Individuals whose platelets lack dense or α-granules suffer various degrees of abnormal bleeding, implying that granule cargo contributes to hemostasis. Despite these clinical observations, little is known regarding the effects of impaired platelet granule secretion on thrombus formation in vivo. In platelets, SNARE proteins mediate the membrane fusion events required for granule cargo release. Endobrevin/VAMP-8 is the primary vesicle-SNARE (v-SNARE) responsible for efficient release of dense and α-granule contents; thus, VAMP-8−/− mice are a useful model to evaluate the importance of platelet granule secretion in thrombus formation. Thrombus formation, after laser-induced vascular injury, in these mice is delayed and decreased, but not absent. In contrast, thrombus formation is almost completely abolished in the mouse model of Hermansky-Pudlak syndrome, ruby-eye, which lacks dense granules. Evaluation of aggregation of VAMP-8−/− and ruby-eye platelets indicates that defective ADP release is the primary abnormality leading to impaired aggregation. These results demonstrate the importance of dense granule release even in the earliest phases of thrombus formation and validate the distal platelet secretory machinery as a potential target for antiplatelet therapies.
Introduction
Abnormal bleeding in patients lacking platelet dense granules (eg, Hermansky-Pudlak syndrome; HPS)1,,–4 or α-granules (Gray platelet syndrome; GPS)5,,–8 suggests an essential role for platelet granules in hemostasis. Yet the role of platelet granule secretion in thrombus formation in vivo is largely inferred from ex vivo experiments. Aggregation studies performed with platelets that lack dense granules vary among individuals. Normal platelet aggregation was observed in 31% of patients with prolonged bleeding times and congenital dense granule deficiency in one series9 and in 35% of patients in a second series.10 When abnormal platelet aggregation is observed in these patients, the defect is highly variable.11 Platelets from patients with GPS are also heterogeneous in aggregation profiles.7 Thus, prolonged bleeding in platelet granule deficiency is not always associated with defects in platelet aggregation
Several mouse strains, which lack dense granules, have been identified and demonstrate prolonged bleeding times.4 However, platelet aggregation defects in these mice are generally overcome by increased agonist concentrations, confounding a simple functional assignment for platelet secretion.12,13 The observation that aggregation defects are not a consistent feature of dense granule–deficient platelets in humans and are overcome by increased agonist concentrations in mice raises the questions of whether and to what degree thrombus formation is affected when granule release is impaired.
If deficiency in granule release results in impaired thrombus formation in a predictable way, then inhibition of granule biogenesis or secretion represents a potential target for modulating thrombosis. This objective may now be tractable given the recent identification of proteins required for platelet granule release. The release of cargo from platelet granules requires a group of membrane proteins called soluble NSF attachment protein receptors (SNAREs) that mediate fusion of granule membranes to the plasma membrane or the open canalicular system. SNAREs are divided into target-membrane SNAREs (t-SNAREs), which in platelets include syntaxins 2, 4, 7, and 11 and SNAP-23, -25, and -29, and the vesicle-membrane SNAREs (v-SNAREs), which in platelets include VAMP-2, -3, -7, and -8.14,,,,,–20 Using streptolysin-O–permeabilized human platelets, syntaxins 2 and 4 and SNAP-23 have been shown to be important for secretion.15,20,–22 Endobrevin/VAMP-8 appears to be the primary v-SNARE functioning in platelet granule release because platelets from VAMP-8−/− mice have a clear secretion defect ex vivo.19 However, although VAMP-8 is the primary v-SNARE, a secondary, less efficient secretion process involving VAMP-2 or -3 is able to mediate some dense granule release in the absence of VAMP-8.19 This secondary system is slower and requires more agonist to reach maximal release.
A consequence of this partial redundancy is that there is a graded inhibition of granule secretion from VAMP-8−/− platelets. At maximal stimulation, almost normal dense granule release occurs. At low levels of stimulation, there is only limited release of dense granule cargo. Diminished dense granule release is expected to affect platelet responses because secondary signaling from ADP release would be blunted. Although platelet reactivity to strong stimuli may not be affected, such a defect could reduce the rate or extent of thrombus formation after small injuries (eg, laser-induced injury). In this paper, this concept is directly tested by comparing laser injury–induced thrombus formation in VAMP-8−/− mice with that in animals devoid of dense granules.
Given the importance of endobrevin/VAMP-8 in granule release, VAMP-8−/− mice offer a useful model to assess the role of platelet granule secretion in thrombus formation after laser-induced vascular injury. We now show that thrombus formation, in response to injury, is delayed and reduced but not abolished in the cremaster microvasculature of VAMP-8−/− mice. In contrast, thrombus formation is barely detectable in ruby-eye mice, whose platelets lack dense granules. Ex vivo aggregometry studies demonstrate the importance of released ADP to full aggregation of platelets from both VAMP-8−/− and ruby-eye mice. These studies demonstrate the extent to which VAMP-8–mediated secretion contributes to thrombus formation and validate this v-SNARE as a potential target for antiplatelet therapies.
Methods
Mice
Endobrevin/VAMP-8−/− mice, on a mixed C57Bl/6J-129Sv background, were as described.19,23,–25 Heterozygous animals were used for breeding. Littermates were used as controls for the knockout mice in thrombus formation studies. Male mice used for the cremaster injury model were 7 to 14 weeks of age and weighed 20 to 28 g. WT and ruby-eye mice on a C57B1/6J background were previously described.26 They were 7 to 14 weeks of age and weighed 20 to 28 g at time of thrombus formation studies. All animal use was approved by the IACUCs of the University of Kentucky and of Beth Israel Deaconess Medical Center.
Analysis of platelet accrual at sites of vascular injury using videomicroscopy
Mice were anesthetized and prepared as previously described.27,–29 The cremaster muscle was exposed and the microcirculation was observed with an Olympus AX70 fluorescent microscope (60× water-immersion lens, Melville, NY).29 To fluorescently label the platelets, 0.1 μg/g mouse of rat-anti–mouse CD41 (BioPharmingen) and 1 μg/g mouse of Alexa 647–goat-anti–rat IgG (Molecular Probes) were infused through the jugular cannulas. Laser injury was created with a nitrogen dye laser (coumarin 440 nm; Photonics) using a single laser pulse and with blinding to genotype to avoid operator bias. Platelet accumulation after injury was imaged continuously for 3 minutes using an Olympus AX microscope (Olympus) with a 40×/ 0.8 NA or 60×/ 0.9 NA water-immersion objective (Olympus). Digital images were captured with a Cooke Sensicam CCD camera (COOKE) in 640 × 480 format. The total thrombus fluorescence in each video frame was analyzed using Slidebook software (Intelligent Imaging Innovations). Platelet accrual at each time point was calculated by determining the median platelet accumulation at that time point.
Blood collection
Mice were euthanatized by CO2 inhalation. Blood was collected from the right ventricle and was mixed with sodium citrate to a final concentration of 0.38%. The citrated blood was mixed with an equal volume of PBS, pH 7.4. Platelet-rich plasma (PRP) was prepared by centrifugation at 250g for 10 minutes. After adding 10 ng/mL prostaglandin I2 (Sigma-Aldrich) for 5 minutes, the PRP was centrifuged at 500g for 15 minutes, and the platelet pellet was gently resuspended in HEPES/Tyrode buffer (10 mM HEPES/NaOH, pH 7.4, 5.56 mM glucose, 137 mM NaCl, 12 mM NaHCO3, 2.7 mM KCl, 0.36 mM NaH2PO4, 1 mM MgCl2) in the presence of 0.2 U/mL apyrase (Sigma-Aldrich) and 1 mM EGTA. Platelets were pooled from 3 to 8 individual mice of the same genotype to dilute potential effects of differences in genetic background.
Platelet aggregation
Platelet aggregation was measured using model 460Vs Lumi-Dual or Chrono-Log 680 aggregometers (Chronolog). Mouse platelets were prepared as indicated in “Blood collection” and recalcified with 1 mM CaCl2. WT, VAMP-8−/−, or ruby-eye platelet suspensions (250 μL; 2.5 × 108/mL) were put into siliconized cuvettes and stirred for 5 minutes at 37°C and 800 rpm before addition of indicated concentrations of agonist. Chronolume reagent (Chronolog) was added to each cuvette during the preincubation period and used to monitor ATP release from dense granules.
Coagulation studies
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were measured from plasma using assay kits (Helena Laboratories).
P-selectin surface expression
Platelets (2.5 × 108/mL) from WT or ruby-eye mice were incubated in the presence or absence of 2 μM ADP and 1 mM Ca2+. These concentrations of ADP and Ca2+ did not stimulate α-granule release. Platelets were subsequently stimulated with 100 mU/mL thrombin. Platelet activation was measured by P-selectin surface expression using phycoerythrin-anti–human P-selectin (BD Pharmingen) and flow cytometry as previously described.29
Quantification of platelet v-SNAREs
The v-SNAREs in mouse and human platelets were measured by quantitative Western blotting using enhanced chemifluorescence (ECF) and the levels were calculated by comparison with a standard curve generated with the recombinant cytoplasmic domains of VAMP-2, -3, -7, and -8.6
Statistical analysis
Two independent analyses were performed to determine whether differences in thrombus size between mutant genotypes and their WT controls were statistically significant. As established previously in several laboratories,27,29,,,,–34 analysis of median thrombus size after laser injury was performed using a nonparametric analysis, the Mann-Whitney test, to reduce outlier bias. We have also evaluated the thrombus formation data by determining the mean of more than 3 thrombi in each individual mouse to calculate thrombus size per mouse. The significance of the differences in the mean of thrombus size in mutant mice compared with their WT controls was determined using a 2-tailed t test. Values of P less than .05 were considered statistically significant. To evaluate the role of VAMP-8−/− in thrombus formation, fluorescence intensity of thrombi generated in WT mice (26 thrombi, n = 3) and VAMP-8−/− mice (23 thrombi, n = 5) was compared. For evaluation of thrombus formation in ruby-eye mice, maximal fluorescence intensity in WT (34 thrombi, n = 3) and ruby-eye mice (38 thrombi, n = 3) was compared. For measurement of platelet aggregation, mean + standard deviations were calculated from 3 to 8 independent studies, as indicated in the figure legends.
Results
Thrombus formation is defective in VAMP-8−/− mice
Platelets contain at least 4 v-SNAREs: VAMP-2, -3, -7, and -8. Quantitative Western blotting was performed to compare the relative abundance of VAMP isoforms in human and murine platelets. VAMP-8 was the most prevalent in both species (Table 1). In human platelets, VAMP-3 and -7 were expressed at comparable levels, but the expression of VAMP-2 was markedly decreased. In mouse platelets, VAMP-2 was expressed at levels nearly equal to that of VAMP-8. VAMP-3 and VAMP-7 were expressed at comparable levels. In functional studies, anti–VAMP-7 antibody and a VAMP-7–based peptide inhibitor have no effect on permeabilized human platelets (Ren et al19 and Q.R., unpublished observations, 2008). Absence of VAMP-3, reduced levels of VAMP-2, or the combination has no effect on secretion, ex vivo.19 Absence of VAMP-8, however, results in impaired platelet granule secretion in response to thrombin and collagen.19
Quantification of VAMP isoforms in human and mouse platelets
| . | Human, n=3 . | WT mouse, n=3 . | VAMP-8−/− mouse, n=3 . | |||
|---|---|---|---|---|---|---|
| ng/5×107* . | Molecules/plt† . | ng/5×107 . | Molecules/plt . | ng/5×107 . | Molecules/plt . | |
| VAMP-2 | < 1 | < 806 | 9.46 ± 0.11 | 7629 ± 89 | 9.06 ± 0.51 | 7306 ± 411 |
| VAMP-3 | 4.59 ± 0.66 | 4588 ± 658 | 2.20 ± 0.12 | 2197 ± 120 | 1.92 ± 0.19 | 1920 ± 190 |
| VAMP-7 | 7.84 ± 1.45 | 3766 ± 696 | 5.65 ± 0.49 | 2712 ± 230 | 5.56 ± 1.30 | 2669 ± 624 |
| VAMP-8 | 6.6 ± 0.37 | 6590 ± 370 | 8.36 ± 0.11 | 8360 ± 110 | ND | ND |
| . | Human, n=3 . | WT mouse, n=3 . | VAMP-8−/− mouse, n=3 . | |||
|---|---|---|---|---|---|---|
| ng/5×107* . | Molecules/plt† . | ng/5×107 . | Molecules/plt . | ng/5×107 . | Molecules/plt . | |
| VAMP-2 | < 1 | < 806 | 9.46 ± 0.11 | 7629 ± 89 | 9.06 ± 0.51 | 7306 ± 411 |
| VAMP-3 | 4.59 ± 0.66 | 4588 ± 658 | 2.20 ± 0.12 | 2197 ± 120 | 1.92 ± 0.19 | 1920 ± 190 |
| VAMP-7 | 7.84 ± 1.45 | 3766 ± 696 | 5.65 ± 0.49 | 2712 ± 230 | 5.56 ± 1.30 | 2669 ± 624 |
| VAMP-8 | 6.6 ± 0.37 | 6590 ± 370 | 8.36 ± 0.11 | 8360 ± 110 | ND | ND |
ND indicates not detected.
Mass of the VAMP in nanograms per 5 × 107 platelets was determined by quantitative Western blotting using recombinant VAMPs to generate a standard curve.
Number of molecules per platelet was calculated using the following molecular weights for the indicated VAMP: VAMP-2, 13.5 kDa; VAMP-3, 12 kDa; VAMP-7, 25 kDa; VAMP-8, 12 kDa.
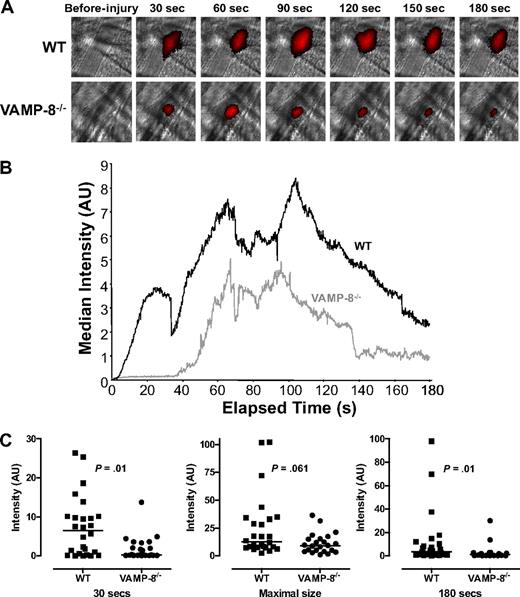
The observation that VAMP-8 mediates platelet granule secretion enabled us to use VAMP-8−/− animals to assess the role of granule release in normal thrombus formation. We determined the effect of VAMP-8 deficiency on the extent and kinetics of thrombus formation after vascular injury. Platelet accumulation at sites of laser-induced arteriolar injury was directly observed in real time in the cremaster microvasculature using an IgG directed at CD41 and an Alexa 647–labeled secondary IgG (Figure 1A). Fluorescence intensity was consistently decreased in VAMP-8−/− mice, demonstrating that platelet accumulation was less in VAMP-8−/− mice compared with WT controls.
Platelet accumulation in VAMP-8−/− mice after laser-induced injury of cremaster arterioles. (A) Platelet accumulation was imaged at the indicated time intervals after injury in both wild-type (WT) and VAMP-8–deficient (VAMP-8−/−) mice. (B) Median integrated platelet fluorescence intensity was plotted at each second for 180 seconds after laser injury in VAMP-8−/− mice. (C) At 30 seconds, median platelet accumulation in VAMP-8−/− mice was 4% of WT (P = .01). There was a trend toward a decrease in maximal thrombus size in VAMP-8−/− mice compared with WT mice, but the difference was not statistically significant (74% of WT; P = .061). By 180 seconds, median platelet accumulation in VAMP-8−/− mice was 28% of WT mice (P = .01).
Platelet accumulation in VAMP-8−/− mice after laser-induced injury of cremaster arterioles. (A) Platelet accumulation was imaged at the indicated time intervals after injury in both wild-type (WT) and VAMP-8–deficient (VAMP-8−/−) mice. (B) Median integrated platelet fluorescence intensity was plotted at each second for 180 seconds after laser injury in VAMP-8−/− mice. (C) At 30 seconds, median platelet accumulation in VAMP-8−/− mice was 4% of WT (P = .01). There was a trend toward a decrease in maximal thrombus size in VAMP-8−/− mice compared with WT mice, but the difference was not statistically significant (74% of WT; P = .061). By 180 seconds, median platelet accumulation in VAMP-8−/− mice was 28% of WT mice (P = .01).
For kinetic studies, thrombus formation occurring immediately after laser injury was evaluated in WT and VAMP-8−/− mice. The median fluorescence intensity at each second after injury was calculated and plotted (Figure 1B). Platelet accumulation in wild-type mice followed the kinetic pattern previously observed in this model.29 Immediately after laser injury in WT mice, there was a net increase in platelet accumulation that continued for approximately 60 seconds, when the thrombus appears to reach its maximal size (Figure 1B; supplemental Video 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). After approximately 100 seconds, a net decrease in platelet accumulation was observed until approximately 160 seconds. Recordings were performed for a total of 180 seconds after injury, because little change in platelet accumulation occurs after this time point.29
Distinct differences in thrombus formation were observed in mice lacking VAMP-8. Thrombus formation after vascular injury was both delayed and decreased in VAMP-8−/− mice (Figure 1B; supplemental Video 2). Analysis of individual thrombi from WT and VAMP-8−/− mice at 30 seconds showed that median platelet accumulation in VAMP-8−/− mice was 4% of accumulation in WT mice (P = .01, Figure 1C). Once initiated, platelet accumulation occurred at approximately the same rate as in WT mice. There was a trend toward smaller maximal thrombus size in VAMP-8−/− mice, but the difference did not reach statistical significance (P = .061, Figure 1C). By 180 seconds, median platelet accumulation in VAMP-8−/− mice was 28% of WT mice (P = .01, Figure 1C). An independent statistical analyses of thrombus size in individual VAMP-8−/− and WT mice demonstrated that mean platelet accrual at 30 seconds and 80 seconds was significantly lower in VAMP-8−/− mice compared with WT controls (supplemental Figure 1). These data confirm that thrombus formation after laser-induced injury of cremaster arterioles is significantly delayed and decreased in VAMP-8−/− mice.
Aggregation defect of VAMP-8−/− platelets
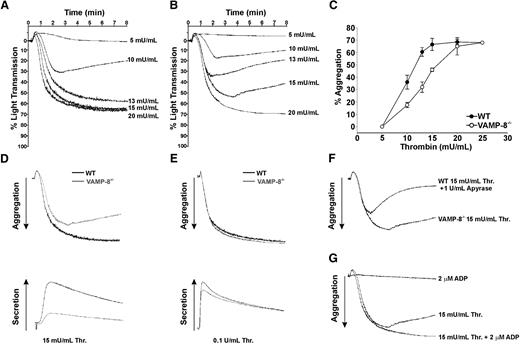
Thrombus formation after laser-induced injury of arterioles within the cremaster muscle is dependent on thrombin-induced platelet activation. In this model, collagen-induced platelet stimulation is not essential.31,35 Therefore, we evaluated the ability of VAMP-8−/− platelets to aggregate in response to thrombin. WT platelets demonstrated a dose-dependent stimulation curve with irreversible aggregation occurring in response to 13 to 15 mU/mL thrombin (Figure 2A). VAMP-8−/− platelets demonstrated a different dose-dependent response, requiring 20 mU/mL or more thrombin for irreversible aggregation (Figure 2B). Summary data of aggregation studies with WT and VAMP-8 platelets is shown in Figure 2C. By comparing the dose responsiveness of aggregation between WT and null platelets, it is clear that deletion of VAMP-8 causes a rightward shift in the curve as well as a change in the slope of the curve (6.9% aggregation/mU/mL for WT vs 4.5% aggregation/mU/mL for VAMP-8−/−; Figure 2C). These data indicate that VAMP-8−/− platelets are less responsive to thrombin stimulation, which is consistent with the in vivo phenotype shown in Figure 1.
Role of ADP in aggregation of VAMP-8−/− platelets. Wild-type (A) and VAMP-8−/− (B) platelets were incubated with the indicated concentrations of thrombin and percentage of light transmission was recorded. Panel C represents the mean percentage of aggregation versus thrombin concentration with SD indicated (3 aggregations/condition, n = 8 mice). Thrombin (D: 15 mU/mL; E: 100 mU/mL) was used to stimulate WT (black line) or VAMP-8−/− platelets (gray line). Aggregation (upper trace) and release of ATP (lower trace) were monitored. (F) Aggregations were measured for VAMP-8−/− platelets stimulated with 15 mU/mL thrombin and wild-type platelets stimulated with 15 mU/mL thrombin plus 1 U/mL apyrase. (G) Aggregations were measured for VAMP-8−/− platelets stimulated with 15 mU/mL thrombin alone, 15 mU/mL thrombin plus 2 μM ADP, or 2 μM ADP alone. All aggregation measurements were performed with constant stirring.
Role of ADP in aggregation of VAMP-8−/− platelets. Wild-type (A) and VAMP-8−/− (B) platelets were incubated with the indicated concentrations of thrombin and percentage of light transmission was recorded. Panel C represents the mean percentage of aggregation versus thrombin concentration with SD indicated (3 aggregations/condition, n = 8 mice). Thrombin (D: 15 mU/mL; E: 100 mU/mL) was used to stimulate WT (black line) or VAMP-8−/− platelets (gray line). Aggregation (upper trace) and release of ATP (lower trace) were monitored. (F) Aggregations were measured for VAMP-8−/− platelets stimulated with 15 mU/mL thrombin and wild-type platelets stimulated with 15 mU/mL thrombin plus 1 U/mL apyrase. (G) Aggregations were measured for VAMP-8−/− platelets stimulated with 15 mU/mL thrombin alone, 15 mU/mL thrombin plus 2 μM ADP, or 2 μM ADP alone. All aggregation measurements were performed with constant stirring.
Because ADP is released from dense granules and functions in thrombin-induced aggregation, we evaluated ADP release in VAMP-8−/− platelets after thrombin stimulation. A defect in ADP/ATP release accompanied impairment of aggregation at 15 mU/mL (Figure 2D); but, both defects could be overcome when 100 mU/mL thrombin was added (Figure 2E). Consistently, exposure of WT platelets to 1 U/mL apyrase resulted in reversible aggregation in response to 15 mU/mL thrombin, similar to the response seen in VAMP-8−/− platelets (Figure 2F). To determine whether the loss of ADP/ATP release accounted for the aggregation defect at low doses of thrombin, VAMP-8−/− platelets were stimulated with 15 mU/mL thrombin and also treated with 2 μM ADP (Figure 2G). The resulting aggregometry traces indicate that exogenous ADP can compensate for impaired dense granule release and cause full aggregation. When used alone, however, 2 μM ADP failed to stimulate aggregation of either WT (data not shown) or VAMP-8−/− platelets (Figure 2G). These data confirm the importance of ADP in promoting irreversible aggregation at low thrombin doses and support the premise that defective ADP release from VAMP-8−/− platelets is responsible for impaired platelet aggregation in vivo after vascular injury.
Thrombus formation and aggregation are defective in ruby-eye mice
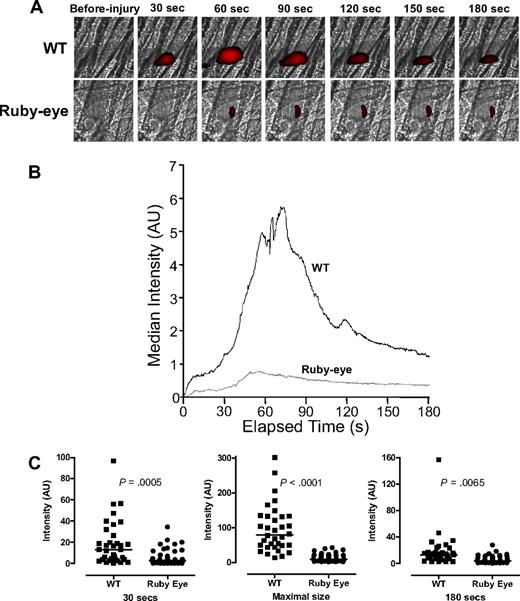
By inference from the data in Figures 1 and 2, aggregation and thrombus formation appear dependent on the extent of ADP release. To further evaluate the role of dense granule release in laser-induced thrombus formation in vivo, we examined thrombus formation in ruby-eye mice, which are an HPS model with either empty or diminutive dense granules.26,36 Thrombus formation was markedly impaired in these mice compared with WT (Figure 3A; supplemental Videos 3-4). Kinetic studies demonstrated marked differences in the initial rate of platelet accumulation after laser injury. In addition, maximum platelet accumulation was decreased to 12% of that of WT (P < .001; Figure 3B). An independent statistical analysis of thrombus size in individual ruby-eye and WT mice demonstrated that mean platelet accrual at 30 seconds and 80 seconds in ruby-eye mice is significantly lower in ruby-eye versus WT controls (supplemental Figure 2). These data demonstrate the importance of dense granule release to thrombus formation and suggest that the partial defect in dense granule release from VAMP-8−/− platelets (Figure 1) is relevant in vivo.
Platelet accumulation in ruby-eye mice after laser-induced injury of cremaster arterioles. (A) Platelet accumulation was imaged at the indicated time intervals, after injury, in both wild-type (WT) and ruby-eye mice. (B) Median integrated platelet fluorescence intensity was recorded at each second for 180 seconds after laser injury in ruby-eye mice. (C) At 30 seconds, median platelet accumulation in ruby-eye mice was 22% of WT (P = .001). Maximal thrombus size in ruby-eye mice was decreased compared with WT mice (12% of WT; P = .001). By 180 seconds, median platelet accumulation in ruby-eye mice was 31% of WT mice (P = .007).
Platelet accumulation in ruby-eye mice after laser-induced injury of cremaster arterioles. (A) Platelet accumulation was imaged at the indicated time intervals, after injury, in both wild-type (WT) and ruby-eye mice. (B) Median integrated platelet fluorescence intensity was recorded at each second for 180 seconds after laser injury in ruby-eye mice. (C) At 30 seconds, median platelet accumulation in ruby-eye mice was 22% of WT (P = .001). Maximal thrombus size in ruby-eye mice was decreased compared with WT mice (12% of WT; P = .001). By 180 seconds, median platelet accumulation in ruby-eye mice was 31% of WT mice (P = .007).
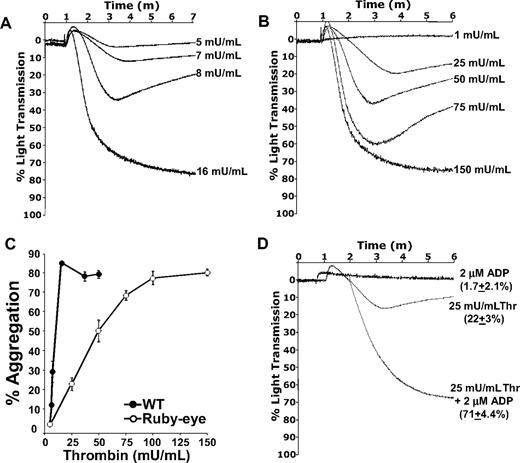
Because release of ADP/ATP from ruby-eye platelets was undetectable (data not shown), these platelets could be used to quantitatively assess the function of dense granule secretion in thrombin-mediated platelet aggregation ex vivo. Although complete aggregation of ruby-eye platelets could be elicited by thrombin, nearly 10-fold higher concentrations of thrombin were required, compared with WT (Figure 4A-B). Summary data of aggregation studies with WT and ruby-eye platelets is shown in Figure 4C. Loss of dense granules in ruby-eye platelets markedly increased the dose range of thrombin required to aggregate platelets. In WT platelets, there was an approximately 3-fold difference between the highest thrombin dose that failed to cause any aggregation and the dose required for full aggregation (Figure 4C). In ruby-eye platelets, this difference was greater than 20-fold. Thus, as shown in the VAMP-8−/− mice, loss of dense granules decreases the slope of the aggregation response curve relative to WT (7.6% aggregation/mU thrombin for WT vs 0.82% aggregation/mU thrombin for ruby-eye). This implies an increase in the dynamic range of aggregation in response to thrombin.
Thrombin-mediated aggregation of ruby-eye platelets. Wild-type (A) and ruby-eye (B) platelets were incubated with the indicated concentrations of thrombin and percentage of light transmission was recorded. (C) Mean percentage aggregation in wild-type and ruby-eye platelets was plotted as a function of thrombin concentration with standard deviation indicated (n = 3-8 aggregations/condition, n = 5 mice). (D) Ruby-eye platelets were incubated with 2 μM ADP alone, 25 mU/mL thrombin alone, or 25 mU/mL thrombin plus 2 μM ADP as indicated. Platelet aggregation was measured with constant stirring.
Thrombin-mediated aggregation of ruby-eye platelets. Wild-type (A) and ruby-eye (B) platelets were incubated with the indicated concentrations of thrombin and percentage of light transmission was recorded. (C) Mean percentage aggregation in wild-type and ruby-eye platelets was plotted as a function of thrombin concentration with standard deviation indicated (n = 3-8 aggregations/condition, n = 5 mice). (D) Ruby-eye platelets were incubated with 2 μM ADP alone, 25 mU/mL thrombin alone, or 25 mU/mL thrombin plus 2 μM ADP as indicated. Platelet aggregation was measured with constant stirring.
As with VAMP-8−/− platelets, the severe defect in aggregation of ruby-eye platelets in response to 25 mU/mL thrombin was overcome by addition of 2 μM ADP (Figure 4D); 2 μM ADP alone, however, did not cause aggregation. Incubation of platelets with ADP also reversed the defect in thrombin-induced α-granule release observed in ruby-eye platelets (supplemental Figure 3). These data demonstrate that although dense granule release is not strictly required for thrombin-induced aggregation, it increases the sensitivity of platelets to thrombin. ADP appears to be the primary dense granule constituent responsible for this increased sensitivity.
Discussion
These studies show that the participation of VAMP-8 in platelet granule release facilitates thrombus formation after laser-induced vascular injury. Previous work using this model demonstrated that significant α-granule secretion, as detected by P-selectin exposure, does not occur during the first 2 minutes after injury.37 Therefore, the defective α-granule release observed in VAMP-8−/− platelets is unlikely to substantially contribute to the delay in the initiation of thrombus formation seen in the VAMP-8−/− mice. Leukocyte recruitment occurs 2 to 3 minutes after injury, after P-selectin expression. Therefore, although leukocyte secretion might be defective in VAMP-8−/− mice, such deficiency would not be expected to contribute to the impaired thrombus formation observed. The thrombus formation defect is not due to deficient hepatic secretion of soluble clotting factors because PT (11.0 ± 1.18 seconds vs 10.3 ± 0.65 seconds, n = 11) and aPTT (30.8 ± 2.9 seconds vs 28.5 ± 1.4 seconds, n = 10) values were comparable between WT and VAMP-8−/− plasma. Although it is possible that exocytosis of Weibel-Palade bodies from endothelial cells requires VAMP-8−/−, plasma von Willebrand factor (VWF) can support normal thrombus formation even in the absence of endothelial cell VWF.38 Thus, even if VAMP-8−/− mice have impaired endothelial cell secretion, the significance of this defect for thrombus formation in this model would be unclear. These considerations and the data presented in this paper support the conclusion that impaired dense granule release is primarily responsible for the delayed and decreased thrombus formation in VAMP-8−/− animals. This is strengthened by our analysis of the ruby-eye mice whose platelets lack dense granules but have normal α-granules. Thrombus formation in these animals was severely impaired (Figure 3). Similarly, PKCα−/− mice have impaired dense granule biogenesis and secretion and demonstrate a defect in thrombus formation.39
Platelets possess 3 to 8 dense granules that contain approximately 2.2 M Ca2+, 653 mM ADP, 436 mM ATP, and 65 mM serotonin.40 Although inhibition of dense granule secretion during thrombus formation in vivo has not been directly evaluated until now, inhibition and/or deletion of receptors for dense granule contents (eg, ADP, serotonin) have been. Inhibition or deletion of the ADP receptors P2Y12, P2Y1, or P2X1 suppresses thrombus formation in vivo.41 Inhibition of the 5-HT2A receptor also hampers thrombus formation.42,,,–46 Other dense granule components such as polyphosphate47,48 and Ca2+49 have been proposed to contribute to thrombus formation based on in vitro studies.
Mathematic modeling of the dilution of the platelet releasate under physiologic flow rates suggests that ADP is the only major dense granule constituent to achieve an interfacial fluid concentration capable of stimulating platelets.50 Although a role for other dense granule constituents in thrombus formation cannot be excluded, the role of ADP in thrombus formation is the best understood of all dense granule constituents and inhibition of ADP-induced signaling is an important target for antiplatelet therapy.51,52 Addition of ADP to PKCα−/− platelets or to HPS platelets reverses the defect in thrombus formation under flow conditions in vitro.39,53 Aggregometry analysis of VAMP-8−/− and ruby-eye platelets (Figures 2,Figure 3–4) was consistent with the premise that deficient ADP release is responsible for impaired aggregation. Exogenous ADP was sufficient to reverse the aggregation defect in both VAMP-8−/− and ruby-eye platelets. Thus defective release of ADP appears to be the primary defect responsible for the in vitro phenotype of both VAMP-8−/− and ruby-eye platelets.
Taken as a whole, the present studies show that initial thrombus formation, in vivo, is inversely related to the degree to which dense granule release is attenuated. When there is no dense granule release, as in the ruby-eye mice, there is only limited thrombus formation. When dense granule release is deficient, but not ablated, as in the VAMP-8−/− mice, thrombus formation is delayed and inefficient but still occurs. However, both ruby-eye and VAMP-8−/− platelets could be fully aggregated, ex vivo, given sufficient stimulation with thrombin. For the ruby-eye platelets, a 10-fold higher concentration of thrombin was required for irreversible aggregation compared with wild-type platelets. Ruby-eye platelets also required at least 5-fold more thrombin to induce aggregation than did VAMP-8−/− platelets. The intermediate sensitivity of VAMP-8−/− platelets to thrombin compared with ruby-eye platelets likely contributes to the partial defect in thrombus formation observed in vivo (Figure 1).
Tissue factor–mediated generation of thrombin, resulting in stimulation of platelets through PAR4, is required for thrombus formation after laser-induced injury.54,55 In contrast, collagen-mediated stimulation of platelets through GPVI is not required.31 Our present studies demonstrate that, in vivo, positive feedback from released dense granule cargo is required for normal thrombus formation in the laser injury model. Our in vitro aggregometry studies show that this requirement can be overcome with increased thrombin. However, the thrombus formation defects in both VAMP-8−/− and ruby-eye mice imply that the degree of thrombin-mediated platelet activation after laser-induced vascular injury is relatively low or short-lived and insufficient to completely compensate for the lack of the secondary signals provided by platelet dense granule release. Whether dense granule release serves as critical a role in thrombus formation after exposure to rose bengal or FeCl3, which cause endothelial injury and appear to be more reliant on collagen-mediated platelet activation,31 is not known. However, a role for ADP-mediated stimulation of P2Y1 and P2Y12 in FeCl3-induced thrombus formation is indicated by the observation that occlusion times are increased in P2Y1- or P2Y12-null mice and in WT mice infused with MRS2179, a selective P2Y1 inhibitor, or the P2Y12 antagonist clopidogrel.56,57
Our in vitro observations further suggest that dense granule release increases the sensitivity of platelets to thrombin and narrows the dynamic range of platelet responses to thrombin. The dynamic range of responses to thrombin was markedly expanded (∼ 6-fold) in ruby-eye platelets where dense granule release is absent (Figure 4). The dynamic range was moderately increased (∼ 2-fold) in VAMP-8−/− platelets (Figure 2). Our in vivo observation suggests that modulating dense granule release may substantially modify thrombosis without completely preventing it. Manipulating the dynamic range of platelet responses, instead of just the sensitivity of response, may enable maintenance of some degree of hemostasis while preventing pathologic thrombus formation. These data demonstrate that targeting the distal secretory machinery directly may be a valid therapeutic approach to modulate thrombus formation without ablating it.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Glenn Merrill-Skoloff for his expert assistance with intravital microscopy studies. The authors acknowledge Drs Cheng-Chun Wang and Wanjin Hong for the generous gift of the VAMP-8−/− mice.
This work was supported by National Institutes of Health (NIH; Bethesda, MD) grants HL56652 and HL091893 (S.W.W.), HL63250 (R.F.), and T32 HL07917 (P.B.). R.F. is a recipient of a Special Project Award from Bayer Healthcare (Leverkusen, Germany) and an Established Investigator Award from the American Heart Association (Dallas, TX). Q.R. is the recipient of a Pre-Doctoral Fellowship (0615238B) from the Ohio Valley Affiliate of the American Heart Association (Baltimore, MD).
National Institutes of Health
Authorship
Contribution: G.J.G. performed intravital microscopy and data analysis for thrombus formation studies; Q.R. measured the PT and aPTT, performed quantification of VAMP isoforms in human and mouse platelets, and performed aggregation studies of VAMP-8−/− platelets; J.R.D. and P.B. performed aggregation and P-selectin expression studies of ruby-eye platelets; and S.W.W. and R.F. initiated and supervised the project and drafted and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sidney W. Whiteheart, Department of Molecular and Cellular Biochemistry, University of Kentucky College of Medicine, 741 S Limestone St, Biomedical Biological Sciences Research Bldg, Lexington, KY 40536-0509; e-mail: whitehe@uky.edu.