Leukocyte extravasation involves interdependent signaling pathways underlying the complex dynamics of firm adhesion, crawling, and diapedesis. While signal transduction by agonist-bound chemokine receptors plays a central role in the above responses, it is unclear how it contributes to the sustained and concurrent nature of such responses, given the rapid kinetics of chemokine-induced trimeric G protein coupling and homologous desensitization. Our findings unveil a novel role of β-arrestins in regulating the activation of signaling pathways underlying discrete integrin-mediated steps in CXCR2-driven leukocyte extravasation. By combining in vivo approaches in β-arrestin knockout mice with in vitro studies in engineered cellular models, we show that membrane-recruited β-arrestin 2 is required for the onset and maintenance of shear stress-resistant leukocyte adhesion mediated by both β1 and β2 integrins. While both β-arrestin isoforms are required for rapid keratinocyte-derived chemokine (KC)–induced arrest onto limiting amounts of vascular cell adhesion molecule-1 (VCAM-1), adhesion strengthening under shear is selectively dependent on β-arrestin 2. The latter synergizes with phospholipase C in promoting activation of Rap1A and B, both of which co-operatively control subsecond adhesion as well as postarrest adhesion stabilization. Thus, receptor-induced Gαi and β-arrestins act sequentially and in spatially distinct compartments to promote optimal KC-induced integrin-dependent adhesion during leukocyte extravasation.
Introduction
Leukocyte extravasation in response to both homeostatic and inflammatory cues is a multistep process involving rapid integrin-mediated arrest to the adluminal side of postcapillary venules, as a prerequisite for both transcellular and paracellular diapedesis across the vascular endothelium.1,–3 Capture of circulating leukocytes and subsequent rolling, primarily mediated by selectins,4 precede and facilitate integrin-dependent firm adhesion, which is dynamically regulated by the interplay of chemokines and adhesion molecules. Circulating leukocytes maintain their integrins in a low-adhesive state that can undergo in situ up-regulation by chemokine-bound Gαi protein-coupled receptors (GPCRs) via cytoplasmic changes (“inside-out” signaling).5,–7 Concurrently, binding of endothelial adhesion molecules stabilizes the active conformation of integrins by “outside-in” signaling, further contributing to the dynamics of leukocyte adhesion under flow.8,9 Adhesion strengthening at the adluminal side of the endothelium in postcapillary venules allows firmly adherent leukocytes to resist hemodynamic shear forces in the order of 0.1 to 1 pN/μm2.10
Signaling cascades originating from chemokine-bound GPCRs orchestrate the complex dynamics of leukocyte adhesion, crawling, and directed migration, that concur in promoting the extravasation of leukocytes.3,7 Several interdependent pathways invoked by chemokine receptor triggering have been shown to effect selected steps in this process. Intriguingly, recent evidence suggests that molecular scaffolds, either preexisting to chemokine stimulation or rapidly induced by GPCR engagement, are required to effect subsecond information transfer between the GPCR and the adhesion receptor.11
Among GPCR regulators, the ubiquitously expressed intracellular proteins β-arrestin 1 (β-arr 1) and β-arrestin 2 (β-arr 2) have been extensively characterized. β-arrestins were originally identified as mediators of agonist-activated GPCR desensitization, leading to the termination of G protein-dependent signals.12 Recent evidence indicates that GPCR-associated β-arrestins may also initiate signal transduction pathways downstream of G protein activation, by scaffolding signaling intermediates into functional complexes.13 Indeed, by acting as adaptors for several signaling molecules, including cytoskeletal regulators,14 Src family kinases (SFKs),15 phosphatidylinositol 3-kinase (PI3K),16 RhoA,17 and mitogen-activated protein kinases (MAPKs) such as extracellular signal-regulated kinases 1 and 2 (ERK1/2),18,19 c-Jun N-terminal kinase (JNK),20 and p38,21 β-arrestins can spatially and temporally control their activity. In agreement with these findings, β-arr 1 and β-arr 2 have emerged as key regulators of cellular processes such as epithelial permeability, cytoskeletal reorganization, and chemotaxis.22,23 Although the above evidence suggests that β-arrestins play a key role in chemokine-dependent events, it is unclear whether they are involved at defined steps in leukocyte extravasation and to what extent the 2 ubiquitous β-arrestins exert redundant functions.
In this study, we addressed the pathophysiologic significance of β-arrestins in keratinocyte-derived chemokine (KC)-induced leukocyte-endothelial interactions implicated in leukocyte transmigration across inflamed blood vessels. By both in vivo and in vitro approaches, we found a novel role of β-arrestins in several discrete steps of CXCR2-driven adhesion dynamics mediated by integrins. By modulating selectively the expression of either β-arr isoform both in vitro and in vivo, we demonstrate a requirement for both β-arr 1 and β-arr 2 in earliest KC-triggered integrin activation events obligatory for leukocyte arrest on diluted endothelial ligands. In addition, we find a unique requirement for β-arr 2 in postarrest adhesion strengthening, which involves activation of the small GTPase Rap1. This β-arr 2–dependent process is necessary for arrested leukocytes to establish prolonged shear force resistance without which subsequent leukocyte diapedesis fails. Collectively, these results implicate β-arrestins as new modulators of GPCR signaling to integrins, acting downstream of chemokine-triggered Gαi activation.
Methods
Reagents and antibodies
Reagents and antibodies used in this study are reported in the online supplemental materials (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Animals
β-Arrestin 2–deficient (β-arr 2−/−) mice (C57BL/6 background) were kindly provided by Robert J. Lefkowitz (Howard Hughes Medical Institute, Duke University Medical Center, Durham, NC). Male β-arr 2−/− and littermate wild-type (WT) mice aged 8 to 10 weeks were used in the study. All experiments were performed with the approval of the local animal protection legislation (Bezirksregierung Muenster).
Intravital microscopy
Surgical preparation of cremaster muscles and intravital microscopy were essentially done as described previously.24,25 Inflammatory stimulation was achieved by superfusion of the cremaster muscle with 6.25 nM KC. In each animal (n = 5 per group of mice), 3 to 5 single unbranched postcapillary venules were analyzed. Leukocyte rolling, adhesion, and emigration were assessed and quantified as described previously.24,25 Leukocyte responses were quantified at 30-minute intervals for 60 minutes.
Cell culture
Rat basophilic leukemia (RBL-2H3) cells were cultured in Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 20% heat-inactivated fetal bovine serum (FBS; Euroclone) and 1% penicillin/streptomycin (Invitrogen). For generation of mouse CXCR2 stable transfectants, RBL-2H3 cells were transfected with the mouse CXCR2 cDNA. Geneticin (G418; Invitrogen)-resistant cells were subcloned and tested for mouse CXCR2 surface expression by flow cytometry. Mouse myeloid 32D cells were maintained undifferentiated in DMEM supplemented with 10% FBS and 10% WEHI conditioned medium as a source of interleukin-3 (IL-3). Differentiation of 32D cells into polymorphonuclear granulocytes was obtained by adding 20 ng/mL G-CSF to the medium in the absence of IL-3 for 10 to 12 days.26
Small interfering RNAs and cDNA constructs
Small interfering RNAs (siRNAs) and cDNA constructs used in this study are reported in the online supplemental materials.
Cell transfection
Both siRNA and plasmid transfection of RBL-2H3 cells was performed by electroporation using the Gene-Pulser system (Bio-Rad Laboratories). siRNA-mediated silencing of differentiated 32D cells was performed by nucleoporation with the Amaxa Nucleofector (Amaxa Biosystems, Lonza Cologne). Expression of β-arrestins was maximally suppressed at 48 hours after siRNA transfection. Endogenous β-arr level was determined by immunoblotting of protein extracts from 7 × 104 cells lysed in reducing 2× sodium dodecyl sulfate (SDS) sample buffer (Laemmli buffer). RBL-2H3 cells transfected with cDNA constructs were used within 36 hours after electroporation. RBL-2H3 transfectants used in functional assays were serum-starved for 3 hours, harvested in phosphate-buffered saline (PBS) supplemented with 4 mM ethylenediaminetetraacetic acid (EDTA)/1 mM ethyleneglycoltetraacetic acid (EGTA), and resuspended in H/H medium (Hank buffered salt solution [HBSS] containing 2 mg/mL bovine serum albumin [BSA] and 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], pH 7.4) supplemented with 1 mM Ca2+/Mg2+, unless differently indicated.
Under flow adhesion assays to vascular cell adhesion molecule-1
Various concentrations of mouse vascular cell adhesion molecule-1 (VCAM-1)/Fc were coimmobilized with either heat-inactivated or intact KC (2 μg/mL) on polystyrene plates as previously described.6 The polystyrene plates were each assembled on the lower wall of the flow chamber (260-μm gap).27 RBL-2H3 transfectants suspended in H/H medium supplemented with 1 mM Ca2+/Mg2+ were perfused through the flow chamber at the desired shear stress. All flow experiments were conducted at 37°C. “Transient” tethers were defined as cells that attached briefly (< 3 seconds) to the substrate; “arrest” (firm) tethers were defined as tethered cells that immediately stopped for at least 3 seconds.28 Frequencies of adhesive categories were determined as a percentage of cells flowing immediately over the substrates.6 All cellular interactions with the adhesive substrates were determined by a MatLab-based cell tracking software (Mathworks).
For analysis of integrin-mediated adhesion strengthening at short stationary contacts, RBL-2H3 transfectants were perfused into the flow chamber and allowed to settle onto the substrate for 1 minute. Flow was then initiated and increased stepwise every 5 seconds through a programmed set of flow rates. At the end of each 5-second interval, the number of cells that remained bound was expressed relative to the number of cells originally settled on the substrate.
Under flow adhesion assays to intercellular adhesion molecule-1
Mouse E-selectin/Fc (1 mg/mL) and various concentrations of mouse intercellular adhesion molecule-1 (ICAM-1)/Fc were coimmobilized with either heat-inactivated or intact KC (2 μg/mL) on 100-μL microcap glass capillary tubes as previously described.29 Cellular interactions were recorded and scored as described.29 Cells arrested for up to 1 second (see Figure 2F-H) or at least 10 seconds (Figure 2I-K) were scored separately and plotted as independent groups.
Confocal microscopy
The method for cell staining is described in the online supplemental materials.
Intracellular calcium mobilization
RBL-2H3 transfectants were loaded with 5 μM Fura-2-acetoxymethyl ester (Fura-2AM) for 40 minutes at 37°C. Changes of calcium-bound Fura-2AM fluorescence in cells (106/2 mL Krebs-Ringer-HEPES [KRH]) stimulated with 0.5 μg/mL KC were monitored at 37°C in a LS 50B spectrofluorometer (Perkin-Elmer). Intracellular calcium concentrations [Ca2+]i were calculated as previously described.30
Rap1 activation assay
RBL-2H3 transfectants were lysed for 7 minutes with ice-cold lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1% Triton X-100, 10 mM MgCl2, complete protease inhibitor cocktail [Roche Diagnostics], phosphatase inhibitor cocktail 1 and 2 [Sigma-Aldrich]). Cleared lysates were incubated for 30 minutes with GST-RalGDS-RBD bound to glutathione-Sepharose 4B beads (Amersham Biosciences, GE Healthcare) and washed 3 times in lysis buffer. GST-RalGDS-RBD pulldowns (active Rap1) and lysates (total Rap1) were resolved by 12% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Hybond C Extra; GE Healthcare). Rap1 levels were detected by immunoblotting with an anti-Rap1 antibody and revealed using the Enhanced Chemoluminescence (ECL) detection reagent plus (GE Healthcare). Immunoblots were quantified by densitometry using the ImageQuant software (GE Healthcare).
ERK/Akt activation assay
The method for assessing ERK/Akt activation is described in the online supplemental materials.
Statistical analysis
Statistical significance was determined by unpaired 2-tailed Student t test with unequal variance. P values less than .05 were considered statistically significant.
Results
β-arr 2–null leukocytes are impaired in KC-induced firm adhesion and diapedesis in vivo
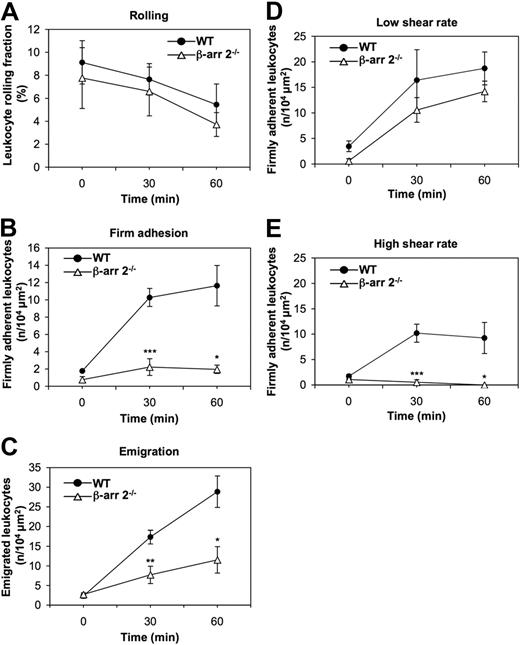
β-Arrestins have recently emerged as key regulators of chemotaxis.23,31,32 As leukocyte-endothelial interactions are controlled by chemokines independently of their role in directional motility, we addressed whether β-arrestins are required at defined steps in chemokine-induced leukocyte adhesion to and motility across endothelial barriers and to what extent the 2 ubiquitous β-arrestins are functionally redundant in these processes. To this aim, we examined the consequence of genetic inactivation of β-arr 2 on leukocyte extravasation by in vivo microscopy in the mouse cremaster muscle model.24 We compared leukocyte rolling (Figure 1A), firm adhesion (Figure 1B), and emigration (Figure 1C) induced by exposure to the proinflammatory chemokine KC in cremasteric postcapillary venules of β-arr 2−/− mice and WT littermates. KC superfusion induced a time-dependent decrease of leukocytes rolling along the vessel wall at defined shear rate conditions (supplemental Table 1), with no significant differences between β-arr 2−/− and control mice (Figure 1A). In contrast, KC-induced firm adhesion of leukocytes in β-arr 2−/− mice was markedly reduced at both 30 and 60 minutes of observation compared with WT littermates (Figure 1B). Consistent with the adhesion response profile, leukocyte transmigration elicited by KC was also defective in β-arr 2−/− animals (Figure 1C). Next, we compared the levels of shear-resistant adhesion strengthening developed by β-arr 2−/− and control leukocytes upon KC exposure in both high- and low-shear rate postcapillary venules. While no significant differences in firm leukocyte adhesion over time were observed in the 2 groups of mice in low-shear rate venules (Figure 1D), the number of leukocytes that developed high resistance to detachment in vessels exhibiting high blood shear flow was markedly reduced in β-arr 2−/− compared with control mice (Figure 1E). Collectively, these results indicate that β-arr 2 contributes selectively to KC-induced adhesion strengthening of leukocytes by finely modulating the establishment of shear-resistant firm adhesion to the endothelium in postcapillary venules.
Defective KC-induced leukocyte firm adhesion and diapedesis in β-arr 2−/− mice. (A-C) Rolling (A), firm adhesion (B), and emigration (C) of WT and β-arr 2−/− leukocytes examined by intravital microscopy of cremaster muscles superfused with 6.25 nM KC. The sequential steps of leukocyte extravasation were analyzed in cremasteric venules at a defined shear rate (WT venule shear rate = 1095.5 ± 118.9 s−1, β-arr 2−/− venule shear rate = 1101.8 ± 26.4 s−1, supplemental Table 1). (D-E) WT and β-arr 2−/− leukocytes were examined for the ability to firmly adhere to either low (D) shear rate venules (WT venule shear rate = 756.2 ± 186.6 s−1, β-arr 2−/− venule shear rate = 716.1 ± 63 s−1) or high (E) shear rate venules (WT venule shear rate = 1506.6 ± 138.6 s−1, β-arr 2−/− venule shear rate = 1516.1 ± 7.1 s−1) by intravital microscopy of cremaster muscles superfused with 6.25 nM KC. Data are expressed as mean ± SEM of either 5 (A-C) or 3 (D-E) mice per group (*P < .05, **P < .01, ***P < .001 for β-arr 2−/− vs WT leukocyte responses at the same experimental time point).
Defective KC-induced leukocyte firm adhesion and diapedesis in β-arr 2−/− mice. (A-C) Rolling (A), firm adhesion (B), and emigration (C) of WT and β-arr 2−/− leukocytes examined by intravital microscopy of cremaster muscles superfused with 6.25 nM KC. The sequential steps of leukocyte extravasation were analyzed in cremasteric venules at a defined shear rate (WT venule shear rate = 1095.5 ± 118.9 s−1, β-arr 2−/− venule shear rate = 1101.8 ± 26.4 s−1, supplemental Table 1). (D-E) WT and β-arr 2−/− leukocytes were examined for the ability to firmly adhere to either low (D) shear rate venules (WT venule shear rate = 756.2 ± 186.6 s−1, β-arr 2−/− venule shear rate = 716.1 ± 63 s−1) or high (E) shear rate venules (WT venule shear rate = 1506.6 ± 138.6 s−1, β-arr 2−/− venule shear rate = 1516.1 ± 7.1 s−1) by intravital microscopy of cremaster muscles superfused with 6.25 nM KC. Data are expressed as mean ± SEM of either 5 (A-C) or 3 (D-E) mice per group (*P < .05, **P < .01, ***P < .001 for β-arr 2−/− vs WT leukocyte responses at the same experimental time point).
β-arr 2 is required for KC-induced very late antigen-4 and β2 integrin functional regulation
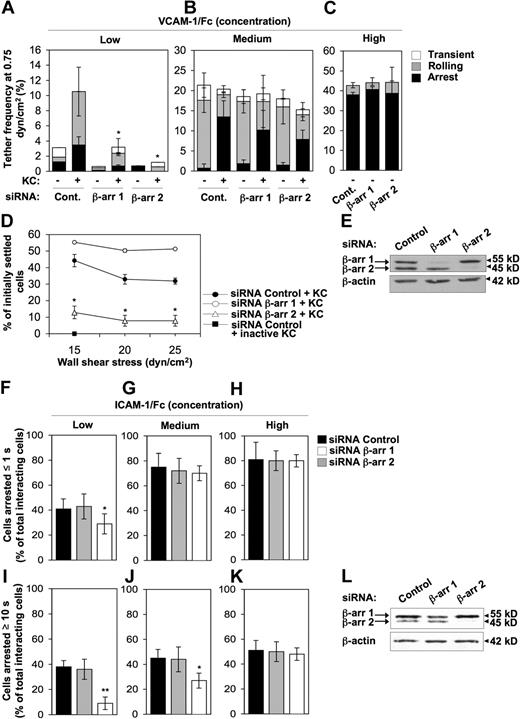
To investigate the requirement for ubiquitous β-arrestins in KC-induced leukocyte adhesion, we developed several cellular models. First, we stably expressed the mouse CXCR2 receptor in the RBL-2H3 myeloid cell line, which has been used extensively in cell migration studies and displays physiologic levels of integrins33 (data not shown). Using such model, we assessed the consequence of selective down-regulation of either β-arr isoform on the ability of KC to trigger in situ very late antigen-4 (VLA-4; α4β1) activation under physiologic shear flow (Figure 2A-C) and to stabilize VLA-4–mediated adhesions against detaching shear forces (Figure 2D). Endogenous expression of β-arrestins was down-regulated by transient siRNA-mediated silencing, which consistently and selectively reduced β-arr 1 and β-arr 2 protein levels to less than 5% of control values (Figure 2E). Both spontaneous and KC-triggered adhesion of CXCR2 expressing (CXCR2+) RBL-2H3 cells to VCAM-1 were first confirmed to be VLA-4–dependent by using a highly specific α4β1 blocker, BIO-1211, which abrogated all cell interactions with VCAM-1 (supplemental Figure 1). To address the specific role of β-arr 1 and β-arr 2 in VLA-4 adhesiveness under shear, we compared β-arr–depleted and control CXCR2+ RBL-2H3 cells for the ability to tether onto increasing concentrations of VCAM-1 (Figure 2A-C). While in the absence of KC siRNA control-treated cells adhered poorly onto a low VCAM-1 concentration, in the presence of immobilized KC in situ subsecond signals induced robust VLA-4-dependent cell interactions with VCAM-1, as shown by the relative increase in rolling and firm arrest (Figure 2A). Both β-arr 2 and β-arr 1 down-regulation markedly decreased KC-triggered RBL-2H3 cells' binding to a low VCAM-1 concentration. Importantly, β-arr depletion did not significantly affect either α4 integrin or CXCR2 surface expression (supplemental Figure 2A). Likewise, neither β-arr isoform was involved in KC-dependent modulation of VLA-4 affinity to soluble VCAM-1 under shear-free conditions (supplemental Figure 3). These results indicate that both β-arr 1 and β-arr 2 are required for optimal KC-mediated activation of VLA-4 adhesiveness to highly diluted VCAM-1. Medium VCAM-1 concentration supported spontaneous, KC-independent VLA-4 adhesions of control RBL-2H3 cells with only a small fraction of cells forming stable adhesions onto the ligand (Figure 2B). At variance with low VCAM-1 concentration, immobilized KC did not enhance the frequency of VLA-4 tethers to medium VCAM-1 but rather converted weak VLA-4 attachments into firm interactions with the ligand, known to require high affinity VLA-4.27,34 Notably, both β-arr 1–depleted and β-arr 2–depleted cells retained spontaneous VLA-4 adhesiveness to a medium VCAM-1 concentration. Down-regulation of either β-arr isoform decreased KC-triggered arrest to a variable extent compared with control cells. Finally, a high VCAM-1 concentration mediated KC- and β-arrestin–independent arrest of RBL-2H3 cells (Figure 2C), consistent with previous findings indicating that α4 integrins can support spontaneous T-cell rolling and firm arrests on high density VCAM-1.35 Collectively, these findings demonstrate that β-arrestins do not affect intrinsic VLA-4 adhesiveness but rather modulate KC-induced formation of adhesive tethers at subsecond contacts, especially under limiting ligand densities. Next, we examined the contribution of β-arrestins to VLA-4 adhesion stabilization under disruptive shear forces. To this aim, we analyzed the resistance of β-arr–depleted or control CXCR2+ RBL-2H3 cells to shear-induced detachment from a medium VCAM-1 concentration coimmobilized with KC (Figure 2D). Interestingly, β-arr 2–depleted RBL-2H3 cells, but not control or β-arr 1–depleted cells, were severely impaired in their ability to develop KC-dependent VLA-4–mediated resistance to detachment by increasing shear forces.
Impaired KC-induced VLA-4 and β2 integrin functional regulation in β-arr 2–depleted cells. (A-C) Effect of either β-arr isoform depletion on frequency and strength of tethers mediated by CXCR2+ RBL-2H3 cells with (A) low (0.025 μg/mL), (B) medium (0.05 μg/mL), or (C) high (0.2 μg/mL) concentrations of VCAM-1/Fc coimmobilized with heat-inactivated (−) or functional (+) KC (2 μg/mL), determined at a shear stress of 0.75 dyn/cm2. Data are expressed as mean plus or minus the range of 2 experimental fields. Results are representative of 3 independent experiments (*P < .05 relative to control cells for each adhesive category). (D) Effect of either β-arr isoform depletion on resistance to detachment by incremental shear stresses of CXCR2+ RBL-2H3 cells. Cells were accumulated on a medium VCAM-1/Fc concentration (0.05 μg/mL) coimmobilized with heat-inactivated or functional KC (2 μg/mL) for 1 minute under a shear stress of 0.75 dyn/cm2 and then subjected to the indicated shear stresses, incremented at 5-second intervals. The percentage of cells resisting detachment by the indicated shear stresses is shown. Results are expressed as mean ± SEM of 4 experimental fields in 2 independent experiments (*P < .05 for β-arr 2–depleted vs control RBL-2H3 cells). (E) Representative immunoblot analysis of endogenous expression of β-arr 1 and β-arr 2 in CXCR2+ RBL-2H3 cells transfected with either control, β-arr 1 or β-arr 2 siRNAs. Whole cell lysates were immunoblotted with a β-arr–specific antibody. Expression of β-actin was used as protein loading control. (F-K) Effect of either β-arr isoform depletion on KC-stimulated adhesion of differentiated 32D cells to (F,I) low (0.1 μg/mL), (G,J) medium (0.5 μg/mL), or (H,K) high (1 μg/mL) concentrations of ICAM-1/Fc under flow. Data are expressed as mean plus or minus the standard deviation (± SD) of 8 to 12 experimental fields in 3 independent experiments (*P < .02, **P < .001 relative to control cells; paired 2-tailed Student t test). (L) Representative immunoblot analysis of endogenous expression of β-arr 1 and β-arr 2 in differentiated 32 D cells transfected with either control, β-arr 1, or β-arr 2 siRNAs. Whole cell lysates were immunoblotted with a β-arr–specific antibody. Expression of β-actin was used as protein loading control.
Impaired KC-induced VLA-4 and β2 integrin functional regulation in β-arr 2–depleted cells. (A-C) Effect of either β-arr isoform depletion on frequency and strength of tethers mediated by CXCR2+ RBL-2H3 cells with (A) low (0.025 μg/mL), (B) medium (0.05 μg/mL), or (C) high (0.2 μg/mL) concentrations of VCAM-1/Fc coimmobilized with heat-inactivated (−) or functional (+) KC (2 μg/mL), determined at a shear stress of 0.75 dyn/cm2. Data are expressed as mean plus or minus the range of 2 experimental fields. Results are representative of 3 independent experiments (*P < .05 relative to control cells for each adhesive category). (D) Effect of either β-arr isoform depletion on resistance to detachment by incremental shear stresses of CXCR2+ RBL-2H3 cells. Cells were accumulated on a medium VCAM-1/Fc concentration (0.05 μg/mL) coimmobilized with heat-inactivated or functional KC (2 μg/mL) for 1 minute under a shear stress of 0.75 dyn/cm2 and then subjected to the indicated shear stresses, incremented at 5-second intervals. The percentage of cells resisting detachment by the indicated shear stresses is shown. Results are expressed as mean ± SEM of 4 experimental fields in 2 independent experiments (*P < .05 for β-arr 2–depleted vs control RBL-2H3 cells). (E) Representative immunoblot analysis of endogenous expression of β-arr 1 and β-arr 2 in CXCR2+ RBL-2H3 cells transfected with either control, β-arr 1 or β-arr 2 siRNAs. Whole cell lysates were immunoblotted with a β-arr–specific antibody. Expression of β-actin was used as protein loading control. (F-K) Effect of either β-arr isoform depletion on KC-stimulated adhesion of differentiated 32D cells to (F,I) low (0.1 μg/mL), (G,J) medium (0.5 μg/mL), or (H,K) high (1 μg/mL) concentrations of ICAM-1/Fc under flow. Data are expressed as mean plus or minus the standard deviation (± SD) of 8 to 12 experimental fields in 3 independent experiments (*P < .02, **P < .001 relative to control cells; paired 2-tailed Student t test). (L) Representative immunoblot analysis of endogenous expression of β-arr 1 and β-arr 2 in differentiated 32 D cells transfected with either control, β-arr 1, or β-arr 2 siRNAs. Whole cell lysates were immunoblotted with a β-arr–specific antibody. Expression of β-actin was used as protein loading control.
To investigate the specific requirement for β-arrestins in KC-induced β2 integrin activation and dynamics, we used the IL-3–dependent mouse 32D cell line, which, upon G-CSF stimulation undergoes polymorphonuclear cell differentiation,26 acquires KC responsiveness, and expresses higher levels of β2 integrins compared with RBL-2H3 cells (supplemental Figure 2B and data not shown). Using G-CSF–induced 32D cells, we examined the effect of selective down-regulation of either β-arr isoform on both rapid onset (up to 1 second, Figure 2F-H) and long-lasting (at least 10 seconds, Figure 2I-K) arrest to increasing ICAM-1 concentrations under shear. In the absence of KC, 32D cells transiently tether and roll but fail to arrest onto ICAM-1/E-selectin cocoated surfaces (data not shown). Conversely, in the presence of immobilized KC, control cells undergo firm arrest onto the ICAM-1–coated surface in a ligand concentration-dependent manner (Figure 2F-K). Importantly, β-arr 2, but not β-arr 1, depletion mediated by siRNAs (achieving more than 90% and 55% down-regulation of the respective proteins, see Figure 2L), significantly inhibited both rapid arrest onto a low ICAM-1 concentration (Figure 2F) and long-lasting arrest onto low and medium ligand concentrations (Figure 2I-J). Notably, this was not due to altered β2 integrin or CXCR2 surface expression (supplemental Figure 2B). These results indicate that, consistent with its role in VLA-4–dependent adhesion, β-arr 2 is required for both β2 integrin-mediated initial arrest and the stabilization of adhesion to limiting ICAM-1 concentration under shear. At present, we cannot rule out that the variable effect of β-arr 1 knockdown on KC-induced VLA-4 versus β2 integrin activation under limiting ligand concentrations (Figure 2A,F) can be accounted for by the reduced efficiency of β-arr 1 silencing in 32D cells, compared with RBL-2H3 cells.
Functional interactions between KC-activated CXCR2 and β-arrestins
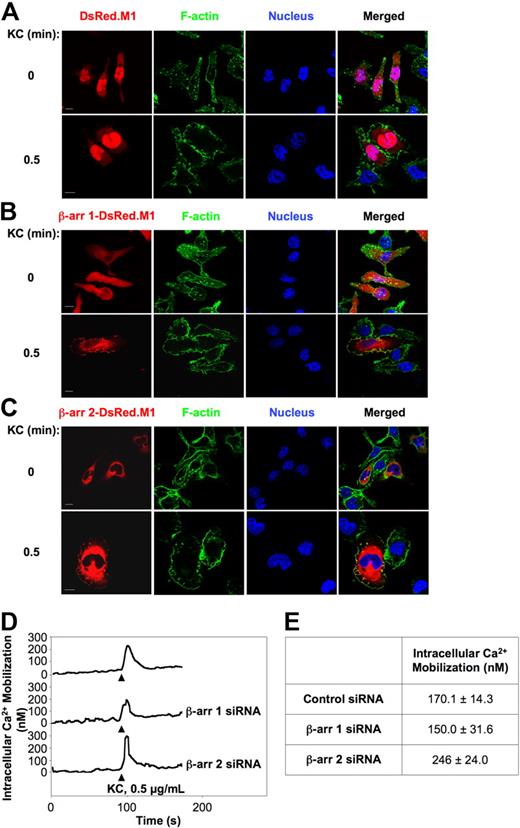
To elucidate the mechanisms underlying the regulatory role of β-arrestins in KC-induced integrin activation, we assessed the spatiotemporal as well as the functional interplay between agonist-stimulated CXCR2 and β-arrestins. To this aim, we first examined by confocal microscopy the time-dependent response to KC of RBL-2H3 cells coexpressing CXCR2 and either β-arr 1–DsRed-monomer (DsRed.M1) or β-arr 2–DsRed.M1 chimeric proteins (Figure 3A-C). In the absence of agonist stimulation β-arr 1–DsRed.M1 was uniformly distributed both in the nucleus and in the cytoplasm, whereas β-arr 2–DsRed.M1, which has a nuclear export sequence, was mainly cytosolic (Figure 3A-C, 0 minutes). Treatment with 0.5 μg/mL KC stimulated rapid and time-dependent membrane ruffling, indicative of cytoskeletal reorganization (Figure 3A-C, 0.5 minutes, and supplemental Figure 4). Importantly, a fraction of both β-arr 1 and β-arr 2, but not DsRed.M1 alone, was observed to localize in ruffling membranes. At later time points, both β-arr isoforms became enriched in endocytic structures (supplemental Figure 4A-C, 30 minutes). These findings support the notion that upon KC stimulation, β-arrestins localize to areas of the plasma membrane that are actively involved in integrin adhesion and in the generation of signals that affect cytoskeletal changes and cell polarization.
Functional interactions between KC-activated CXCR2 and β-arrestins. (A-C) Subcellular localization of β-arr 1 and β-arr 2 in RBL-2H3 cells. RBL-2H3 cells were transiently transfected with CXCR2 along with either DsRed.M1 (A), β-arr 1-DsRed.M1 (B), or β-arr 2–DsRed.M1 (C). Cells were left unstimulated (0 minutes) or stimulated with 0.5 μg/mL KC for the indicated times (0.5 minutes and supplemental Figure 4), fixed, permeabilized, stained, and analyzed by confocal microscopy. Scale bars represent 10 μm. Representative images from one of 4 separate experiments are shown. (D-E) Retained KC-induced calcium transients in either β-arr isoform-depleted CXCR2+ RBL-2H3 cells. (D) Cells were loaded with the calcium indicator Fura 2-AM. Changes in free intracellular calcium levels induced by stimulation with 0.5 μg/mL KC were measured by fluorimetry. Representative curves from one of 3 experiments are shown. (E) Quantitative analysis of KC-induced intracellular calcium mobilization in control and β-arr–depleted CXCR2+ RBL-2H3 cells. Results are expressed as mean ± SEM of 3 independent experiments.
Functional interactions between KC-activated CXCR2 and β-arrestins. (A-C) Subcellular localization of β-arr 1 and β-arr 2 in RBL-2H3 cells. RBL-2H3 cells were transiently transfected with CXCR2 along with either DsRed.M1 (A), β-arr 1-DsRed.M1 (B), or β-arr 2–DsRed.M1 (C). Cells were left unstimulated (0 minutes) or stimulated with 0.5 μg/mL KC for the indicated times (0.5 minutes and supplemental Figure 4), fixed, permeabilized, stained, and analyzed by confocal microscopy. Scale bars represent 10 μm. Representative images from one of 4 separate experiments are shown. (D-E) Retained KC-induced calcium transients in either β-arr isoform-depleted CXCR2+ RBL-2H3 cells. (D) Cells were loaded with the calcium indicator Fura 2-AM. Changes in free intracellular calcium levels induced by stimulation with 0.5 μg/mL KC were measured by fluorimetry. Representative curves from one of 3 experiments are shown. (E) Quantitative analysis of KC-induced intracellular calcium mobilization in control and β-arr–depleted CXCR2+ RBL-2H3 cells. Results are expressed as mean ± SEM of 3 independent experiments.
Next, we addressed whether depletion of β-arrestins perturbed the activation and uncoupling of Gαi protein by CXCR2. To this aim, we compared KC-induced intracellular Ca2+ mobilization in control and β-arr–suppressed CXCR2+ RBL-2H3 cells, as this provides a measure of Gαi protein activation by agonist-engaged receptor. As shown in Figure 3D and E, stimulation with 0.5 μg/mL KC induced a rapid rise in intracellular calcium concentration [Ca2+]i, which peaked within 12 to 15 seconds after activation, both in control and in β-arr–depleted cells. Cells deficient of β-arr 2, but not β-arr 1, displayed a modest but reproducible increase of [Ca2+]i peak levels, along with a slightly prolonged response to KC compared with controls. Finally, KC-induced internalization of CXCR2 was marginally (< 5%) affected by down-regulation of either β-arr isoform (data not shown). Collectively, these results suggest that β-arrestins play a signaling role in KC-driven adhesion that is largely independent of their established involvement in the modulation of G-protein coupling and receptor desensitization.
Depletion of β-arr 2 selectively inhibits KC-induced activation of Rap-1
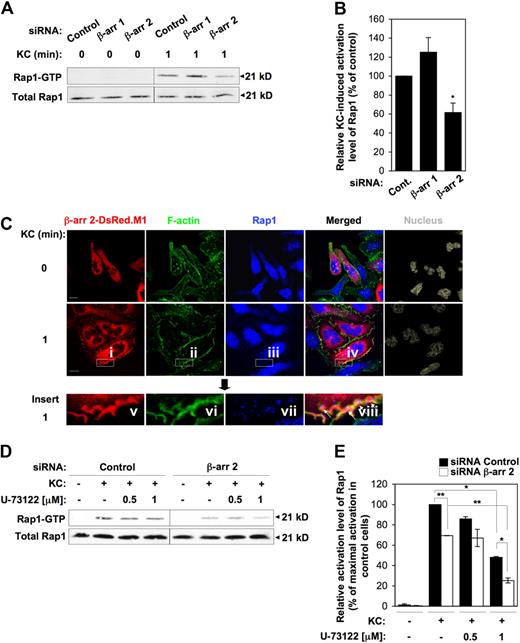
To gain further insight into the mechanistic role of β-arr 1 and β-arr 2 in regulating leukocyte adhesion dynamics, we examined their requirement in KC-driven signaling pathways controlling cell adhesion and motility. Since the small GTPase Rap1 has emerged as a key regulator of integrin function, actin cytoskeletal dynamics, and cell adhesion,36,37 we tested whether β-arrestins were required for KC-induced activation of Rap1. To this aim, we assessed the fraction of GTP-bound Rap1 in control versus chemokine-stimulated cells using an affinity pull-down assay with the Ras-binding domain (RBD) of Ral-GDS. Stimulation of CXCR2+ RBL-2H3 cells with 0.5 μg/mL KC triggered a rapid and time-dependent activation of Rap1, which peaked within 1 minute upon activation but was still evident at 2 minutes after stimulation (supplemental Figure 5). Importantly, suppression of β-arr 2 reproducibly inhibited KC-induced Rap1 activation by approximately 40% compared with control cells (Figure 4A-B). Thus, in parallel with the aforementioned findings demonstrating a specific role of β-arr 2 in leukocyte adhesion strengthening under shear flow, these data indicate that β-arr 2 is also selectively required for Rap1 activation in response to KC. Notably, both responses appear to be enhanced, although not to a statistically significant level, by selective depletion of β-arr 1, in keeping with prior observations indicating that endogenous β-arr 1 may interfere with signaling responses selectively requiring β-arr 2 by competing for receptor association.38 The functional interaction between β-arr 2 and Rap1 is further supported by the experiments reported in Figure 4C, showing that at early time points, KC stimulation leads to relocalization of a fraction of endogenous Rap1 to F-actin–enriched membrane ruffles, where it colocalizes with β-arr 2.
Defective Rap1 activation by KC in β-arr 2–depleted cells. (A,B) Effect of either β-arr isoform depletion on Rap1 activation by KC. (A) Cells were stimulated with 0.5 μg/mL KC for 1 minute. Rap1-GTP was determined in pull down assays with the effector protein GST-RalGDS-RBD and detected by immunoblotting with a Rap1-specific antibody. Total Rap1 levels in whole cell lysates were used as loading control. (B) Quantitative analysis of KC-induced Rap1 activation in control and β-arr–depleted CXCR2+ RBL-2H3 cells. Rap1-GTP levels were quantified by densitometry and normalized both to the total Rap1 and to the GST-RalGDS-RBD signals. Values represent the percentage of normalized Rap1-GTP levels relative to control siRNA-transfected cells. Data are expressed as mean ± SEM of at least 8 independent experiments (*P < .001 for β-arr 2–depleted vs control RBL-2H3 cells). (C) Subcellular localization of Rap1 in RBL-2H3 cells. RBL-2H3 cells were transiently transfected with CXCR2 along with β-arr 2–DsRed.M1. Cells were left unstimulated (0 minutes) or stimulated with 0.5 μg/mL KC for 1 minute, fixed, permeabilized, stained, and analyzed by confocal microscopy. Boxed inserts i-iv represent areas enlarged in images v-viii. Arrows indicate colocalization of Rap1, β-arr 2, and F-actin in ruffling membranes. Scale bars represent 10 μm. Representative images from one of 4 separate experiments are shown. (D-E) Additive effects of PLC inhibition and β-arr 2 depletion on Rap1 activation. CXCR2+ RBL-2H3 transfectants were pretreated for 30 minutes at 37°C with either vehicle control or increasing concentrations of U-73122 (0.5 and 1 μM) before addition of 0.5 μg/mL KC for 1 minute. Rap1-GTP was determined in pull down assays and quantified as described above. Quantitated values represent the percentage of normalized Rap1-GTP levels relative to vehicle-treated control cells stimulated with KC. Results are expressed as mean ± SEM of 4 independent experiments (*P < .05; **P < .005).
Defective Rap1 activation by KC in β-arr 2–depleted cells. (A,B) Effect of either β-arr isoform depletion on Rap1 activation by KC. (A) Cells were stimulated with 0.5 μg/mL KC for 1 minute. Rap1-GTP was determined in pull down assays with the effector protein GST-RalGDS-RBD and detected by immunoblotting with a Rap1-specific antibody. Total Rap1 levels in whole cell lysates were used as loading control. (B) Quantitative analysis of KC-induced Rap1 activation in control and β-arr–depleted CXCR2+ RBL-2H3 cells. Rap1-GTP levels were quantified by densitometry and normalized both to the total Rap1 and to the GST-RalGDS-RBD signals. Values represent the percentage of normalized Rap1-GTP levels relative to control siRNA-transfected cells. Data are expressed as mean ± SEM of at least 8 independent experiments (*P < .001 for β-arr 2–depleted vs control RBL-2H3 cells). (C) Subcellular localization of Rap1 in RBL-2H3 cells. RBL-2H3 cells were transiently transfected with CXCR2 along with β-arr 2–DsRed.M1. Cells were left unstimulated (0 minutes) or stimulated with 0.5 μg/mL KC for 1 minute, fixed, permeabilized, stained, and analyzed by confocal microscopy. Boxed inserts i-iv represent areas enlarged in images v-viii. Arrows indicate colocalization of Rap1, β-arr 2, and F-actin in ruffling membranes. Scale bars represent 10 μm. Representative images from one of 4 separate experiments are shown. (D-E) Additive effects of PLC inhibition and β-arr 2 depletion on Rap1 activation. CXCR2+ RBL-2H3 transfectants were pretreated for 30 minutes at 37°C with either vehicle control or increasing concentrations of U-73122 (0.5 and 1 μM) before addition of 0.5 μg/mL KC for 1 minute. Rap1-GTP was determined in pull down assays and quantified as described above. Quantitated values represent the percentage of normalized Rap1-GTP levels relative to vehicle-treated control cells stimulated with KC. Results are expressed as mean ± SEM of 4 independent experiments (*P < .05; **P < .005).
Because β-arr 2 depletion only partially inhibits KC-induced Rap1 activation, we addressed the interdependence of β-arr 2 with known signaling pathways linking chemokine stimulation to the activation of Rap1. The trimeric G protein effector phospholipase C (PLC) is a major determinant of Rap1 activation downstream of several chemokine receptors, acting via the Rap1 guanine exchange factor (GEF) CalDAG-GEFI.11 Consistently, the PLC inhibitor U-73122 induced a dose-dependent inhibition of Rap1 activation by KC in CXCR2+ RBL-2H3 cells (Figure 4D-E and data not shown). To assess if the β-arr 2 and PLC pathways are functionally interdependent, we combined depletion of β-arr 2 with U-73122 treatment before assessing KC-induced Rap1 activation. Figure 4D and E shows that a strong, additive inhibition of Rap1 activity is achieved when the 2 treatments are combined. KC-induced membrane recruitment of β-arr 2 was unaffected by U-73122 treatment (supplemental Figure 6), further suggesting that β-arr 2 and PLC act independently to affect Rap1 activation upon KC stimulation.
Rap1 is essential for both KC-induced VLA-4 activation and the stabilization of integrin-dependent adhesion under flow
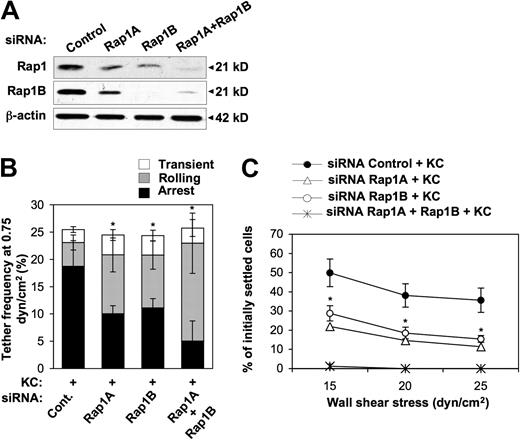
Rap1 is a Ras family member that exists in 2 ubiquitously expressed isoforms, Rap1A and Rap1B, sharing 95% amino acid identity, but being encoded by separate genes.39 To independently assess the functional role of the 2 Rap1 isoforms in VLA-4–mediated leukocyte adesion, we examined the consequence of their selective down-regulation both on rapid onset adhesion (Figure 5B) and on adhesion stabilization (Figure 5C) under shear flow in KC-stimulated cells. Endogenous expression of Rap1A and Rap1B was down-regulated by RNA interference (RNAi), which effectively reduced Rap1 levels (Figure 5A). Down-regulation of either Rap1A or Rap1B significantly impaired the ability of CXCR2+ RBL-2H3 cells to arrest onto a medium VCAM-1 concentration (Figure 5B). Likewise, we observed a marked suppression of resistance to shear-induced detachment from medium VCAM-1 concentration after depletion of each Rap1 isoform (Figure 5C). Both rapid onset adhesion and resistance to detachment were more significantly impaired by the combined depletion of both Rap1 isoforms (Figure 5B-C), suggesting that they act cooperatively to control multiple steps in chemokine-induced integrin activation.
Reduced KC-triggered rapid arrest and shear-resistant adhesion to VCAM-1 of Rap1-depleted cells. (A) Representative immunoblot analysis of endogenous expression of Rap1A and Rap1B in CXCR2+ RBL-2H3 cells transfected with either control, Rap1A, Rap1B, or both siRNAs. Whole cell lysates were immunoblotted with total Rap1A/B- and Rap1B-specific antibodies. Expression of β-actin was used as protein loading control. (B) Effect of either or both Rap1 isoform depletion on frequency and strength of tethers mediated by CXCR2+ RBL-2H3 cells with a medium concentration (0.05 μg/mL) of VCAM-1/Fc coimmobilized with heat-inactivated (data not shown) or functional KC (2 μg/mL) under flow conditions. Data are expressed as mean ± SD of 4 experimental fields in 2 independent experiments (*P < .05 relative to control cells for the arrest fraction). Results are representative of 3 independent experiments. (C) Effect of either or both Rap1 isoform depletion on KC-induced shear-resistant adhesion of CXCR2+ RBL-2H3 cells to a medium VCAM-1/Fc concentration (0.05 μg/mL), determined as described in the legend to Figure 2D. Results are expressed as mean ± SEM of 4 experimental fields in 2 independent experiments (*P < .05 for either or both Rap1 isoform–depleted vs control RBL-2H3 cells).
Reduced KC-triggered rapid arrest and shear-resistant adhesion to VCAM-1 of Rap1-depleted cells. (A) Representative immunoblot analysis of endogenous expression of Rap1A and Rap1B in CXCR2+ RBL-2H3 cells transfected with either control, Rap1A, Rap1B, or both siRNAs. Whole cell lysates were immunoblotted with total Rap1A/B- and Rap1B-specific antibodies. Expression of β-actin was used as protein loading control. (B) Effect of either or both Rap1 isoform depletion on frequency and strength of tethers mediated by CXCR2+ RBL-2H3 cells with a medium concentration (0.05 μg/mL) of VCAM-1/Fc coimmobilized with heat-inactivated (data not shown) or functional KC (2 μg/mL) under flow conditions. Data are expressed as mean ± SD of 4 experimental fields in 2 independent experiments (*P < .05 relative to control cells for the arrest fraction). Results are representative of 3 independent experiments. (C) Effect of either or both Rap1 isoform depletion on KC-induced shear-resistant adhesion of CXCR2+ RBL-2H3 cells to a medium VCAM-1/Fc concentration (0.05 μg/mL), determined as described in the legend to Figure 2D. Results are expressed as mean ± SEM of 4 experimental fields in 2 independent experiments (*P < .05 for either or both Rap1 isoform–depleted vs control RBL-2H3 cells).
β-arr–dependent early activation of PI3K and ERK1/2 MAPKs is not involved in rapid activation of VLA-4 adhesiveness by KC
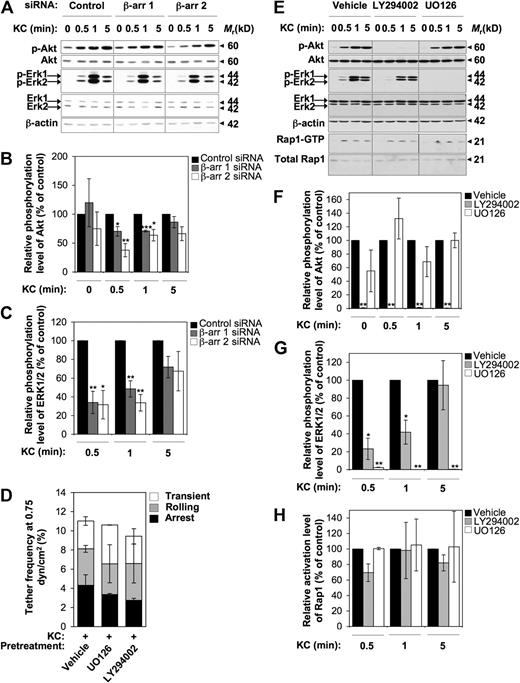
To further dissect the β-arr–dependent signaling pathways regulating integrin-mediated cell adhesion, we examined the functional contribution of either β-arr isoform to KC-induced activation of PI3K and ERK1/2 MAPKs, as both of these pathways are involved in chemokine-driven directional motility.9 As shown in Figure 6A, treatment of control CXCR2+ RBL-2H3 cells with 0.5 μg/mL KC induced a rapid and transient activation of both ERK1/2 and Akt, a downstream effector of PI3K, as determined by the use of phospho-specific antibodies. Importantly, suppression of either β-arr isoform resulted in a time-dependent inhibition of both ERK1/2 and Akt (Figure 6A-C). Interestingly, maximal inhibition was observed at early time points after KC exposure (30 seconds), while the activation profile of both kinases was not significantly affected by β-arr depletion at later time points after activation. However, the activation of PI3K and ERK1/2 does not appear to be involved in the early steps of CXCR2-mediated integrin activation in our model, as shown by the failure of either the specific MAPK inhibitor U0126 or the PI3K inhibitor LY294002 to modulate KC-triggered rapid adhesive interactions to low VCAM-1 concentrations (Figure 6D). Finally, by using U0126 and LY294002 in CXCR2+ RBL-2H3 cells stimulated by KC at various time points, we assessed whether CXCR2-triggered activation of Rap1 is downstream of PI3K and MAPK signaling pathways (Figure 6E-H). Notably, while treatment with U0126 did not significantly alter KC-induced activation of Akt, PI3K inhibition reduced KC-triggered activation of ERK1/2 MAPKs at early time points, suggesting that ERK1/2 activity is at least partially distal to PI3K in CXCR2-triggered signaling (Figure 6E-G). Importantly, neither ERK1/2 nor PI3K inhibition impaired Rap1 activation by KC at any experimental time point (Figure 6E,H), indicating that KC-triggered Rap1 activation occurs independently of the above signaling pathways. Consistently, the ability of KC-stimulated CXCR2+ RBL-2H3 cells to resist shear-induced detachment from VCAM-1 was unaffected upon either U0126 or LY294002 treatment (data not shown).
KC-triggered rapid activation of VLA-4 adhesiveness does not involve β-arr–dependent early activation of PI3K and ERK1/2 MAPKs. (A-C) Effect of either β-arr isoform depletion on KC-induced activation of Akt and ERK1/2. (A) Cells were stimulated with 0.5 μg/mL KC for the indicated times. Phosphorylated Akt (p-Akt) and phosphorylated ERK1/2 (p-ERK1/2) were detected by immunoblotting with phospho-specific antibodies. The levels of total Akt, ERK1/2, and β-actin in whole-cell lysates were used as protein loading control. (B-C) Quantitative analysis of KC-stimulated activation of Akt (B) and ERK1/2 (C) in control and β-arr–depleted CXCR2+ RBL-2H3 cells. The levels of p-Akt and p-ERK1/2 were quantified by densitometry and normalized to the total Akt and ERK1/2, respectively. Values at each time point represent the percentage of normalized p-Akt (B) and p-Erk1/2 (C) compared with siRNA control-transfected cells. Data are expressed as mean ± SEM of 3 independent experiments (*P < .05, **P < .01, ***P < .001 relative to control RBL-2H3 cells at the same time point). (D) Effect of inhibitors of PI3K and MAPKs on frequency and strength of tethers mediated by CXCR2+ RBL-2H3 cells with a low concentration (0.025 μg/mL) of VCAM-1/Fc coimmobilized with heat-inactivated (data not shown) or functional KC (2 μg/mL) under flow conditions. Data are expressed as mean plus or minus the range of 2 experimental fields. Results are representative of 3 independent experiments. (E-H) Effect of inhibitors of PI3K and MAPKs on KC-induced activation of Akt, ERK1/2, and Rap1. (E) CXCR2+ RBL-2H3 cells were pretreated for 30 minutes at 37°C with either vehicle control, 15 μM LY294002, or 10 μM U0126 before addition of 0.5 μg/mL KC for the indicated times. p-Akt and p-ERK1/2 were detected as described above. Rap1-GTP was determined in pull down assays as described in the legend to Figure 4A. (F-H) Quantitative analysis of KC-stimulated activation of Akt (F), ERK1/2 (G), and Rap1 (H) in vehicle- and inhibitor-treated CXCR2+ RBL-2H3 cells. The levels of p-Akt and p-ERK1/2 were quantified as described above. The levels of Rap1-GTP were quantified as described in the legend to Figure 4B. Values at each time point represent the percentage of normalized p-Akt (F), p-ERK1/2 (G), and Rap1-GTP (H) relative to vehicle-treated cells. Data are expressed as mean ± SEM of 3 independent experiments (*P < .05, **P < .01 relative to vehicle-treated RBL-2H3 cells at the same time point).
KC-triggered rapid activation of VLA-4 adhesiveness does not involve β-arr–dependent early activation of PI3K and ERK1/2 MAPKs. (A-C) Effect of either β-arr isoform depletion on KC-induced activation of Akt and ERK1/2. (A) Cells were stimulated with 0.5 μg/mL KC for the indicated times. Phosphorylated Akt (p-Akt) and phosphorylated ERK1/2 (p-ERK1/2) were detected by immunoblotting with phospho-specific antibodies. The levels of total Akt, ERK1/2, and β-actin in whole-cell lysates were used as protein loading control. (B-C) Quantitative analysis of KC-stimulated activation of Akt (B) and ERK1/2 (C) in control and β-arr–depleted CXCR2+ RBL-2H3 cells. The levels of p-Akt and p-ERK1/2 were quantified by densitometry and normalized to the total Akt and ERK1/2, respectively. Values at each time point represent the percentage of normalized p-Akt (B) and p-Erk1/2 (C) compared with siRNA control-transfected cells. Data are expressed as mean ± SEM of 3 independent experiments (*P < .05, **P < .01, ***P < .001 relative to control RBL-2H3 cells at the same time point). (D) Effect of inhibitors of PI3K and MAPKs on frequency and strength of tethers mediated by CXCR2+ RBL-2H3 cells with a low concentration (0.025 μg/mL) of VCAM-1/Fc coimmobilized with heat-inactivated (data not shown) or functional KC (2 μg/mL) under flow conditions. Data are expressed as mean plus or minus the range of 2 experimental fields. Results are representative of 3 independent experiments. (E-H) Effect of inhibitors of PI3K and MAPKs on KC-induced activation of Akt, ERK1/2, and Rap1. (E) CXCR2+ RBL-2H3 cells were pretreated for 30 minutes at 37°C with either vehicle control, 15 μM LY294002, or 10 μM U0126 before addition of 0.5 μg/mL KC for the indicated times. p-Akt and p-ERK1/2 were detected as described above. Rap1-GTP was determined in pull down assays as described in the legend to Figure 4A. (F-H) Quantitative analysis of KC-stimulated activation of Akt (F), ERK1/2 (G), and Rap1 (H) in vehicle- and inhibitor-treated CXCR2+ RBL-2H3 cells. The levels of p-Akt and p-ERK1/2 were quantified as described above. The levels of Rap1-GTP were quantified as described in the legend to Figure 4B. Values at each time point represent the percentage of normalized p-Akt (F), p-ERK1/2 (G), and Rap1-GTP (H) relative to vehicle-treated cells. Data are expressed as mean ± SEM of 3 independent experiments (*P < .05, **P < .01 relative to vehicle-treated RBL-2H3 cells at the same time point).
Discussion
Shear-resistant adhesion to the endothelium and diapedesis are integrin-mediated events that direct efficient leukocyte trafficking. Sustained and dynamic control of integrin-mediated adhesion involves concurrent signals originating from chemokine-bound GPCRs and ligand-engaged integrins.3,8,9 β-Arrestins have been extensively characterized as GPCR activity regulators12 and have been recently implicated in the modulation of chemotaxis.23 However, their specific involvement in the distinct steps of leukocyte extravasation, including the paradigmatic adhesion cascade,3 has not been previously investigated.
In the present study, we addressed the role of β-arrestins in the higher order response of leukocytes to the inflammatory chemokine KC, both in vivo and in vitro under shear flow conditions. Our results show that β-arr 2 is selectively required to modulate the strengthening of KC-induced adhesive interactions with the endothelium in high shear postcapillary venules. The in vivo results in β-arr 2−/− mice were confirmed by the in vitro findings, which addressed the induction of both β1 and β2 integrin–dependent adhesive interactions by KC under shear flow conditions. Using cellular models, we show that β-arr 2, but not β-arr 1, depletion affects selectively the ability of arrested cells to resist detachment from VCAM-1 or ICAM-1 under shear, a finding that is consistent with the in vivo observation in β-arr 2−/− mice. As a consequence of defective shear-resistant firm adhesion, leukocyte transmigration is impaired. Consistent with these findings, previous studies indicate that β-arr 2−/− lymphocytes fail to accumulate in mouse airways in an in vivo model of allergic asthma and are defective in in vitro chemotaxis to CXCL12.31,32 However, another study showed increased CXCR2-mediated recruitment of β-arr 2−/− neutrophils to sites of inflammation using the air pouch model.40 To reconcile this evidence with our findings, we argue that the requirement for β-arr in leukocyte transendothelial migration may vary depending on the inflammatory milieu. Further, as different agonists (macrophage-inflammatory protein-2 [MIP-2] and KC) activate CXCR2 in the 2 experimental models, their downstream consequences cannot be directly compared as, according to recent evidence, they might stabilize distinct GPCR active conformations, thus eliciting different downstream cell responses.41 Although our in vivo data do not formally rule out that β-arr 2−/− endothelial cells may affect leukocyte extravasation, our in vitro findings show that leukocyte β-arr 2 does play a cell autonomous role in controlling KC-induced adhesion to integrin ligands.
Stable adhesion under flow involves rapid inside-out activation of integrin combined with postligand binding mechanisms leading to adhesion strenghtening.8,42 Defective KC-triggered rapid VLA-4 and β2 integrin adhesiveness observed in β-arr 2–depleted cells under limiting endothelial ligand concentrations, might be due to impaired chemokine-driven integrin clustering, as previously shown for lymphocyte function-associated antigen-1 (LFA-1) avidity modulation.5 Impaired KC-triggered rapid arrest onto highly diluted VCAM-1 is similarly observed in β-arr 1–depleted cells, suggesting that β-arr 1 and β-arr 2 may cooperatively control signaling pathways leading to rapid VLA-4 activation by CXCR2 engagement (Figure 7).
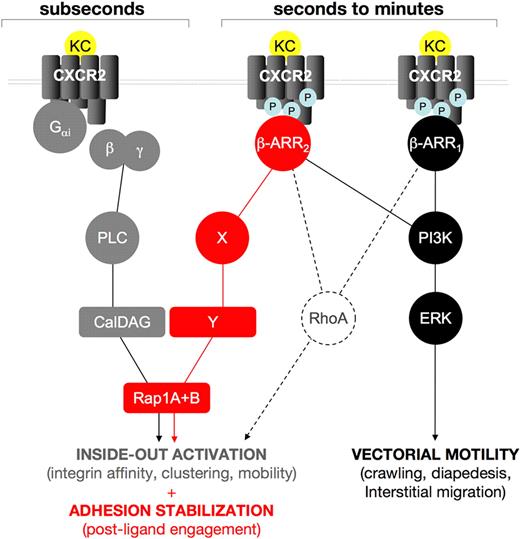
A working model for the role of β-arrestins in CXCR2-driven responses. Our findings support the hypothesis that β-arrestins act sequentially to Gαi-driven signaling in regulating CXCR2-driven responses. Rapid onset (subsecond) adhesion requires Rap1 activity, cooperatively induced by Gαi-triggered PLC, via the CalDAG GEF (gray lines and symbols) and by CXCR2-associated β-arrestins 1 and 2. A plausible (dashed lines) intermediate in the β-arr–dependent component of KC-driven rapid onset adhesion is RhoA, as it is involved in inside-out activation of integrins43 and has been previously shown to be induced by β-arr signaling in several model systems.17,44 Later events (seconds to minutes after agonist binding) in KC-induced leukocyte extravasation are differentially affected by the 2 β-arr isoforms, which become independently associated to phosphorylated CXCR2; both β-arr isoforms are involved in triggering a PI3K-ERK1/2 cascade that may control leukocyte motility during the crawling, diapedesis, and interstitial migration steps (black lines and symbols). Conversely, β-arr 2 specifically controls a postintegrin binding Rap1 activation step, which is involved in the stabilization of integrin-dependent adhesion, (red lines and symbols). Intermediates in this pathway, including potential Rap1 GEFs, have yet to be identified.
A working model for the role of β-arrestins in CXCR2-driven responses. Our findings support the hypothesis that β-arrestins act sequentially to Gαi-driven signaling in regulating CXCR2-driven responses. Rapid onset (subsecond) adhesion requires Rap1 activity, cooperatively induced by Gαi-triggered PLC, via the CalDAG GEF (gray lines and symbols) and by CXCR2-associated β-arrestins 1 and 2. A plausible (dashed lines) intermediate in the β-arr–dependent component of KC-driven rapid onset adhesion is RhoA, as it is involved in inside-out activation of integrins43 and has been previously shown to be induced by β-arr signaling in several model systems.17,44 Later events (seconds to minutes after agonist binding) in KC-induced leukocyte extravasation are differentially affected by the 2 β-arr isoforms, which become independently associated to phosphorylated CXCR2; both β-arr isoforms are involved in triggering a PI3K-ERK1/2 cascade that may control leukocyte motility during the crawling, diapedesis, and interstitial migration steps (black lines and symbols). Conversely, β-arr 2 specifically controls a postintegrin binding Rap1 activation step, which is involved in the stabilization of integrin-dependent adhesion, (red lines and symbols). Intermediates in this pathway, including potential Rap1 GEFs, have yet to be identified.
Mechanistically, β-arrestins have been implicated in the control of directional migration both as regulators of GPCR desensitization, based on the hypothesis that desensitization and recycling of chemokine-receptors are essential for chemotaxis, and as scaffold intermediates in signaling cascades leading to directional motility.23 While in our model β-arr depletion has minimal consequences on CXCR2 internalization (data not shown), it does lead to impaired signal transduction by agonist-engaged CXCR2. Such an impairment appears to be downstream of Gαi activation, as KC-induced [Ca2+]i transients are not impaired by β-arr depletion. As previously described for other GPCRs,23,45 in this study we observe partial impairment of CXCR2-induced ERK1/2 and Akt activation, particularly at early time points after stimulation, by interfering with either isoform of β-arr. However, chemical inhibition of either PI3K or ERK1/2 does not affect the functional activation of VLA-4 by KC under flow, suggesting that the above signaling pathways may be involved at later stages in the leukocyte extravasation process, as previously suggested.9,19,23 Conversely, the activation of the small GTPase Rap1 downstream of CXCR2 stimulation is selectively affected by depletion of β-arr 2, as is the strengthening of adhesion under shear stress, both in vivo and in vitro. The selective involvement of β-arr 2 in the above processes is further underscored by the finding that down-regulation of β-arr 1 results in enhanced, even though not statistically significant, Rap1 activity as well as higher resistance to detachment from VCAM-1 upon KC stimulation. Prior reports have actually shown that endogenous β-arr 1 may interfere with signaling responses selectively requiring β-arr 2, possibly by competing for receptor association.38
Existing evidence indicates that Rap1 leads to an increase of integrin function either by inducing a conformational change or by promoting clustering of integrins, 2 nonexclusive modes of integrin activation.37,46 Importantly, the effect of Rap1 on integrin function occurs with both β1 and β2 integrins.36,47 Prior studies have demonstrated that a major pathway leading to chemokine-induced activation of Rap1 involves phospholipase C-γ (PLC-γ)48 and the Rap1-specific GEF CalDAG-GEFI, directly downstream of Gαi protein activation.49 Consistent with these findings, a deficiency of CalDAG-GEFI leads to defective chemokine-induced adhesion of lymphocytes and neutrophils to both β1 and β2 integrin ligands.28,49 In our study, we show additive effects of sub-μM concentration of the specific PLC inhibitor U-73122 and β-arr 2 depletion on CXCR2-induced Rap1 activation. Because in this work we demonstrate that the 2 ubiquitous isoforms of Rap1, Rap1A and B, are essential for both rapid onset and prolonged, shear-resistant VLA-4–mediated adhesion in KC-stimulated myeloid cells, we postulate that a β-arr 2–dependent pathway may cooperate with, and act sequentially to G protein-associated signaling pathways in inducing sustained Rap1 activation, possibly leading to integrin-dependent adhesion strengthening (Figure 7). In apparent contrast, a recent report has shown that LFA-1– but not VLA-4–dependent adhesion requires CXCL12-induced Rap1 activity in T lymphocytes.50 In view of our findings, we suggest that the involvement of Rap1, as well as additional pathways controlling chemokine-triggered integrin regulation, may vary to a large extent depending on the cell lineage (eg, lymphoid vs myeloid) and the chemokine involved. Our findings also rule out that the activation of Rap1 by CXCR2 is downstream of ERK1/2 or PI3K, as specific and highly effective inhibitors of the above pathways do not impair the activation of Rap1 by KC stimulation in RBL-2H3 cells.
In conclusion, we identify β-arrestins as novel key intermediates of discrete integrin-mediated adhesive and migratory responses critical for leukocyte trafficking. Interestingly, selected steps in this process emerge to be β-arr isoform–specific, supporting the possibility that β-arr 1 and β-arr 2 play distinct spatial and/or temporal roles in chemokine-induced responses. It will be particularly relevant to dissect the structural basis of the nonredundant function of the 2 β-arr isoforms in integrin-mediated adhesion, as this process could be targeted by novel and selective anti-inflammatory drugs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr R. Lefkowitz for providing the β-arrestin knockout mice.
This work was supported in part by grants from the European Union (MAIN LSHG-CT-2003-502935) and Ministry for Education, University, and Research (PRIN 2005 and 2007) to R.P.
Authorship
Contribution: R.M. performed the experiments, collected and analyzed the data, and contributed to writing the paper; C.L.C. and M.F. performed the experiments and collected the data; C.M., S.F., V.G., and C.L. performed the intravital and shear flow experiments, respectively, and collected and analyzed the data; R.A. and F.K. designed the shear flow and intravital experiments, respectively; and R.P. designed the study and contributed to writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruggero Pardi, Professor of Pathology, Department of Immunology, Transplantation and Infectious Diseases, San Raffaele University Medical School, via Olgettina 58, 20132 Milan, Italy; e-mail: pardi.ruggero@hsr.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal