weeks in primary transplant recipients. Similar data were seen in secondary recipients (data not shown). Flow at low (3 Pa) and high (28 Pa) shear stress is represented by shaded areas. (F) RBC half-life (determined by in vivo biotin labeling) in the GbG mice, mock/BERK mice, and normal mice after primary transplantations. Similar results were seen in secondary recipients (data not shown).
High levels of HbF prevent chronic organ damage associated with SCA.
Bone marrow, spleen, liver, and kidneys at 24 weeks showed complete prevention of organ pathology. There was reduced erythroid hyperplasia in bone marrow and spleen, decreased spleen size, and preservation of the splenic follicular architecture, compared with obliterated follicular architecture from the severe erythroid hyperplasia in mock mice. The focal tubular atrophy and segmental glomerular infarction seen in mock mice were absent in the GbG mouse kidneys. Infarctions and extramedullary hematopoiesis seen in livers of mock mice were absent in livers of GbG mice (supplemental Figure 3 shows representative sections; Table 2 summarizes the data in all groups of mice). Overall, except for a mild erythroid hyperplasia no organ pathology was observed in the GbG mice.
Correction of organ pathology in GbG mice that underwent transplantation after myeloablative conditioning
| . | Kidney . | Liver . | Spleen . | Bone marrow . |
|---|---|---|---|---|
| Mock mice, n = 5 | Focal tubular atrophy (1/5), mild congestion (3/5) | 2+ liver infarction (1/5), 3+ liver infarction (1/5), E-M hematopoiesis (2/5) | Weight 500 ± 60 mg, severe erythroid hyperplasia (5/5) | Severe erythroid hyperplasia (5/5) |
| GbG mice, n = 5 | Normal kidney (5/5) | Normal liver (5/5) | Weight 256 ± 51 mg, mild erythroid hyperplasia (5/5), mild congestion (5/5) | Mild erythroid hyperplasia (5/5) |
| . | Kidney . | Liver . | Spleen . | Bone marrow . |
|---|---|---|---|---|
| Mock mice, n = 5 | Focal tubular atrophy (1/5), mild congestion (3/5) | 2+ liver infarction (1/5), 3+ liver infarction (1/5), E-M hematopoiesis (2/5) | Weight 500 ± 60 mg, severe erythroid hyperplasia (5/5) | Severe erythroid hyperplasia (5/5) |
| GbG mice, n = 5 | Normal kidney (5/5) | Normal liver (5/5) | Weight 256 ± 51 mg, mild erythroid hyperplasia (5/5), mild congestion (5/5) | Mild erythroid hyperplasia (5/5) |
2+ liver infarction indicates 2 to 3 infarctions/section; 3+ liver infarction, more than 3 infarctions/section; and E-M, extramedullary.
Mild congestion of the spleen vessels with sickle RBCs is seen when splenic architecture is restored. This is not noted when the splenic architecture is effaced by extramedullary erythropoiesis. Splenic erythroid hyperplasia: severe is complete obliteration of splenic follicles; moderate, more than 1 follicle present/section; and mild, preservation of follicles with evidence of erythroid islands.
Bone marrow: normal erythropoiesis indicates M/E = 5:2; mild erythroid hyperplasia, M/E = 2:1; moderate erythroid hyperplasia, M:E = 1:1; and severe erythroid hyperplasia, M/E = 1:3. Bone marrow erythropoiesis expressed as myeloid-erythroid ratio (M/E). Numbers in parentheses indicate the histologic feature seen in the number of mice/total number of mice analyzed in that group.
High HbF expression improves survival of sickle mice.
The life span of BERK sickle mice is significantly reduced, as in humans with SCA before modern treatment. Kaplan-Meier survival curves showed a 100% survival of the GbG mice at 24 weeks, in contrast to 20% survival in mock mice (n = 14, P < .001; supplemental Figure 3C).
Minimal parameters required correction of SCA
Myeloablative conditioning allows noncompetitive repopulation of gene-corrected donor HSCs, resulting in high transgene-modified HSC engraftment and transgene expression. We hypothesized that high levels γ-globin expression achieved by myeloablative conditioning may not be necessary for correction, and if so, would reduce transplantation-related morbidity.17,18
We performed reduced-intensity transplantation by transplanting gene-modified BERK LSK cells into sublethally irradiated, but with significantly high radiation dose, BERK mice. The proportion of transduced HSCs and vector copy/cell in the graft was varied by transducing LSK cells with at a range of MOI (30-100). Since the half-life of BERK RBCs was 1.5 to 2 days (Figure 3G-H), mice were transfused in the peritransplantation period and analyzed after 12 weeks. Three serial experiments were carried out with mice followed for 1 year. GbG mice were analyzed by separating them into 3 groups based upon percentage of HbF at 18 weeks: HbF = 0% (mock, n = 4), HbF less than 10% (termed GbG<10; n = 17), and HbF of 10% or more (termed GbG≥10; n = 9); (Figure 4A). The cutoff at 10% HbF was selected as this appeared to be a threshold level of HbF that reflected correction of disease: GbG<10 mice showed a higher mortality and inconsistent hematologic correction, compared with GbG≥10 described in the following paragraph. The mouse numbers in the groups changed with time primarily due to the increased mortality related to SCA in mice with no/low HbF.
HbF expression and functional correction in GbG mice that underwent transplantation after reduced-intensity conditioning, separated into 2 groups: mice with HbF of 10% or more (GbG≥10) and mice with HbF of less than 10% (GbG<10). (A) HbF in individual BERK mice 18 weeks after transplantation of sGbG-transduced BERK HSCs, after reduced-intensity conditioning. (B-C) Stable and high HbF expression and F-cell repopulation in long-term survivors analyzed at 11 months. (D) Box and whisker plot showing vector copy numbers in GbG<10 and GbG≥10 mice, with mean vector copy number denoted by the line. Symbols in panels A through C represent mouse groups: ○ = mock (HbF 0%), ▽ = GbG<10 (HbF < 10%), and ● = GbG≥10 (HbF ≥ 10%). (E) The proportion of ISCs was reduced (P < .04) in GbG<10 mice, but was markedly reduced in GbG≥10 mice (P < .001), compared with mock mice. (F) Graded deoxygenation via tonometry demonstrates significant reduction in sickling at physiologically relevant partial oxygen pressures (PO2) in GbG≥10 mice, whereas GbG<10 mice RBC sickled similar to controls. (G-H) RBC deformability showed highly variable improvement in deformability in GbG<10 mice. In contrast, RBC deformability in GbG≥10 mice was highly significantly improved at low and high shear stress (P < .001). Symbols represent mouse groups: ○, mock; ▽, GbG<10; ●, GbG≥10; and ⊗, wild-type mice (C57BL/6). Gray shaded rectangles are representative of low and high shear stress through microvessels and large vessels, respectively. Error bars indicate SEM.
HbF expression and functional correction in GbG mice that underwent transplantation after reduced-intensity conditioning, separated into 2 groups: mice with HbF of 10% or more (GbG≥10) and mice with HbF of less than 10% (GbG<10). (A) HbF in individual BERK mice 18 weeks after transplantation of sGbG-transduced BERK HSCs, after reduced-intensity conditioning. (B-C) Stable and high HbF expression and F-cell repopulation in long-term survivors analyzed at 11 months. (D) Box and whisker plot showing vector copy numbers in GbG<10 and GbG≥10 mice, with mean vector copy number denoted by the line. Symbols in panels A through C represent mouse groups: ○ = mock (HbF 0%), ▽ = GbG<10 (HbF < 10%), and ● = GbG≥10 (HbF ≥ 10%). (E) The proportion of ISCs was reduced (P < .04) in GbG<10 mice, but was markedly reduced in GbG≥10 mice (P < .001), compared with mock mice. (F) Graded deoxygenation via tonometry demonstrates significant reduction in sickling at physiologically relevant partial oxygen pressures (PO2) in GbG≥10 mice, whereas GbG<10 mice RBC sickled similar to controls. (G-H) RBC deformability showed highly variable improvement in deformability in GbG<10 mice. In contrast, RBC deformability in GbG≥10 mice was highly significantly improved at low and high shear stress (P < .001). Symbols represent mouse groups: ○, mock; ▽, GbG<10; ●, GbG≥10; and ⊗, wild-type mice (C57BL/6). Gray shaded rectangles are representative of low and high shear stress through microvessels and large vessels, respectively. Error bars indicate SEM.
The GbG≥10 group of mice had 16% (± 1.2%), 17% (± 1.8%), and 21% (± 2.3%) HbF, whereas the GbG<10 group of mice had 5% (± 1.4%), 4% (± 0.6%), and 4% (± 0.5%) HbF at 12, 18, and 24 weeks, respectively, that was stable up to 1 year (Figure 4B). F-cell repopulation was significantly higher in GbG≥10 mice (65% ± 14%) compared with GbG<10 mice (30% ± 9.4%; Figure 4C). GbG≥10 mice had 2 to 2.5 vector copies/cell, whereas the GbG<10 mice had 1.4 copies/cell (Figure 4D).
Hematologic improvement occurred with reduced-intensity transplantations.
Hematologic parameters stabilized at 18 weeks, due to persistent transfused RBCs in the early posttransplantation period. There was a significant improvement in hematologic parameters in the GbG≥10 group of mice (Table 2), in contrast to a small and inconsistent improvement in GbG<10 mice.
Improvement in RBC function occurs with reduced-intensity transplantations.
(1) Sickling: There was a very significant reduction in ISCs in GbG≥10 mice (P < .005) and a small, but significant reduction in ISCs in GbG<10 mice compared with mock/BERK controls (P < .05, Figure 4E). RBCs from GbG≥10 mice showed reduced sickling when exposed to graded hypoxia, compared with RBCs from GbG<10 or mock/BERK mice (n = 20, P < .01; Figure 4F). In contrast, there was no significant difference in sickling between GbG<10 and mock/BERK mice. (2) RBC membrane deformability: Surprisingly, despite similar degree of sickling with hypoxia in RBCs from GbG<10 mice and mock/BERK mice, there was slight improvement in RBC deformability in the GbG<10 mice. However, these differences were not statistically significant from the mock/BERK mice due to the high variance (Figure 4G). In contrast, there was a consistent significant improvement in RBC deformability in GbG≥10 mice (P < .001, Figure 4H). The deformability pattern suggested improved RBC flow through large vessels and microvessels.19 (3) RBC survival: RBC half-life of BERK mice was 1.5 days. RBCs of GbG mice with 1%, 3%, and 7% HbF had a slightly higher half-life (2 days). Two GbG mice with 18% HbF showed an RBC half-life of 6 days, a 4-fold increase, similar to that seen in mice carrying 40% HbF in the myeloablative transplantation model.
Taken together, the sGbG vector resulted in significant and consistent hematologic and functional correction of SCA, when the HbF production exceeded 10% of the total hemoglobin. Notably, the improvement in phenotype was comparable with that achieved with myeloablative conditioning.
Remarkable improvement in organ pathology when HbF concentrations exceed 10%.
The unique feature of this BERK→BERK transplantation model was presence of significant sickle pathology in recipients at the time of transplantation (determined using BERK controls of comparable age as recipient mice when they underwent transplantation). Therefore, the potential for reversal of organ pathology after gene therapy could be assessed. Organ pathology in the surviving mice at approximately 50 weeks after transplantation was compared with 3-month-old BERK mice that did not undergo transplantation (Figure 5A; Table 3).
Correction of organ pathology in GbG≥10 mice that underwent transplantation after reduced-intensity conditioning and improved overall survival. (A) Representative hematoxylin-eosin–stained sections of a kidney, liver, and spleen of GbG≥10 and GbG<10 mice 48 to 50 weeks after reduced-intensity conditioning transplantation and a 3-month-old BERK control. Image acquisition information is available in supplemental data. (B) Kaplan-Meier survival curve showed significantly improved survival of the GbG≥10 mice compared with mock/GbG<10 mice at 50 weeks. Survival at 24 weeks is denoted by a dashed vertical line to compare with survival of the GbG mice in the myeloablative transplantation model (supplemental Figure 3c).
Correction of organ pathology in GbG≥10 mice that underwent transplantation after reduced-intensity conditioning and improved overall survival. (A) Representative hematoxylin-eosin–stained sections of a kidney, liver, and spleen of GbG≥10 and GbG<10 mice 48 to 50 weeks after reduced-intensity conditioning transplantation and a 3-month-old BERK control. Image acquisition information is available in supplemental data. (B) Kaplan-Meier survival curve showed significantly improved survival of the GbG≥10 mice compared with mock/GbG<10 mice at 50 weeks. Survival at 24 weeks is denoted by a dashed vertical line to compare with survival of the GbG mice in the myeloablative transplantation model (supplemental Figure 3c).
Hematologic parameters of GbG mice that underwent transplantation following reduced-intensity conditioning
| Time point/mouse type . | n . | WBC, 103/μL . | RBC, 106/μL . | Hb, g/dL . | MCV, fL . | MCH, pg . | RDW, % . | Plt, 103/μL . | Reticulocyte, % . |
|---|---|---|---|---|---|---|---|---|---|
| Week 12 | |||||||||
| Mock | 6 | 21.7 ± 3.8 | 6.1 ± 0.7 | 7.9 ± 0.7 | 46.2 ± 2.3 | 12.5 ± 0.2 | 27.0 ± 1.4 | 839 ± 86 | 53.2 ± 3.9 |
| GbG≥10 | 14 | 32.3 ± 4.6 | 7.4 ± 0.3 | 7.9 ± 0.4 | 41.7 ± 0.7 | 10.7 ± 0.3 | 29.1 ± 0.5 | 737 ± 36 | 20.2 ± 2.5 |
| P* | .6 | .16 | .96 | .12 | .001 | .2 | .3 | .001 | |
| Week 18 | |||||||||
| Mock | 4 | 32.3 ± 4.6 | 5.6 ± 0.1 | 6.8 ± 0.2 | 46.9 ± 1.3 | 12.2 ± 0.3 | 31.4 ± 0.9 | 802 ± 91 | 54.5 ± 2.3 |
| GbG≥10 | 9 | 12.9 ± 1.4 | 7.7 ± 0.4 | 8.9 ± 0.4 | 43.2 ± 1.5 | 11.7 ± 0.6 | 28.8 ± 0.8 | 798 ± 97 | 26 ± 4.1 |
| P† | .01 | .002 | .001 | .09 | .57 | .07 | .97 | .0001 | |
| Week 24 | |||||||||
| Mock | 4 | 34.1 ± 9.4 | 5.6 ± 0.3 | 7.4 ± 0.3 | 48.7 ± 2.4 | 13.2 ± .0.3 | 30.1 ± 1.9 | 780 ± 100 | 50.8 ± 1.9 |
| GbG≥10 | 5 | 13.4 ± 1.1 | 8.1 ± 0.5 | 9.3 ± 0.6 | 43.9 ± 1.2 | 11.3 ± 0.2 | 29.8 ± 1.1 | 764 ± 61 | 21.2 ± 1.9 |
| P‡ | .07 | .003 | .04 | .1 | .002 | .9 | .9 | .001 |
| Time point/mouse type . | n . | WBC, 103/μL . | RBC, 106/μL . | Hb, g/dL . | MCV, fL . | MCH, pg . | RDW, % . | Plt, 103/μL . | Reticulocyte, % . |
|---|---|---|---|---|---|---|---|---|---|
| Week 12 | |||||||||
| Mock | 6 | 21.7 ± 3.8 | 6.1 ± 0.7 | 7.9 ± 0.7 | 46.2 ± 2.3 | 12.5 ± 0.2 | 27.0 ± 1.4 | 839 ± 86 | 53.2 ± 3.9 |
| GbG≥10 | 14 | 32.3 ± 4.6 | 7.4 ± 0.3 | 7.9 ± 0.4 | 41.7 ± 0.7 | 10.7 ± 0.3 | 29.1 ± 0.5 | 737 ± 36 | 20.2 ± 2.5 |
| P* | .6 | .16 | .96 | .12 | .001 | .2 | .3 | .001 | |
| Week 18 | |||||||||
| Mock | 4 | 32.3 ± 4.6 | 5.6 ± 0.1 | 6.8 ± 0.2 | 46.9 ± 1.3 | 12.2 ± 0.3 | 31.4 ± 0.9 | 802 ± 91 | 54.5 ± 2.3 |
| GbG≥10 | 9 | 12.9 ± 1.4 | 7.7 ± 0.4 | 8.9 ± 0.4 | 43.2 ± 1.5 | 11.7 ± 0.6 | 28.8 ± 0.8 | 798 ± 97 | 26 ± 4.1 |
| P† | .01 | .002 | .001 | .09 | .57 | .07 | .97 | .0001 | |
| Week 24 | |||||||||
| Mock | 4 | 34.1 ± 9.4 | 5.6 ± 0.3 | 7.4 ± 0.3 | 48.7 ± 2.4 | 13.2 ± .0.3 | 30.1 ± 1.9 | 780 ± 100 | 50.8 ± 1.9 |
| GbG≥10 | 5 | 13.4 ± 1.1 | 8.1 ± 0.5 | 9.3 ± 0.6 | 43.9 ± 1.2 | 11.3 ± 0.2 | 29.8 ± 1.1 | 764 ± 61 | 21.2 ± 1.9 |
| P‡ | .07 | .003 | .04 | .1 | .002 | .9 | .9 | .001 |
Hematologic parameters and abbreviations as stated in Table 1. P values represent comparisons of mock mice with GbG≥10 at 12 (*), 18 (†), and 24 weeks (‡).
The GbG<10 group of mice showed slight improvement in organ pathology: There was a slight reduction in spleen weight (717 ± 162 mg in GbG<10 vs 870 ± 71 mg in BERK/mock mice; P value, NS). Bone marrow and spleens showed moderate to severe erythroid hyperplasia; livers had infarctions and extramedullary hematopoiesis; and the kidneys showed occasional focal segmental lesions, focal tubular atrophy, and vascular congestion (Table 4).
Organ pathology in GbG mice that underwent transplantation after reduced-intensity transplantation
| Mouse type . | n . | Pathology . | |||
|---|---|---|---|---|---|
| Kidney . | Liver . | Spleen . | Bone marrow . | ||
| BERK/mock | 7 | Mesangial proliferation (2/7), E-M hematopoiesis (7/7), focal ischemic lesion (2/7), cystic kidney dilation (1/7), congestion* (7/7) | E-M hematopoiesis (7/7), 1+ liver infarction (3/7), 3+ liver infarction (4/7) | Weight 870 ± 71 mg, severe erythroid hyperplasia (6/7), moderate erythroid hyperplasia (1/7), obliteration of lymphoid follicles (7/7) | Severe erythroid hyperplasia (6/7), moderate erythroid hyperplasia (1/7) |
| GbG<10 | 4 | Focal segmental lesion (1/4), focal tubular atrophy (1/4), congestion (4/4) | 1+ liver infarction (2/4), 2+ liver infarction (1/4), hemosiderosis (1/4) | Weight 717 ± 162 mg, severe erythroid hyperplasia (1/4), moderate erythroid hyperplasia (2/4), mild erythroid hyperplasia (1/4), occasional lymphoid follicles (2/4) | Mild erythroid hyperplasia (1/3), moderate erythroid hyperplasia (2/3) |
| GbG≥10 | 4 | Mild congestion (1/4), focal tubular atrophy (1/4), normal kidney (2/4) | Normal liver (4/4) | Weight 363 ± 85 mg, mild erythroid hyperplasia (4/4), multiple lymphoid follicles (4/4), congestion (4/4) | Mild erythroid hyperplasia (2/2) |
| Mouse type . | n . | Pathology . | |||
|---|---|---|---|---|---|
| Kidney . | Liver . | Spleen . | Bone marrow . | ||
| BERK/mock | 7 | Mesangial proliferation (2/7), E-M hematopoiesis (7/7), focal ischemic lesion (2/7), cystic kidney dilation (1/7), congestion* (7/7) | E-M hematopoiesis (7/7), 1+ liver infarction (3/7), 3+ liver infarction (4/7) | Weight 870 ± 71 mg, severe erythroid hyperplasia (6/7), moderate erythroid hyperplasia (1/7), obliteration of lymphoid follicles (7/7) | Severe erythroid hyperplasia (6/7), moderate erythroid hyperplasia (1/7) |
| GbG<10 | 4 | Focal segmental lesion (1/4), focal tubular atrophy (1/4), congestion (4/4) | 1+ liver infarction (2/4), 2+ liver infarction (1/4), hemosiderosis (1/4) | Weight 717 ± 162 mg, severe erythroid hyperplasia (1/4), moderate erythroid hyperplasia (2/4), mild erythroid hyperplasia (1/4), occasional lymphoid follicles (2/4) | Mild erythroid hyperplasia (1/3), moderate erythroid hyperplasia (2/3) |
| GbG≥10 | 4 | Mild congestion (1/4), focal tubular atrophy (1/4), normal kidney (2/4) | Normal liver (4/4) | Weight 363 ± 85 mg, mild erythroid hyperplasia (4/4), multiple lymphoid follicles (4/4), congestion (4/4) | Mild erythroid hyperplasia (2/2) |
E-M indicates extramedullary; and 1+ liver infarction, 1 infarction/section.
Congestion of vessels and presence of sickle RBC in vessels. Notably, congested vessels were visible in spleens only when erythroid hyperplasia effacing splenic architecture was reduced. The terminology used to quantify organ pathology is the same as documented in Table 2.
In contrast, a dramatic reversal of organ pathology was seen in GbG≥10 mice: there was a 50% reduction in spleen weight to 363 plus or minus 85 mg, preservation of splenic follicles, and mild erythroid hyperplasia in bone marrow and spleen. Remarkably, no liver infarctions and no kidney pathology were detected, except in one mouse with a single focus of focal tubular atrophy.
Overall, GbG≥10 mice showed correction of organ pathology. The lack of organ pathology in GbG mice at 15 months of age compared with 3-month-old BERK controls demonstrates that gene therapy with the sGbG vector in a reduced-intensity transplantation setting prevents any further organ damage, and the existent organ damage at the time of transplantation probably reverses from regeneration.
Survival.
There was a significant improvement in overall survival in the GbG≥10 mice compared with GbG<10 or mock mice (Figure 5B; P < .05). Indeed, at 24 weeks, survival of the GbG≥10 mice was comparable with survival in mice with approximately 40% HbF in the myeloablative transplantation model that were followed for 24 weeks (supplemental Figure 3). There was some improvement in early survival in GbG<10 mice compared with mock mice (P < .05). However, by 1 year, there was no difference in survival of GbG<10 mice over mock mice.
F cells and HbF/F cell critical for improved RBC survival and correction of SCA
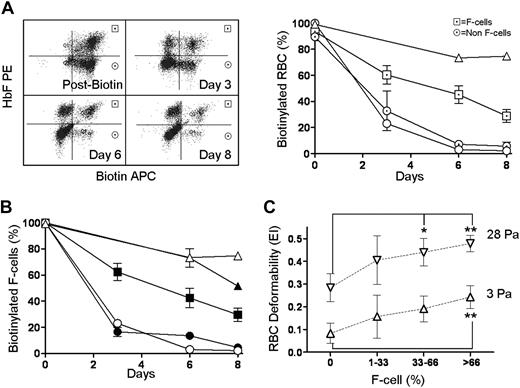
Using biotin surface labeling and intracellular HbF staining, we studied survival of F cells and non-F cells in the same animal, which allowed quantification of the HbF/F cell necessary for improved sickle RBC survival and deformability. F cells showed a selective prolonged survival, as anticipated (Figure 6A). The average HbF/F cell20 in GbG mice in the BERK→C57Bl/6 model was 64% (in these mice, HbF was 41% ± 5%, F cells were 64% ± 6%). In the reduced-intensity transplantation model, GbG≥10 mice had 32% HbF/F cell (in these mice HbF was 21% ± 2%, F cells were 65% ± 14%), and GbG<10 mice had 13% HbF/F cell (HbF, 4% ± 0.1%; F cells, 30% ± 9.4%). Note that GbG mice in the myeloablative model and GbG≥10 mice had similar F-cell repopulation (64%-65%), suggesting that 32% HbF/F cell was sufficient to correct the sickle phenotype. However GbG<10 mice with 13% HbF/F cell and 30% F cells had inconsistent and insignificant amelioration of the disease phenotype.
Effect of HbF, F cells, and percentage HbF/F cell required for functional improvement in RBC survival and deformability. (A) RBC half-life. Left panel shows a representative GbG mouse injected with biotin, with biotin-labeled F cells (upper right quadrants) and non-F cells (lower right quadrants) determined by FACS. Right panel shows survival of F cells (⊡), compared with the non-F cells (⊙) in GbG mice (n = 4); wild-type mice (△); and Berkeley mice (○). (B) A cohort of GbG mice analyzed for RBC survival in vivo, based upon the percentage of HbF/F cell. Each symbol represents a mouse group with HbF percentage and number of mice listed in the adjacent table legend. (C) All GbG and mock mice (n = 34) that were analyzed for RBC deformability were divided into groups based on proportion of F cells: 0%, 1% to 33%, 33% to 66%, and more than 66%, and deformability of total RBC in these mice was plotted at low (3 Pa, △) and high (28 Pa, ▽) shear stress. Significantly improved deformability over mock controls is denoted by *(P < .05) and **(P < .01). Error bars indicate SEM.
Effect of HbF, F cells, and percentage HbF/F cell required for functional improvement in RBC survival and deformability. (A) RBC half-life. Left panel shows a representative GbG mouse injected with biotin, with biotin-labeled F cells (upper right quadrants) and non-F cells (lower right quadrants) determined by FACS. Right panel shows survival of F cells (⊡), compared with the non-F cells (⊙) in GbG mice (n = 4); wild-type mice (△); and Berkeley mice (○). (B) A cohort of GbG mice analyzed for RBC survival in vivo, based upon the percentage of HbF/F cell. Each symbol represents a mouse group with HbF percentage and number of mice listed in the adjacent table legend. (C) All GbG and mock mice (n = 34) that were analyzed for RBC deformability were divided into groups based on proportion of F cells: 0%, 1% to 33%, 33% to 66%, and more than 66%, and deformability of total RBC in these mice was plotted at low (3 Pa, △) and high (28 Pa, ▽) shear stress. Significantly improved deformability over mock controls is denoted by *(P < .05) and **(P < .01). Error bars indicate SEM.
We therefore determined the half-life of F cells in mice grouped by the percentage of HbF/F cell. GbG mice with low (16%; n = 2), intermediate (33%; n = 4), and very high (89%; n = 2) HbF/F cell was injected with biotin and followed by periodic blood sampling. We found mice with low HbF/F cell had no improvement in RBC half-life over BERK controls (Figure 6B), those with 33% HbF/F cell had a 3- to 4-fold improvement in half-life, and mice with very high amounts of HbF/F cell showed RBC survival similar to normal mice. These data demonstrate that if one-third of the hemoglobin within a sickle RBC is HbF, there is significant improvement in RBC survival. Note that mice with these levels of HbF/F cell showed approximately 65% F cells, more than 10% HbF.
To confirm the impact of percentage of circulating F cells on overall RBC deformability, we grouped mice from both the myeloablative and reduced-intensity experiments (n = 34) into 3 groups: mice with less than 33% circulating F cells, 33% to 65% F cells, and 66% or more F cells and measured RBC deformability. Only data from the low (3 Pa) and high (28 Pa) shear rates are plotted in Figure 6C. Mice with more than 66% F cells had a highly significant improvement in RBC deformability at both high and low shear stress (P < .01). Mice with 33% to 66% F cells had significantly improved RBC deformability only at high shear stress (P < .05). Mice with less than 33% F cells showed inconsistent improvement in RBC deformability at low or high shear stress, which was not significantly different from mock controls. These data quantify the critical amount of HbF/F cell, the proportion of F cells, and overall HbF that are necessary for correction of sickle cell disease.
Proportion of transduced HSCs required for phenotypic correction
The proportion of HSCs transduced with sGbG in GbG mice was analyzed by the secondary spleen colony-forming unit (CFU-S) assay performed at 6 months in both models (Figure 7A-B). Bone marrow aspirates were performed at 6 months in the BERK→BERK mice that were followed for 1 year. The proportion of transduced CFU-S's was determined by HbF expression. We have previously shown that all vector-positive CFUs express the transgene in an identical vector that encodes β-globin.13 GbG mice in the myeloablative conditioning group had 16% to 87% sGbG-transduced CFU-S's (average HSC transduction was ∼ 50%), and those in the reduced-intensity group had 5% to 60% transduced HSCs (average HSC transduction was ∼ 30%). It is to be noted that in the reduced-intensity model, HSC transduction is overestimated, secondary to the higher mortality of GbG<10% mice in the first 6 months.
Proportion of transduced HSCs in GbG mice. Proportions in the myeloablative (A) and reduced-intensity (B) transplantation models are shown. The proportion of sGbG-transduced HSCs was determined by spleen colonies (30-36 colonies/mouse) by intracellular staining with HbF and HbS. Each bar represents an individual mouse. (A) In the myeloablative transplantation model, symbols beneath each bar (representing one mouse) are consistent with the symbols in mice labeled in Figures 1 and 2. (B) In the reduced-intensity group, bone marrow was successfully aspirated from 8 mice at 24 weeks and mice were followed for an additional 24 weeks. The HbF expression in peripheral blood by HPLC and bone marrow copy number of the respective mice at 24 weeks are labeled under each bar.
Proportion of transduced HSCs in GbG mice. Proportions in the myeloablative (A) and reduced-intensity (B) transplantation models are shown. The proportion of sGbG-transduced HSCs was determined by spleen colonies (30-36 colonies/mouse) by intracellular staining with HbF and HbS. Each bar represents an individual mouse. (A) In the myeloablative transplantation model, symbols beneath each bar (representing one mouse) are consistent with the symbols in mice labeled in Figures 1 and 2. (B) In the reduced-intensity group, bone marrow was successfully aspirated from 8 mice at 24 weeks and mice were followed for an additional 24 weeks. The HbF expression in peripheral blood by HPLC and bone marrow copy number of the respective mice at 24 weeks are labeled under each bar.
Importantly, 3 mice with 16%, 20%, and 22% transduced CFU-S's had more than 10% HbF (HbF was 20%, 11%, and 18%, respectively) and showed complete phenotypic correction. A vector copy number analysis was performed concurrently at 24 weeks on bone marrow cells and showed 1 to 3 copies/cell and 1 to 2.5 copies/cell in GbG mice that underwent transplantation using the myeloablative conditioning and reduced-intensity conditioning models, respectively. When corrected for HSC transduction, there were 1.5 to 5 vector copies/cell.
Transduction of human CD34+ cells.
The percentage of gene-modified HSCs necessary for effective gene therapy is critical in this disease. In vitro studies on SCA marrow can be done only on a small scale, and would read out correction in progenitors, not HSCs. We have shown HSC correction in humanized models of SCA with long-term analysis. The extremely limited numbers of RBCs we were able to produce from injecting human thalassemia bone marrow CD34+ cells10 are prohibitive for studies on sickling. Therefore, we optimized lentivirus transduction into normal human CD34+ cells for a preclinical scale-up, using a GFP lentivirus vector and the severe combined immunodeficient (SCID)–repopulating assay. Granulocyte colony-stimulating factor–mobilized peripheral blood CD34+ cells transduced with a GFP lentivirus vector were transplanted into nonobese diabetic (NOD)/LtSz-scid IL2Rγnull (NOG) mice. Here, mock mice were those that received a transplant of untransduced CD34+ cells immediately after selection, as controls for the effect of transduction on engraftment and clonogenicity. At 6 weeks, CFUs were plated from bone marrow derived from NOG mice, and 36 individual CFUs/mouse were analyzed for the percentage of gene-marked colonies (supplemental Figure 4). The 18-hour transduction did not affect engraftment or clonogenicity (data not shown). We observed a 77% gene transfer on average in the SCID-repopulating cell assay, similar to our previous data in human thalassemia CD34+ cells.10
Discussion
We show that lentiviral delivery human γ-globin under β-globin regulatory control elements in HSCs results in sufficient postnatal HbF expression to correct SCA in mice. We then de-escalated the amount of HbF and transduced HSCs, using reduced-intensity conditioning and varying MOI, to assess critical parameters needed for correction. A systematic quantification of functional and hematologic RBC indices, organ pathology, and life span were critical to determine the minimal amount of HbF, F cells, HbF/F cell, and gene-modified HSCs required for reversing the sickle phenotype. We show the following: (1) Amelioration of disease occurred when HbF exceeded 10%, F cells constituted two-thirds of the circulating RBCs, and HbF/F cell was one-third of the total hemoglobin in RBCs; and when approximately 20% sGbG-modified HSCs repopulated the marrow. (2) Genetic correction was sustained in primary or secondary transplant recipients followed long-term. (3) There is a method of determining minimum HSC chimerism for correction of a hematopoietic disease in an in vivo model, which would contribute to design of cell dose and conditioning regimens to achieve equivalent genetically corrected HSCs in human clinical trials.
The novel aspect of our study is that it addresses, for the first time, the gene dosage and the gene-modified hematopoietic stem cell dosage required for correction of a genetic defect. Expressing a tremendous amount of fetal/antisickling hemoglobin will undoubtedly correct disease, as has been shown by others,8,9,21 but is not practically possible in a clinical setting. As an example, an initial gene therapy for adenosine deaminase (ADA) deficiency was performed using no conditioning, and was not therapeutic, even though few gene-marked stem cells engrafted, and a selective advantage to gene-corrected lymphocytes was evident upon withdrawal of ADA.22 In a subsequent trial, 4 mg/kg busulfan was used before transplantation, as conditioning, resulting in adequate gene-corrected stem cell dose and gene-modified T cells.23,24 Although these pioneering studies provided us with invaluable information, they underscore the critical importance of determining thresholds for genetic correction before embarking on clinical studies.
Previous laboratories have shown genetic correction of SCA with antisickling globins using myeloablative transplantation models. Pawliuk et al showed correction of marine SCA in 3 mice using a lentivirus encoding βT87Q, a recombinant antisickling β-globin. They demonstrated reduction in ISCs and spleen size after genetic correction in a BERK to C57/BL6 transplantation model.8 Levasseur et al further modified β-globin with 3 mutations (βAS3) and corrected disease in the Townes knockout-transgenic (KO-TG) to C57Bl/6 model.9 Townes KO-TG mice are extremely anemic but showed increased hemoglobin to nearly 80 g/L (8 g/dL) with 2 to 2.5 vector copies/cell, corrected organ pathology, and sustained correction in secondary mice. Both of these studies showed a significant antisickling effect of recombinant β-globins. Recently, Pestina et al showed correction of BERK mice with a γ-globin lentivirus.21 Using the sickle to C57BL/6 transplantation model, we show proof-of-concept studies of correction of SCA with a novel β/γ-hybrid vector and determine functional and quantifiable assays of disease correction. Although we and others correct disease at 1 to 3 copies/cell, we show that the percentage of transduced stem cells in this setting of lethal irradiation/transplantation is very high (average HSCs transduced are 50%, as analyzed by a stringent secondary CFU-S assay). This level of HSC transduction would likely not be achieved in the clinical setting unless myeloablation is performed.
Therefore a novel model (BERK to BERK transplantation) was developed to address the minimal gene transfer needed, and answer questions of correction of SCA in a mouse with significant sickle pathology at 12 weeks of life (Figure 5). Notably, a sickle to normal myeloablative transplantation, used by other groups showing correction of SCD,8,9,21 is a disease prevention model, where there was no underlying pathology at time of transplantation. Our studies show that repair of preexisting pathology can occur, if genetic correction results in more than 10% HbF.
A specific concern of using antisickling β-globins is a potential to illicit an immune response. The long history of chronic transfusions suggests that globin proteins have low immunogenicity. However, development of antibodies against hemoglobin A has been reported in 3 multiply transfused SCA patients, with the epitope directed to the sixth codon.25 This report suggests a single base pair difference between β and βS globin can be immunogenic. How immunogenic endogenous production of recombinant β-globins by erythroid progenitors occurs is an unknown, though given the overall low immunogenicity of globin proteins, immune responses against antisickling β-globins may not occur. In this regard, production of the normally expressed γ-globin would be devoid of any immunologic sequelae. Recently, Samakoglu et al used a lentivirus encoding human γ-globin and a small hairpin RNA (shRNA) against βS-globin and showed reduction in βS-globin mRNA in primary human cells in vitro.26 Whether these changes in MRNA expression resulted in changes in protein expression or functional improvement in sickle RBC was not demonstrated.
Blouin et al generated SAD mice27 transgenic for human γ-globin with 9% to 16% HbF, without significant hematologic improvement.28 Fabry et al produced sickle mice that exclusively produced HbS but varying amounts of HbF and observed significant improvement in hematologic/pathologic features only in the high HbF mice.29 It is to be noted that in transgenic mice, HbF expression is pancellular, whereas in genetic correction of HSCs, there is heterocellular correction. The results from HbF-inducing agents such as hydroxyurea are not entirely applicable to HbF induction via gene therapy, because stress erythropoiesis and RBC macrocytosis occur with hydroxyurea and ameliorate sickling partly from a dilutional effect of HbS. Some comparisons on HSC chimerism can be drawn in patients with partial chimerism after allogeneic transplantation, although these patients have normal RBCs that contain HbA, rather than HbF-containing sickle RBCs amid sickle RBCs.
BERK mice have some degree of thalassemia. Therefore one concern in using this model for genetic therapy studies for sickle cell anemia is that correction of thalassemia would obscure improvements made by the antisickling effects of HbF. Surprisingly we saw no significant change in MCH in GbG<10 or GbG≥10 mice (Table 3), including mice with HbF/F cell as high as 89% (Figure 6). These results were surprising, but showed that the correction of sickling in RBCs was not secondary to correction of thalassemia, as seen in murine thalassemia model,30 where increasing MCH was seen with increases in HbF of 4% or higher.30 Conceivably, HbF is produced at the expense of HbS.
Although BERK mice exclusively carry human hemoglobin, the total hemoglobin in the mouse RBCs is one-third of a human RBC. Therefore, HbF and HbF/F cell were expressed as a percentage, rather than in absolute amounts, to best compare murine data to human. An increase of HbF from 3.6% to 13.6% has been shown to reduce acute sickle events in patients on decitabine.31 Similar improvement in sickle events occurs with 25% or more HbF/F cell in patients responsive to hydroxyurea.32,33 Our data showing improvement with 33% HbF/F cell is concordant with these reports, but more closely resemble RBCs in infants with SCA, where less than one-third HbF/F cell at 10 to 12 months is considered a threshold for intracellular sickle polymerization.34,35
The most remarkable effect of γ-globin production with the sGbG vector was a dramatic absence of chronic organ damage and an improved survival of the sickle mice when HbF exceeded 10%. Patients with high HbF have an improved survival, confirmed by the multicenter study on hydroxyurea.2,36 HbF expressed from the sGbG vector was comparable with, or even better than, effective hydroxyurea treatment. The potential of a one-time correction, where responsiveness to hydroxyurea and compliance to daily life-long administration would not be limiting factors, would be a tremendous advantage of gene therapy. Indeed, we did not anticipate we would get the same conclusion with gene therapy, as derived from collective knowledge on (1) transgenic mice, in which every RBC has the same amount of HbF although we were imposing HbF on SS RBCs28 ; (2) chimeric transplantations, in which normal amounts of HbA-producing RBCs (AA RBC) are present mixed with SS RBCs17,37,38 ; or (3) SCD patients on hydroxyurea, in whom macrocytosis induced by hydroxyurea would dilute HbS and reduce the threshold for sickling. We expected a much higher threshold of genetically corrected sickle HSCs necessary for F-cell repopulation and correction of SCA phenotype, as we were exogenously imposing HbF into a sickle cell with normal amounts of HbS. We were surprised that despite these distinct differences in transgenics/chimeras, our conclusions were similar with exogenous γ-globin expression: Indeed expressing exogenous HbF in RBCs at concentrations from 33% to as high as 89% resulted in no significant increase in MCV or MCH, yet corrected sickling. Although not formally tested, our data suggest that genetic delivery of HbF decreases endogenous HbS.
We show that the percentage of transduced HSCs in the setting of lethal irradiation/transplantation is very high (50% on average, as analyzed by a stringent secondary CFU-S assay at 24 weeks), a number that would be difficult to achieve in a clinical setting. The BERK→BERK transplantation model, however, shows that 20% autologous HSC correction may suffice for a significant amelioration of sickling, organ damage, and survival. However, whether this percentage of gene-modified HSCs necessary for effective gene therapy is achievable is critical to determine, since there is no survival advantage to the gene-modified HSCs in this disease. High human HSC transduction has been a limitation of gene therapy with the traditional gammaretrovirus vectors. Lentivirus vectors can overcome this barrier: a 20% long-term transduction has been shown in adrenoleukodystrophy with a lentivirus vector.39 We optimized lentivirus transduction into human CD34+ cells using the SCID-repopulating cell assay and can achieve approximately 75% gene transfer in SCID-repopulating cell, on average, similar to our previous data in human thalassemia CD34+ cells, where 70% transduction was seen 3 to 4 months after transplantation into immune-deficient mice.10 Notably, this level of gene transfer in the SCID mice is encouraging, and indeed higher than the gene transfer observed in NOD-SCID mice with the adrenoleukodystrophy lentivirus vector in preclinical studies.40
Gene therapy using this approach could also overcome the toxicity and immunologic consequences of the traditional allogeneic bone marrow transplantation/reduced-intensity transplantation.18,41,42 Mismatched mixed chimerism of normal and sickle marrow in murine transplantations shows that a near complete chimerism is typically necessary for correction of organ damage.39,43 It is encouraging that, in a clinical series, reduced-intensity conditioning (RIC) transplantation with 8 mg/kg busulfan along with fludarabine, antithymocyte globulin, and total lymphoid irradiation in SCA patients has shown an average allogeneic engraftment of 78% at 2 to 8.5 years after transplantation, with correction of SCA phenotype.44 This high level of donor chimerism even in an allogeneic RIC setting, where immune rejection can occur, suggests that high gene transfer efficiency into autologous CD34+ cells followed by RIC may be a potentially safer alternative to myeloablative conditioning. We show 77% gene transfer efficiency in human stem/progenitors using a NOD-SCID repopulating cell assay (supplemental Figure 4) and a correction of phenotype in mice with 1.3 to 1.5 copies per cell and approximately 20% gene-marked CFU-Ss (Figure 7). However, whether RIC will be sufficient for genetic correction of SCA in humans remains to be tested in a clinical trial.
Of note, correction occurred at 1 to 3 vector copies per cell, a clinically achievable goal. Flanking the GbG virus with a chromatin insulator may increase HbF/vector copy by 2- to 4-fold.10,13 In experimental models, the insulator appears to reduce clonal dominance, although whether the insulator element lowers the risk of insertional oncogenesis is unknown.45,46 The risk of insertional oncogenesis observed with randomly integrating vectors has been shown to be lower with a lentivirus vector than a gammaretrovirus vector.47 It would be further lowered when the enhancer element is active only in a restricted erythroid lineage.48
In summary, SCA is a common monogenic blood disorder with potentially devastating consequences due to the chronic and episodic disease; has an enormous impact on the health care system, and is associated with a significant reduction in life span. We show that gene therapy with a γ-globin–expressing lentivirus vector offers the potential of a one-time cure and defines parameters needed to cure the disease. We further demonstrate a preclinical in vivo method of determining the minimal amount of genetically corrected hematopoietic stem cells that would be required to correct disease, important in design of clinical gene therapy trials. We are currently studying whether this vector corrects the functional phenotype in human sickle RBCs as effectively as it does in the humanized mouse models of SCA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We gratefully acknowledge the help of Ping Xia with virus production; Anastacia Loberg, Kristy Lauderback, and Gabriel Estavez-Pegani with mouse genotyping and experiments; Rosalinda Wenby with deformability analysis; Lana Wenckbach, Patricia Adkins, and Licheng Zheng with HPLC analysis; and Peter Ciarolo with tonometry. We owe special thanks to Dr Clinton H. Joiner for a critical review of the paper and helpful comments, and Dr Timothy M. Townes for generously providing the sickle knockin mice.
We thank Jamie Wilhelm and the Hematology Repository and Elke Grassman and Diana Nordling in the Translational Core Laboratories, Tom Leemhuis at the Hoxworth Blood Center, and Carrie Stevens in the Translational Research Trials Office for help with obtaining human samples, apheresis, CliniMacs selections, and gene transfer studies in human CD34+ cells.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants U54-HL070595 (P.M., H.J.M.), RO1-HL70135-01 (P.M.), and U54-HL06-008 (P.M.).
National Institutes of Health
Authorship
Contribution: A.P. performed gene transfer and transplantation experiments, analyzed the hematologic and functional parameters, analyzed the data, and wrote the paper; T.H. performed gene transfer and transplantation experiments, molecular analysis on copy numbers, and CFU-S and the NOD-SCID experiments; F.U. cloned the vector backbone; R.F. supervised tonometry analysis; H.J.M. supervised RBC deformability analysis; D.W. analyzed histopathology of mouse organs; and P.M. contributed to the hypothesis and overall study design and experiments, preparation, and editing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Punam Malik, TCHRF 6564, Division of Experimental Hematology, Cincinnati Children's Hospital Medical Center, ML 7013, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: hhpunam.malik@cchmc.org.
References
Author notes
*A.P. and T.H. contributed equally to this work.