Abstract
Human induced pluripotent stem (iPS) cells derived from somatic cells hold promise to develop novel patient-specific cell therapies and research models for inherited and acquired diseases. We and others previously reprogrammed human adherent cells, such as postnatal fibroblasts to iPS cells, which resemble adherent embryonic stem cells. Here we report derivation of iPS cells from postnatal human blood cells and the potential of these pluripotent cells for disease modeling. Multiple human iPS cell lines were generated from previously frozen cord blood or adult CD34+ cells of healthy donors, and could be redirected to hematopoietic differentiation. Multiple iPS cell lines were also generated from peripheral blood CD34+ cells of 2 patients with myeloproliferative disorders (MPDs) who acquired the JAK2-V617F somatic mutation in their blood cells. The MPD-derived iPS cells containing the mutation appeared normal in phenotypes, karyotype, and pluripotency. After directed hematopoietic differentiation, the MPD-iPS cell-derived hematopoietic progenitor (CD34+CD45+) cells showed the increased erythropoiesis and gene expression of specific genes, recapitulating features of the primary CD34+ cells of the corresponding patient from whom the iPS cells were derived. These iPS cells provide a renewable cell source and a prospective hematopoiesis model for investigating MPD pathogenesis.
Introduction
Recent derivation of human induced pluripotent stem (iPS) cells from patients' somatic cells has made it possible to generate patient- and disease-specific stem cell lines for developing novel cell therapies and disease modeling.1-8 These human iPS cells exhibit characteristics similar to human embryonic stem (hES) cells, including unlimited expansion in culture. Using various vectors to deliver multiple transgenes encoding transcription factors, such as OCT4, SOX2, KLF4, and c-MYC, most published protocols were optimized to reprogram adherent cells, such as fibroblasts and keratinocytes from skin and hair.1-8 It is also highly desirable to reprogram blood cells that are easily accessible and less exposed to environmental mutagens. For example, umbilical cord blood (CB) cells that are collected and stored in multiple cell banks could be used as a source of either autologous or allogeneic but histocompatible iPS cell lines. More critically, the ability to reprogram blood cells is essential if one wishes to generate iPS cells containing somatic mutations that are restricted to the blood cells and found only in acquired hematologic disorders to investigate their pathogenesis. A previous study demonstrated that differentiated mouse B cells could be reprogrammed to iPS cells, primarily using transgenic (reprogramming-ready) mice harboring the 4 reprogramming transgenes that are conditionally active.9 More recently, mouse iPS cell lines were also derived from bone marrow (BM) progenitor cells obtained from a mouse whose hematopoiesis was reconstituted from a single congenic hematopoietic stem cell, providing further evidence that mouse hematopoietic cells can be reprogrammed to pluripotency.10 Derivation of iPS cells from postnatal human blood cells has not been reported until recently when it was reported that granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood (PB) CD34+ cells from a healthy person could be reprogrammed to iPS cells.11 It is unclear, however, whether daily G-CSF treatment for 3 to 5 days could affect the reprogramming process or the properties of blood cell–derived iPS cells.12 Here we report the reprogramming of human CB and adult BM CD34+ cells from healthy donors without any pretreatment. Moreover, we derived multiple iPS cell lines from PB CD34+ cells containing the JAK2-V617F mutation that is commonly found in hematopoietic progenitor cells of adult patients with myeloproliferative disorders (MPDs).13-17 The BCR/ABL-negative MPDs, which include polycythemia vera (PV), essential thrombocytosis (ET), and primary myelofibrosis (PMF), are a heterogeneous group of diseases characterized by increased proliferation of erythroid, megakaryocytic, and myeloid lineages alone or in combination. The acquired common somatic mutation JAK2-V617F is present in more than 95% of PV and approximately 50% of ET and PMF patients.13-19 To determine whether these blood cell–derived iPS cell lines could be used as a model to study normal and abnormal human hematopoiesis, we used an efficient serum-free differentiation protocol to direct iPS cells into hematopoietic lineages. Similar to the increased erythropoiesis of hematopoietic progenitor (CD34+) cells isolated from PV patients,20,21 including one subject whose blood-derived iPS cells were used in this study, redifferentiated hematopoietic progenitor (CD34+CD45+) cells generated from the PV-iPS cells showed enhanced erythropoiesis compared with those from the iPS cells derived from normal blood CD34+ cells.
Methods
Culture media and conditions for expanding human ES cells and iPS cells
Media and culture conditions for derivation, expansion, and karyotyping (G banding) of human iPS cells were described previously.4
Human CD34+ cells and reprogramming by gene transduction
Use of anonymous human samples for laboratory research, such as this study, was approved by Johns Hopkins University Institutional Review Board. Frozen human CD34+ cells from CB and adult BM were purchased (8 years ago) from AllCells and Poietics (now part of Lonza). Previously frozen PB CD34+ cells from 2 patients registered at the Johns Hopkins Center of Chronic MPDs19 were also used in this study and from whom written informed consent was obtained in accordance with the Declaration of Helsinki. Isolated PB CD34+ cells after G-CSF mobilization were purchased from AllCells and used as a normal control for analyzing gene expression. Four classic retroviral vectors pMXs-Oct4, pMXs-Sox2, pMXs-Klf4, and pMXs-c-Myc encoding (mouse) reprogramming factors constructed by the laboratory of Dr Yamanaka were obtained from Addgene (www.addgene.org). Retroviral supernatants were produced by transfection of 293T cells with a mixture of 3 plasmids: one transducing (reprogramming) vector, a plasmid expressing the VSV-G envelope protein, and a helper plasmid expressing the retroviral Gag/Pol gene. After thaw, CD34+ cells were cultured for 2 to 4 days with cytokines stem cell factor (SCF; 100 ng/mL), Flt3-ligand (FL; 50-100 ng/mL), and thrombopoietin (20 ng/mL) before retroviral transduction. Stimulated CD34+ cells (2 × 105) were mixed with the retroviral supernatants supplemented with 4 ng/mL polybrene and cultured with the same medium and the 3 cytokines. After 2 to 3 days of transduction and after culture, transduced cells at day 5 after transduction were plated at a density of 4 × 105 cells/well in 6-well plates. They are cultured on preseeded embryonic fibroblast feeder cells for programming similar to what we previously described.4 At day 7, the hES cell medium was used throughout.
TRA-1-60 live staining
TRA-1-60 antibody (Millipore, 1:300) and Alexa 555–conjugated secondary antibody anti–mouse IgM (Invitrogen, 1:400) were diluted in hES medium and added into the reprogramming plate. The plate was incubated in 37°C for 1 hour before medium was changed to fresh conditioned medium. TRA-1-60+ colonies were identified under an inverted fluorescence microscope.
Immunostaining of undifferentiated iPS cells and their derivatives
Immunostaining of iPS clones for undifferentiated markers and of differentiated cells after embryoid body (EB) formation was performed as previously described.4,22,23 Images were acquired using a Nikon Eclipse TE2000-U microscope equipped with 4×/0.13, 10×/0.3, 20×/0.45, and 40×/0.6 numeric aperture objective lenses. Images were captured with a SPOT7.4 digital camera and SPOT Advanced 3.5.9 software (Diagnostic Instruments) and cropped and labeled with Photoshop 7.0 (Adobe Systems).
Teratoma formation assay of pluripotency
The use of immunodeficient mice for the teratoma formation assay was approved by the Animal Care and Use Committee at Johns Hopkins University. A total of 3 to 5 million iPS cells were harvested by Collagenase IV (Sigma-Aldrich) digestion, washed with phosphate-buffered saline, and resuspended in 200 μL of diluted (1:1) Matrigel solution. Cells were injected intramuscularly into Rag1−/−γC−/− mice or other improved immunodeficient mice with a further reduced level of natural killer cells. Tumors were excised 6 to 10 weeks after injection. Histologic processing was performed as previously described.4,22,23 Teratoma RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer's recommendation. Reverse-transcribed polymerase chain reaction (RT-PCR) of AFP, CD34, PAX6, OCT4, and NANOG human genes was carried out as previously described.4,22,23
DNA fingerprinting and JAK2-V617F allele analysis
Hematopoietic differentiation and analysis
The hematopoietic differentiation potential of CD34+ cell–derived iPS cells was examined by an improved method of EB formation (so called spin-EB) and hematopoietic differentiation under a feeder- and serum-free condition, similar to what was previously described.23-25 The EBs were harvested between weeks 2 and 3 and were analyzed by hematopoietic colony-forming assays and fluorescence-activated cell sorter (FACS) for the presence of hematopoietic markers as previously described.23,26 Cytospin was conducted as previously described,26 and the slides were stained with Hema 3 Stain Set (Fisher Diagnostics).
Erythroid differentiation and expansion in liquid culture
Two to 3 weeks after the spin-EB hematopoietic differentiation, the CD34+CD45+ cell populations were collected using FACS sorting from age-matched normal iPS EBs and PV-iPS EBs. A total of 2 × 104 CD34+CD45+ cells were cultured in serum-free medium containing SCF (20 ng/mL), interleukin-3 (50 ng/mL), and erythropoietin (1 U/mL). Cells were harvested on day 7, and the corresponding cell numbers were counted. The differentiated cells were also stained with anti-CD235a antibody (BD Biosciences) and anti-CD45 antibody (Invitrogen) for FACS analysis.
Quantitative real-time PCR analysis for gene expression
After EB-mediated hematopoietic differentiation (21 days), CD34+CD45+ cells from both normal BM CD34+ cell–derived iPS and MPD183 CD34+ cell–derived iPS were sorted and used for RNA extraction using Trizol reagent following the manufacturer's protocol (Invitrogen). cDNA was prepared using random hexamers, and quantitative PCR was performed with primers and probes for nuclear factor I-B (NFI-B), hemoglobin-γ (HBG), and hemoglobin-β (HBB) genes supplied by Applied Biosystems. Real-time PCR was conducted using the 7500 Real-Time PCR System (Applied Biosystems). The human leukemia cell line DAMI was used to generate a standard curve. Individual gene expression signals were normalized to β-actin in the same cell sample.
Data presentations and statistics
For flow cytometric analysis, at least 10 000 events were collected and analyzed. The percentages of selected cell populations (based on the comparison with background staining shown when isotype-matched antibodies are used) among the total live cells are shown. For histogram presentations, mean and SD were shown. The Wilcoxon 2-sample test was applied when the number of replicates was small (n < 10). This test was run unsupervised with the SAS 9.1 package (SAS Institute). We judged findings to be statistically significant if P was less than or equal to .05.
Results
Reprogramming of CB and adult CD34+ cells
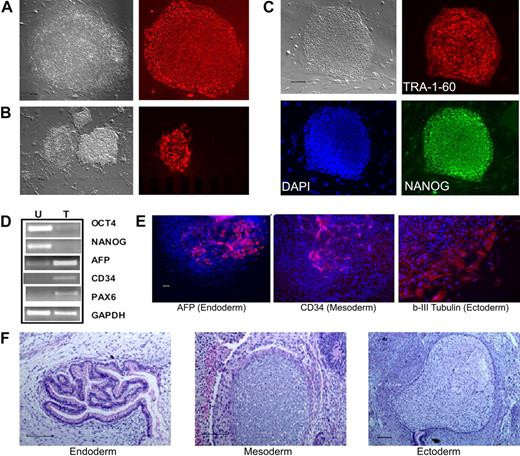
To test whether postnatal human blood cells could be reprogrammed by the conventional 4 reprogramming factors, we started with CD34+ cells purified from CB and adult BM that show extensive proliferative potential in culture.27 After thawing, the CD34+ cells were cultured and activated for 2 to 4 days to stimulate cell proliferation,27 before gene transduction by the4 standard retroviral vectors.1 Colonies resembling human ES/iPS cells emerged approximately day 16 after gene transduction from CB samples and approximately day 21 from adult BM CD34+ cells. One week later, the whole culture was stained live with the TRA-1-60 antibody recognizing a cell-surface epitope on undifferentiated hES and iPS cells (Figure 1A). We found that TRA-1-60 staining is also a convenient and reliable way of identifying candidate iPS colonies derived from human blood cells (Figure 1B). After expansion, the picked TRA-1-60+ clones showed the characteristic hES/iPS cell morphology and the expression of other pluripotency markers in addition to TRA-1-60 (Figure 1C; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). They no longer expressed CD34 or CD45 markers (data not shown), and instead expressed OCT4 and NANOG genes from the endogenous loci (Figure 1D). After expansion for more than or equal to 9 passages, multiple iPS lines examined retain a normal karyotype (supplemental Figure 2). Pluripotency of these iPS clones was demonstrated by differentiation assays, such as in vitro EB formation (Figure 1E) and in vivo teratoma formation (Figure 1F), generating various cell types derived from the 3 embryonic germ layers. Therefore, the iPS cells we derived from human CB and adult BM CD34+ cell lines resemble both hES cells and fibroblast-derived iPS cells morphologically, phenotypically, and functionally.
Reprogramming of human CB and adult BM CD34+ cells to iPS cells. (A) Live culture was stained by the TRA-1-60 antibody, 3 to 4 weeks after transduction of CB CD34+ cells. TRA-1-60+ colony shown on right was first seen at week 3 and was picked at week 4 (after restaining). (B) An illustration of various colonies 4 weeks after transduction of BM CD34+ cells, after live staining with TRA-1-60 and a secondary fluorescent reagent. A smaller fraction of formed colonies are TRA-1-60+. TRA-1-60+ colonies were individually picked and gave rise to iPS clones. (C) Immunofluorescence staining images of expanded iPS cells from CB are shown here (BM-derived iPS cells are shown in supplemental Figure 1). In addition to TRA-1-60, they also express other pluripotency markers NANOG and SSEA4. (D) Gene expression of undifferentiated (OCT4 and NANOG) and differentiation markers in undifferentiated iPS cells (U) derived from CB and teratoma cells (T) after in vivo differentiation. The expression of α-fetoprotein (AFP, endoderm), CD34 (mesoderm), and PAX6 (ectoderm) and a housekeeping gene GAPDH was measured by RT-PCR analysis. (E) Differentiation potential of CB-derived iPS cells after in vitro differentiation by EB formation (10 days). After immunofluorescence staining, differentiated cells expressing AFP, CD34, and β-III-tubulin (an ectoderm marker) were seen. The image of specific staining is overlaid by 4,6-diamidino-2-phenylindole staining of nuclei. (F) In vivo differentiation potential after teratoma formation from CB-derived iPS cells. Hematoxylin and eosin staining of various slides after sectioning show various tissues from the 3 embryonic germ layers: gut epithelium (endoderm), cartilage (mesoderm), and glycogenated epithelium (ectoderm). Scale bar represents 200 μm.
Reprogramming of human CB and adult BM CD34+ cells to iPS cells. (A) Live culture was stained by the TRA-1-60 antibody, 3 to 4 weeks after transduction of CB CD34+ cells. TRA-1-60+ colony shown on right was first seen at week 3 and was picked at week 4 (after restaining). (B) An illustration of various colonies 4 weeks after transduction of BM CD34+ cells, after live staining with TRA-1-60 and a secondary fluorescent reagent. A smaller fraction of formed colonies are TRA-1-60+. TRA-1-60+ colonies were individually picked and gave rise to iPS clones. (C) Immunofluorescence staining images of expanded iPS cells from CB are shown here (BM-derived iPS cells are shown in supplemental Figure 1). In addition to TRA-1-60, they also express other pluripotency markers NANOG and SSEA4. (D) Gene expression of undifferentiated (OCT4 and NANOG) and differentiation markers in undifferentiated iPS cells (U) derived from CB and teratoma cells (T) after in vivo differentiation. The expression of α-fetoprotein (AFP, endoderm), CD34 (mesoderm), and PAX6 (ectoderm) and a housekeeping gene GAPDH was measured by RT-PCR analysis. (E) Differentiation potential of CB-derived iPS cells after in vitro differentiation by EB formation (10 days). After immunofluorescence staining, differentiated cells expressing AFP, CD34, and β-III-tubulin (an ectoderm marker) were seen. The image of specific staining is overlaid by 4,6-diamidino-2-phenylindole staining of nuclei. (F) In vivo differentiation potential after teratoma formation from CB-derived iPS cells. Hematoxylin and eosin staining of various slides after sectioning show various tissues from the 3 embryonic germ layers: gut epithelium (endoderm), cartilage (mesoderm), and glycogenated epithelium (ectoderm). Scale bar represents 200 μm.
Human iPS cell lines derived from MPD patient PB cells
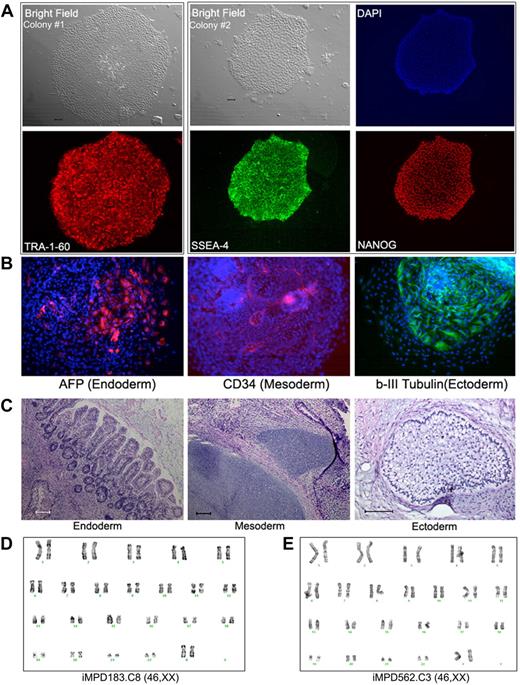
We next used CD34+ cells isolated from the PB of 2 MPD patients (without G-CSF mobilization).19 An acquired common somatic mutation in the JAK2 gene (1849G>T, resulting in a V617F substitution that activates the intracellular kinase) was present in the patients' blood cells as in more than 95% of PV and approximately 50% of ET and PMF patients.13-18,28,29 Both MPD patients (MPD183 with PV and MPD562 with PMF) had a heterozygous JAK2-V617F genotype in 100% of colony-forming erythroid progenitors in their PB CD34+ cells.19 Using the same quantitative allele-specific PCR analysis,18,19 we confirmed that the percentage of the mutated (1849T resulting in V617F) JAK2 allele in the thawed CD34+ cells from each patient was approximately 50% (Table 1). Applying the same reprogramming protocol used for normal human CD34+ cells, we derived multiple iPS clones from each of the 2 patients (Table 1). All of the iPS clones we successfully expanded were heterozygous for JAK2-V617F, identical to the parental CD34+ cells (Table 1). Similar to human iPS cells derived from fibroblasts and normal CD34+ cells, the expanded JAK2-V617F iPS clones displayed the characteristic undifferentiated hES/iPS cell morphology and marker expression (Figure 2A; supplemental Figure 3). Analysis of differentiated cells after EB formation and teratoma formation revealed the presence of various cell types originated from the 3 embryonic germ layers, indicating pluripotency of these patient-specific iPS lines (Figure 2B-C; supplemental Figure 3). The expanded JAK2-V617F iPS cell lines from both MPD patients also showed a normal karyotype by G-banding analysis (Figure 2D).
JAK2-V617F genotyping of iPS cell lines derived from MPD patients
| Cells analyzed . | Wild-type allele (1849G), percentage . | V617F allele (1849T), percentage . |
|---|---|---|
| CD34+ cells from MPD183 | 48 | 52 |
| iPS MPD183 clone 1 | 46 | 54 |
| iPS MPD183 clone 2 | 45 | 55 |
| iPS MPD183 clone 3 | 48 | 52 |
| iPS MPD183 clone 5 | 46 | 54 |
| iPS MPD183 clone 6 | 45 | 55 |
| iPS MPD183 clone 7 | 46 | 54 |
| iPS MPD183 clone 8 | 46 | 54 |
| iPS MPD183 clone 10 | 47 | 54 |
| iPS MPD183 clone 11 | 50 | 50 |
| CD34+ cells from MPD562 | 50 | 50 |
| iPS MPD562 clone 2 | 46 | 54 |
| iPS MPD562 clone 3 | 49 | 51 |
| Cells analyzed . | Wild-type allele (1849G), percentage . | V617F allele (1849T), percentage . |
|---|---|---|
| CD34+ cells from MPD183 | 48 | 52 |
| iPS MPD183 clone 1 | 46 | 54 |
| iPS MPD183 clone 2 | 45 | 55 |
| iPS MPD183 clone 3 | 48 | 52 |
| iPS MPD183 clone 5 | 46 | 54 |
| iPS MPD183 clone 6 | 45 | 55 |
| iPS MPD183 clone 7 | 46 | 54 |
| iPS MPD183 clone 8 | 46 | 54 |
| iPS MPD183 clone 10 | 47 | 54 |
| iPS MPD183 clone 11 | 50 | 50 |
| CD34+ cells from MPD562 | 50 | 50 |
| iPS MPD562 clone 2 | 46 | 54 |
| iPS MPD562 clone 3 | 49 | 51 |
Human iPS lines containing the JAK2-V617F mutation from PB CD34+ cells of 2 MPD patients. (A) Immunostaining of different colonies from a representative iPS line (clone 8) derived from MPD183 shows the expression of undifferentiated cell markers TRA-1-60, SSEA4, and NANOG. (B) A pluripotency test for the iPS clone 8 from MPD183 (iMPD183.C8) after EB formation (day 10) as we did for human ES cells and other (normal) iPS cells, showing that JAK2-V617F iPS cells can also differentiate into various cell types expressing markers of 3 embryonic germ layers. Similar results were obtained from the iPS clone 3 of the second MPD patient (iMPD562.C3) before and after EB-mediated differentiation, as shown in supplemental Figure 4. (C) In vivo differentiation potential after teratoma formation from MPD183-derived iPS cells. Hematoxylin and eosin staining of various slides after sectioning shows various tissues from the 3 embryonic germ layers: gut epithelium (endoderm), cartilage (mesoderm), and glycogenated epithelium (ectoderm). (D-E) Expanded iPS lines from the 2 female MPD patients (D: MPD183; E: MPD562) retained a normal karyotype (46,XX), after 10 and 11 passages, respectively. Scale bar represents 200 μm.
Human iPS lines containing the JAK2-V617F mutation from PB CD34+ cells of 2 MPD patients. (A) Immunostaining of different colonies from a representative iPS line (clone 8) derived from MPD183 shows the expression of undifferentiated cell markers TRA-1-60, SSEA4, and NANOG. (B) A pluripotency test for the iPS clone 8 from MPD183 (iMPD183.C8) after EB formation (day 10) as we did for human ES cells and other (normal) iPS cells, showing that JAK2-V617F iPS cells can also differentiate into various cell types expressing markers of 3 embryonic germ layers. Similar results were obtained from the iPS clone 3 of the second MPD patient (iMPD562.C3) before and after EB-mediated differentiation, as shown in supplemental Figure 4. (C) In vivo differentiation potential after teratoma formation from MPD183-derived iPS cells. Hematoxylin and eosin staining of various slides after sectioning shows various tissues from the 3 embryonic germ layers: gut epithelium (endoderm), cartilage (mesoderm), and glycogenated epithelium (ectoderm). (D-E) Expanded iPS lines from the 2 female MPD patients (D: MPD183; E: MPD562) retained a normal karyotype (46,XX), after 10 and 11 passages, respectively. Scale bar represents 200 μm.
Directed hematopoietic differentiation of blood cell–derived iPS cells
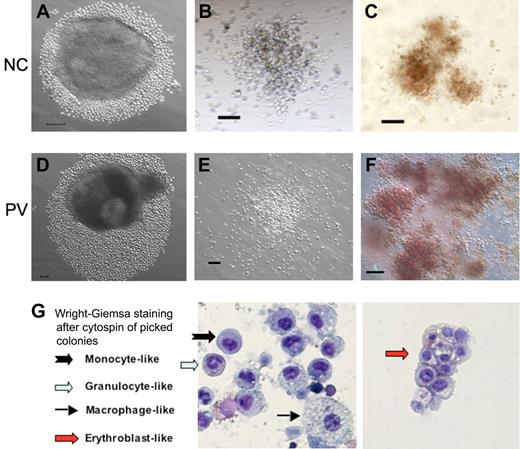
The hematopoietic differentiation potential of human iPS cell lines derived from normal and PV CD34+ cells was examined by an improved method of EB formation and differentiation under a feeder- and serum-free condition.23-25 By week 2, many small cells resembling immature hematopoietic cells grew out of the EBs (Figure 3A,D). The cells were subsequently harvested and analyzed by both hematopoietic colony-forming assays (Figure 3B-C,E-F) and FACS for the presence of hematopoietic markers (supplemental Figure 4). Both myeloid and erythroid colonies were detected (Figure 3B-C,E-F) as we have observed using hES cells.26 This was confirmed when we used the purified CD34+CD45+ cells generated from differentiated iPS cells, which were derived from either normal or the PV CD34+ blood cells (supplemental Figure 5). Staining of individual cells from picked hematopoietic colonies confirmed the presence of various myeloid and erythroid cell types (Figure 3G). In addition, FACS analysis confirmed the presence of differentiated hematopoietic cells at day 13 to 17 after the EB formation (supplemental Figure 4). CD45+ (27%-64%) and CD43+ (36%-60%) hematopoietic cells expressing undetectable to intermediate levels of CD34 marker were also observed (supplemental Figure 4). Gene expression analysis by RT-PCR also showed the up-regulation of hematopoietic markers RUNX1, GATA-1, GATA-2, and HBB genes as well as the down-regulation of the pluripotency-specific marker NANOG gene (supplemental Figure 4). Corroborating a recently published study,11 these results demonstrate that the iPS cells derived from normal and MPD blood CD34+ cells could also be redirected to multiple hematopoietic cell lineages.
Hematopoietic potential of CD34+ cell–derived iPS cells after directed differentiation. Human iPS cells derived from normal control (NC, A) adult CD34+ cells or the PV CD34+ cells (D) were plated in microtiter wells and aggregated for EB formation and directed hematopoietic differentiation. After 10 to 14 days, substantial numbers of small round cells resembling immature hematopoietic cells surrounding EBs were found and increased in the next several days (A,D). Total cells were subsequently harvested and assayed for the presence of hematopoietic markers (supplemental Figure 4) and of hematopoietic colony-forming units (CFUs) formed in semisolid methylcellulose media (B-C: normal control iPS; E-F: PV-iPS). CFU-granulocyte/monocyte (B,E) and CFU-erythroid (C,F) colonies were observed after additional 10 to 14 days in culture. A similar CFU assay using purified CD34+CD45+ cells from an NC and PV sample is shown in supplemental Figure 5. (G) Wright-Giemsa staining after cytospin of individually picked myeloid and erythoid colonies generated from iPS cells derived from normal CD34+ cells. Cells resembling erythroblasts and multiple lineages of myeloid cells were observed. Scale bar represents 100 μm.
Hematopoietic potential of CD34+ cell–derived iPS cells after directed differentiation. Human iPS cells derived from normal control (NC, A) adult CD34+ cells or the PV CD34+ cells (D) were plated in microtiter wells and aggregated for EB formation and directed hematopoietic differentiation. After 10 to 14 days, substantial numbers of small round cells resembling immature hematopoietic cells surrounding EBs were found and increased in the next several days (A,D). Total cells were subsequently harvested and assayed for the presence of hematopoietic markers (supplemental Figure 4) and of hematopoietic colony-forming units (CFUs) formed in semisolid methylcellulose media (B-C: normal control iPS; E-F: PV-iPS). CFU-granulocyte/monocyte (B,E) and CFU-erythroid (C,F) colonies were observed after additional 10 to 14 days in culture. A similar CFU assay using purified CD34+CD45+ cells from an NC and PV sample is shown in supplemental Figure 5. (G) Wright-Giemsa staining after cytospin of individually picked myeloid and erythoid colonies generated from iPS cells derived from normal CD34+ cells. Cells resembling erythroblasts and multiple lineages of myeloid cells were observed. Scale bar represents 100 μm.
Enhanced erythropoiesis of hematopoietic progenitor cells generated from PV iPS cells
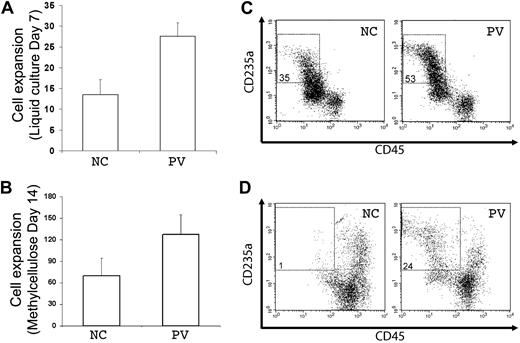
A principal feature of PV is overproduction of red blood cells, which is recapitulated by increased erythropoiesis of purified PB PV CD34+ hematopoietic progenitor cells in vitro. Using the standard colony-forming assay that measures both myeloid and erythroid progenitor cells, we observed that purified (CD34+CD45+) hematopoietic progenitor cells generated from the PV-iPS cells after EB-mediated hematopoietic differentiation formed more erythroid colonies than from the normal control (supplemental Figure 5). To further analyze their erythropoiesis potential, the purified CD34+CD45+ cells differentiated from both iPS cell lines were cultured in 2 types of media containing erythropoietin, SCF, and interleukin-3, the conditions favoring erythropoiesis from CD34+ progenitor cells (Figure 4).20,21 After 7 days in a liquid culture, both normal control and PV cell populations displayed extensive cell proliferation (Figure 4A). However, the proliferation rate with the PV sample was approximately twice that of the normal control (Figure 4A), recapitulating the increased erythropoiesis we observed using primary CD34+ cells from the corresponding PV patient (supplemental Figure 6). To ensure that enhanced cell proliferation observed in PV-iPS cells was associated with erythroid commitment and further differentiation, we analyzed the expression of CD235a (also known as glycophorin A, a specific marker for erythroid differentiation and maturation) and CD45 (a pan-leukocyte marker that is also expressed in progenitor cells but down-regulated after erythroid differentiation). Whereas 35% of the cells in the normal control group showed the erythroid phenotype (CD235a+CD45−), 53% of the cells in the PV sample displayed the erythroid phenotype (Figure 4B), indicating the enhanced erythroid differentiation as well as proliferation.
Increased erythroid differentiation of hematopoietic progenitor cells generated from PV iPS cells. To assess erythroid differentiation potential, purified CD34+CD45+ cells from both normal control (NC) and PV iPS cells after EB-mediated hematopoietic differentiation were plated into a liquid culture medium (A,C), or a serum- and methylcellulose-containing medium (B,D). (A) Fold of cell expansion after 7 days of the liquid culture from the purified CD34+CD45+ cells derived from NC (13.5 ± 3.6-fold) or PV iPS cells (27.6 ± 3.3-fold). (B) Fold of cell expansion of the CD34+CD45+ cells after 14 days of the methylcellulose culture, 69.5 ± 24.7-fold of NC versus 127 ± 28.3-fold of the PV iPS cells. Data in panels A and B are presented as mean ± SD (n = 2). (C) FACS analysis of the 7-day cultured cells for the erythroid phenotype (CD235a+CD45−). The percentages of such cell population are indicated in the top left quadrant based on the gating and comparison with background staining. (D) FACS analysis of the 14-day cells harvested from the methylcellulose-containing medium.
Increased erythroid differentiation of hematopoietic progenitor cells generated from PV iPS cells. To assess erythroid differentiation potential, purified CD34+CD45+ cells from both normal control (NC) and PV iPS cells after EB-mediated hematopoietic differentiation were plated into a liquid culture medium (A,C), or a serum- and methylcellulose-containing medium (B,D). (A) Fold of cell expansion after 7 days of the liquid culture from the purified CD34+CD45+ cells derived from NC (13.5 ± 3.6-fold) or PV iPS cells (27.6 ± 3.3-fold). (B) Fold of cell expansion of the CD34+CD45+ cells after 14 days of the methylcellulose culture, 69.5 ± 24.7-fold of NC versus 127 ± 28.3-fold of the PV iPS cells. Data in panels A and B are presented as mean ± SD (n = 2). (C) FACS analysis of the 7-day cultured cells for the erythroid phenotype (CD235a+CD45−). The percentages of such cell population are indicated in the top left quadrant based on the gating and comparison with background staining. (D) FACS analysis of the 14-day cells harvested from the methylcellulose-containing medium.
A similar enhanced erythropoiesis in the PV sample was observed when the purified CD34+CD45+ cells generated from differentiated iPS cells were grown in a serum and methylcellulose-containing medium. After 14 days of culture, there was a nearly 2-fold proliferation advantage in the PV sample over the normal control (Figure 4C). Flow analysis also showed a greater percentage of cells expressing the erythroid phenotype (Figure 4D).
PV-iPS generated hematopoietic progenitor cells show a PV-unique gene expression pattern similar to the primary CD34+ cells from the PV patient
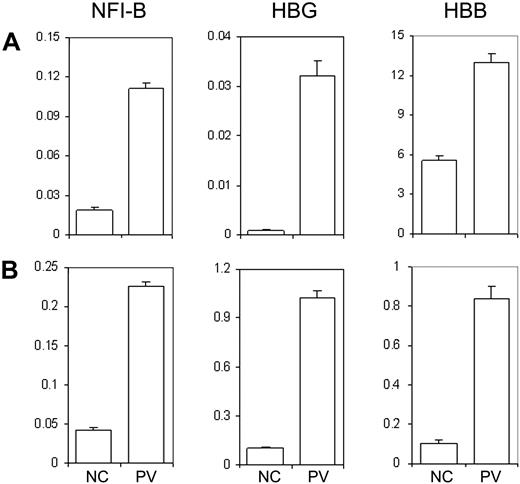
Previously, we conducted whole-genome microarray analysis on purified CD34+ cells from PV patients and normal healthy donors in an effort to identify differentially expressed genes in PV patients. In addition to NFI-B30 and HBG31 genes that have been previously reported, HBB gene expression was also found up-regulated in CD34+ cells of PV patients, including MPD183 (D.M.W., J.L.S., and A.R.M., unpublished data, December 2008). The microarray data of the MPD183 (PV) patient were further confirmed using real-time quantitative PCR analysis of the 3 genes (Figure 5A). Notably, the expression pattern was also observed in the CD34+ cells generated from the PV-iPS cell line that was derived from the corresponding PV patient (Figure 5B). Together, our data shown in Figures 4 and 5 demonstrate that hematopoietic progenitor cells generated from differentiated PV-iPS cells recapitulate principal features of increased erythropoiesis of the primary hematopoietic progenitor cells isolated from the corresponding PV patient.
Human iPS generated hematopoietic progenitor cells exhibit unique gene expression pattern similar to the primary CD34+ cells from the PV patient and a normal control. (A) Total RNA was isolated from primary CD34+ cells from a healthy donor as an NC or from the PV patient (MPD183) where the PV-iPS cell lines were derived from. Gene expression of NFI-B, HBG, and HBB as well as β-actin (as a control) was analyzed by real-time quantitative PCR after reverse transcription of RNA. The normalized level (relative to that of β-actin) is plotted. (B) An identical analysis of purified CD34+CD45+ cells generated from PV iPS (iMPD183) cells and NC iPS cells derived from normal adult CD34+ cells. Data are presented as mean ± SD (n = 2).
Human iPS generated hematopoietic progenitor cells exhibit unique gene expression pattern similar to the primary CD34+ cells from the PV patient and a normal control. (A) Total RNA was isolated from primary CD34+ cells from a healthy donor as an NC or from the PV patient (MPD183) where the PV-iPS cell lines were derived from. Gene expression of NFI-B, HBG, and HBB as well as β-actin (as a control) was analyzed by real-time quantitative PCR after reverse transcription of RNA. The normalized level (relative to that of β-actin) is plotted. (B) An identical analysis of purified CD34+CD45+ cells generated from PV iPS (iMPD183) cells and NC iPS cells derived from normal adult CD34+ cells. Data are presented as mean ± SD (n = 2).
Discussion
In this study, we report the generation of iPS cell lines from CB and adult blood and marrow CD34+ cells as well as their directed hematopoietic differentiation. The technology to derive iPS cells from CB provides the opportunity to generate histocompatible stem cells for many persons because of the large collections in CB banks. Compared with patient-derived fibroblasts and keratinocytes that take weeks to establish, isolated CD34+ cells from blood or marrow (fresh or frozen) just need to be cultured for 2 to 4 days before being reprogrammed by standard protocols. As in vitro expansion of hematopoietic stem/progenitor cells from CB and adult sources remains a challenge, unlimited expansion of derived iPS cells in combination with further optimized hematopoietic differentiation methods should provide a vital alternative way to obtain and amplify histocompatible blood stem cells for blood/BM transplantation purposes. However, more studies are needed to compare the reprogramming efficiency and properties of blood cell–derived iPS cells with those derived from other postnatal cell types.
Our study also demonstrates that MPD-specific iPS cell lines possessing somatic mutations that occur in blood cell lineages can be generated by the standard reprogramming method. The iPS cell lines from MPD (PV and PMF) patient PB displayed the typical morphology and growth pattern as hES and iPS cells when being maintained as undifferentiated pluripotent stem cells. Upon induction, they can differentiate into various cell types originated from the 3 embryonic germ layers, including hematopoietic cells. Notably, the redifferentiated hematopoietic progenitor cells from PV iPS cells showed similarly increased erythropoiesis characteristic of the primary CD34+ cells from patients with PV, a hematopoietic disease signified by overproduction of red blood cells. A full characterization of 2 iPS cell lines derived from a PMF patient (MPD562) is under way. Together, these iPS cell lines will provide a potential model system to study MPD pathogenesis.
Although the identification of JAK2-V617F mutation significantly advanced our understanding of MPD pathogenesis, questions still remain, including how the JAK2-V617F clonal dominance occurs and how 1 mutation contributes to 3 different diseases. Transgenic mouse models suggest that a dosage effect of JAK2-V617F may contribute to different MPD phenotypes.32,33 Consistent with many previous studies indicating that other genetic or epigenetic events could be important to MPD development, recent studies demonstrated that a germline single nucleotide polymorphism is associated with predisposition to the development of JAK2-V617F+ MPDs.34-36 As a complement to these previous studies, the renewable iPS cell lines and subsequent hematopoietic differentiation technologies may provide a novel and prospective model to study MPD pathogenesis. Although the PV iPS-derived hematopoietic progenitor (CD34+CD45+) cells showed a striking similarity in increased erythropoiesis (Figure 4) and gene expression patterns (Figure 5) to the primary CD34+ cells from the same PV patient when being compared with their respective normal controls, we acknowledge a limitation of this study that only a few iPS clones were used in full characterization of their hematopoietic potential. We will need to establish and analyze additional iPS cell lines from more PV and other MPD patients and normal controls. In addition, we are in the process of deriving iPS cell lines from the same patients using both blood cells (that carry the JAK2-V617F mutation) and marrow stromal cells (that lack the JAK2 mutation),37 and we are going to compare their hematopoietic potential after the establishment of paired iPS cell lines. In combination with the improving gene targeting technology in human iPS and ES cells we recently developed,38 these iPS cell lines and their hematopoietic progeny may provide powerful tools to study how JAK2-V617F gene dosages and other genetic variations affect the MPD progenitor cell behavior.
One of the major concerns about the current iPS technology is the use and genomic integration of retroviruses. We predict that the improved methods that achieve reprogramming of human fibroblasts without permanent genome alteration will probably be applicable to that of human blood cells as well.39-43 These methods should allow us to derive adequate patient- or disease-specific iPS cells from blood cells for investigations of various blood diseases with either acquired or inherited mutations.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Ophelia Rogers for conducting quantitative JAK2-V617F allele analyses and Dr Robert Brodsky for discussions and critical reading.
This work was supported by the Johns Hopkins Institute for Cell Engineering, Maryland Cell Research Fund/TEDCO (grant 2009-MSCFII-0047), and the National Institutes of Health (grants R01 HL073781, R01 HL082995, and P01 CA108671).
National Institutes of Health
Authorship
Contribution: Z.Y. designed and performed research, analyzed data, prepared figures, and wrote the paper; H.Z. and P.M. designed and performed research and analyzed data; S.D. conducted research; D.M.W. performed quantitative RT-PCR analysis; Y.-Y.J. contributed experimental/intellectual input in iPS cell culture and hematopoietic differentiation; A.R.M. provided patient samples and clinical guidance and analyzed allele-specific PCR data; J.L.S. and C.V.D. provided patient samples and clinical guidance; L.C. designed research, analyzed data, and wrote the paper; and all authors contributed to writing and editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Linzhao Cheng, Stem Cell Program, Institute for Cell Engineering, Johns Hopkins University School of Medicine, 733 N Broadway, Broadway Research Bldg, Rm 747, Baltimore, MD 21205; e-mail: lcheng@welch.jhu.edu.
References
Author notes
*Z.Y. and H.Z. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal