Abstract
Metastasis-associated antigens 1/2/3 (Mta1/2/3) are components of nucleosome remodeling and deacetylase (NuRD) complexes and have been found to play roles in embryonic development and homeostasis. However, their functions in primitive hematopoiesis are unknown. In this study, we demonstrate that knockdown of mta3 by antisense morpholinos abolishes primitive hematopoietic lineages and causes abnormal angiogenesis in zebrafish embryos. However, the expression of the pronephric duct and paraxial mesoderm markers is unaltered and the specification of angioblasts is unaffected in mta3 morphants. The results suggest that mta3 is specifically required for primitive hematopoiesis. Furthermore, inhibition of deacetylase activity with the inhibitors valproic acid (VPA) or trichostatin A (TSA) in zebrafish embryos completely blocks primitive hematopoiesis, resulting in hematopoietic defects almost identical to those seen in mta3 morphants. Importantly, overexpression of scl or scl and lmo2, 2 master genes for primitive hematopoiesis, is able to overturn effects of mta3 knockdown or VPA/TSA treatment; and overexpression of mta3, and human MBD3 or HDAC1, 2 other components of NuRD complex, enhances the expression of scl and lmo2 in the posterior lateral plate mesoderm during early primitive hematopoiesis. We conclude that Mta3-NuRD complex is essential for the initiation of primitive hematopoiesis. Thus, our findings provide new insight into the regulatory hierarchy of primitive hematopoiesis in vertebrates.
Introduction
Hematopoiesis occurs in 2 successive waves, primitive and definitive hematopoiesis in mammals, birds, and teleosts. In mammalian and avian species, the extraembryonic yolk sac is the site for primitive hematopoiesis that produces primitive macrophages and erythrocytes. In contrast, primitive hematopoiesis in zebrafish progresses in the intermediate cell mass (ICM; an intraembryonic trunk region), which is developed from the ventrolateral plate mesoderm and gives rise to erythrocytes and endothelial linage, as well as in rostral blood island, which arises from the cephalic mesoderm and produces macrophages and endothelial cells.1,2 After the primitive hematopoiesis, definitive hematopoiesis initiates within the ventral wall of dorsal aorta in a region known as aorta-gonad-mesonephros and then transits into fetal liver and bone marrow in mammals, in which long-term hematopoietic stem cells differentiate into erythroid, myeloid, and lymphoid lineages for the life span.3 Zebrafish definitive hematopoiesis also starts in aorta-gonad-mesonephros, but later switches to kidney marrow,3 an analog to bone marrow in mammals.
Zebrafish primitive hematopoietic precursors emerge during early segmentation period from the 1- to 2-somite stage through the 5-somite stage, which is marked by the expression in the anterior lateral mesoderm and/or the posterior lateral mesoderm of several hematopoietic transcription factors, including stem cell leukemia (scl)/T-cell acute lymphocytic leukemia 1 (tal1), lmo2, gata2, and fli1a.4 Studies in mice have demonstrated that Scl plays a critical role in specification and differentiation of primitive hematopoietic precursors, which are responsible for the generation of red blood cells, myeloid cells, megakaryocytes, mast cells, and lymphoid cells.5 In zebrafish, knockdown of scl results in a loss of primitive and definitive hematopoietic cell lineages and abnormal development of endothelial tissues,6-8 whereas overexpression of scl leads to overproduction of hematopoietic and endothelial precursors.9 These findings indicate that zebrafish Scl has a conserved function in hematopoiesis with mammalian species. Scl can form complexes with Lmo2, Gata2, and other transcription factors in hematopoietic cells.5 Consistent with these findings, co-overexpression of scl and lmo2 in zebrafish embryos is able to induce early hematopoietic and endothelial fates throughout the anteroposterior axis.10 Thus, Scl plays a pivotal role in primitive hematopoiesis.
The nucleosome remodeling and deacetylase (NuRD) complexes consist of multiple subunits, including the dermatomyositis-specific autoantigen Mi-2, the histone deacetylases HDAC1/2, the histone binding proteins RbAp46/48, the methyl-CpG binding domain protein MBD3, and the metastasis-associated proteins MTA1/2/3.11,12 The complexes have chromatin remodeling and histone deacetylation properties and function primarily in transcriptional repression. Components of NuRD complexes have been found to play important roles in embryonic development and homeostasis in various organisms13 and in human tumorigenesis.12,14 The exact composition of NuRD complexes varies depending on targets and cell types and plays distinct roles in cell proliferation, survival, and differentiation.
Previous studies have shown that NuRD complexes play critical roles within specific subsets of hematopoietic cells, for example, in megakaryocyte differentiation,15,16 T- and B-lymphocyte development,17-20 erythroid differentiation,21-24 and adult bone marrow hematopoiesis.25 However, it is not known whether NuRD complexes are involved in primitive hematopoiesis. In this study, we investigate the potential roles of NuRD complexes, with a focus on Mta3, in primitive hematopoiesis during zebrafish embryogenesis. We found that knockdown of mta3 inhibited primitive hematopoiesis and this effect was efficiently overridden by overexpression of the hematopoietic master gene scl. Importantly, inhibition of histone deacetylase activity in fish embryos produced primitive hematopoietic defects similar to effects of mta3 knockdown, which were also rescued by scl overexpression. In addition, overexpression of mta3 or other NuRD components enhanced the expression of the earliest hematopoietic markers. Thus, our findings indicate that Mta3-NuRD complex is essential for and acts at the top of the regulatory hierarchy of primitive hematopoiesis.
Methods
Fish maintenance and treatment
Embryos from wild-type Tuebingen strain and Tg(flk1:GFP) transgenic strain were used. Embryos were raised in Holtfreter solution at 28.5°C. For valproic acid (VPA) or trichostatin A (TSA) treatments, embryos grown in Holtfreter solution were dechorionated at the 80% to 90% epiboly stages, transferred to a Holtfreter solution containing indicated concentrations of VPA or TSA, and incubated until desired stages for fixation and in situ hybridization. Ethical approval was obtained from the Animal Care and Use Committee of Tsinghua University.
Generation of constructs
Zebrafish mta2 and mta3 were amplified from a zebrafish cDNA library by polymerase chain reaction and then subcloned into vector pXT7 and pBluescript KS (pBKS) for mRNA and antisense RNA probe synthesis, respectively. The 5′ untranslated region (UTR) and a portion of the coding sequence of zebrafish mta2 or mta3 were amplified and subcloned into vector pEGFP-N3 (Clontech) to generate pmta2-5′UTR–green fluorescent protein (GFP) or pmta3-5′UTR-GFP construct for testing morpholino efficiency. Full-length scl gene was amplified by polymerase chain reaction and subcloned into vector pXT7 for mRNA synthesis. Mouse Lmo2 gene for mRNA synthesis was kindly provided by Dr Feng Liu (State Key Laboratory of Biomembrane and Membrane Biotechnology, Institute of Zoology, Chinese Academy of Sciences, Beijing, People's Republic of China). Human MTA3, HDAC1, and MBD3 cDNA were gifts from Drs Yongfeng Shang (Department of Biochemistry and Molecular Biology, Peking University Health Science Center, Beijing, People's Republic of China) and Jian Zhang (Laboratory of Molecular and Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing, People's Republic of China), and were subcloned in pXT7 for mRNA synthesis.
mRNA synthesis, morpholinos, and microinjection
Capped mRNA was synthesized using Cap-scribe in vitro transcription kits (Roche) following the manufacturer's instruction. Two antisense morpholino oligonucleotides were synthesized by Gene Tools LLC, to target mta3: the translation blocker tMO (5′-CGCCATTTCTGCCCTCCCGGAGTGT-3′), which targets the 5′UTR of mta3, and the splicing blocker sMO (5′-CGTGTTCACTCACTTGCGTGTTTGT-3′), which targets the boundary sequence between the third exon and the third intron. The control morpholino cMO had a sequence identical to tMO except for 6 mismatched nucleotides (underlined; 5′-CGCCATTGCTGAACTCAAGGAGTGT-3′). The translation blocker for mta2 had a sequence of 5′-CATTCTCTCGCTCTCCTAAACAAAC-3′. The morpholino targeting p53 (5′-GCGCCATTGCTTTGCAAGAATTG-3) was purchased from Gene Tool LLC. The mRNAs and morpholinos were injected into embryos from 1-cell to 2-cell stage.
In situ hybridization, TUNEL assay, and histologic staining
Digoxigenin or fluorescein uridine triphosphate–labeled antisense RNA probe was generated by in vitro transcription. Whole-mount in situ hybridization was performed following the standard procedures.
DNA fragmentation was detected by terminal transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) assay using the ApoTag in situ detection kit (Chemicon). Embryos were fixed in 4% paraformaldehyde and dehydrated with methanol at −20°C overnight. After rehydration in phosphate-buffered saline (PBS), embryos were permeabilized by digestion with 10 μg/mL proteinase K (Sigma). Then, the embryos were fixed with 4% paraformaldehyde for 20 minutes and washed with PBS. Next, the embryos were postfixed with prechilled (−20°C) ethanol–acetic acid (2:1) at −20°C for 5 minutes, washed twice with PBS, and quenched in 3.0% hydrogen peroxide in PBS for 5 minutes at room temperature. After washing with PBS, the embryos were equilibrated, labeled, and stained following the manufacturer's instructions.
Staining of phosphohistone H3 in zebrafish embryos was performed as described26 with Ser-10 phosphohistone H3 antibody (Cell signaling) and detected with peroxidase-conjugated antibody (Sigma) and 3,3′-diaminobenzidine (DAB) substrate (Sigma).
For detection of hemoglobin in red blood cells, O-dianisidine was dissolved in 100% ethanol at a final concentration of 1.43 mg/mL and the staining was performed as described.27
Photography
Live embryos and stained embryos after in situ hybridization or immunohistochemical staining were mounted in 5% methylcellulose and glycerol, respectively, and photographed using a SPOT Insight 4MP Firewire Color Mosaic digital camera driven by SPOT advanced 4.6 software (Diagnostic Instruments) under a Leica MZ16 microscope (1× Planapo objective, numerical aperature 0.14, adjustable internal lens ranging from 0.71× to 11.5× magnification; Leica). Tg(flk1:GFP) transgenic embryos were mounted in 5% methylcellulose and photographed using a Photometrics CoolSNAP ES2 digital camera driven by RS Image 1.9.2 software (Roper Scientific) under a Leica MZ16F microscope (1× Planapo objective, numerical aperature 0.14, adjustable internal lens ranging from 0.71× to 11.5× magnification; Leica). Images were captured at 6.3× to 11.5× magnification depending on different requirements and processed with Adobe Photoshop CS2 Version 9.0 software (Adobe Systems).
Results
mta3 is ubiquitously expressed during embryogenesis in zebrafish
In zebrafish, 2 metastasis-associated (MTA) family members, Mta2 and Mta3, have been identified (http://zfin.org).28 Zebrafish Mta2 and Mta3 share an overall identity in amino acid sequence of 81% and 80% to human MTA2 and MTA3, respectively. We cloned zebrafish mta2 and mta3 genes and studied their spatiotemporal expression pattern during embryogenesis by whole-mount in situ hybridization pattern. Maternally supplied mta3 transcripts are distributed evenly in all blastomeres before the midblastula transition (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Zygotic mta3 is ubiquitously expressed from the midblastula onward. From the 18-somite stage to 24 hours after fertilization (hpf), mta3 expression occurs in the brain and spinal cord at higher levels. Notably, it is obviously expressed in the intermediate cell mass (ICM). The expression pattern of mta2 is similar to mta3 (supplemental Figure 2). Because mta2 knockdown did not block primitive hematopoiesis (supplemental Figure 4), we focused on mta3 thereafter.
mta3 is essential for primitive hematopoiesis in zebrafish embryos
To investigate the function of mta3 in development of zebrafish embryos, we first adopted morpholino knockdown approach to block expression of endogenous mta3 gene. We designed 2 morpholinos targeting mta3, tMO that targets 5′ untranslated region and presumably blocks translation of mta3, and sMO that targets the boundary sequence between the third exon and third intron and presumably causes abnormal splicing of primary transcript of mta3. A morpholino (cMO) similar to tMO except for 6 mismatched nucleotides served as a control. As shown in supplemental Figure 3, tMO was indeed able to block translation of mta3, whereas cMO had no effect; and sMO could intervene with splicing of mta3 primary transcript. As observed morphologically at 24 hpf, tMO- and sMO-injected embryos showed gross abnormalities in appearance, with extensive necrosis and growth retardation, whereas cMO-injected embryos appeared normal (supplemental Figure 3). Importantly, tMO and sMO injections produced the same effect on primitive hematopoiesis as shown in Figure 1. Although mta2 morpholino was effective, its injection had little effect on the expression ofthe primitive erythropoietic marker gata1 (supplemental Figure 4), which would exclude the possibility that mta2 is also required for primitive hematopoiesis. Therefore, our analyses were focused on mta3 thereafter.
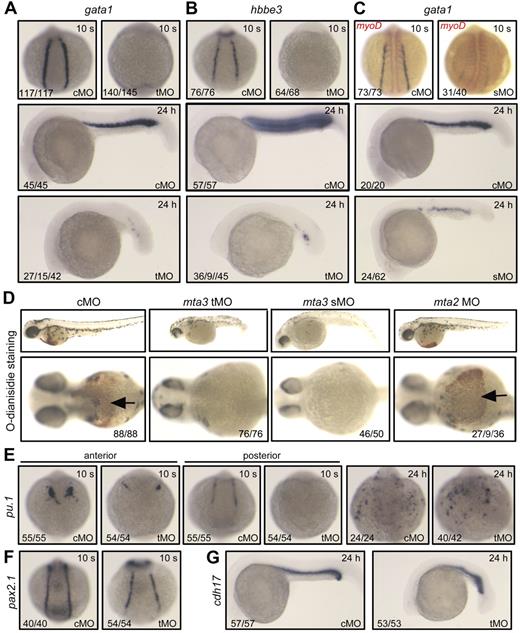
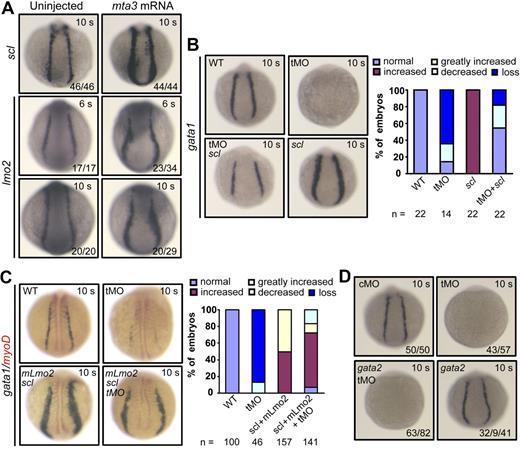
Effect of mta3 knockdown on hematopoietic marker expression. (A-C) The expression of the erythroblast marker gata1 (A,C, in blue), the erythrocyte marker hbbe3 (B), and the paraxial mesoderm marker myod (C, in red) in cMO-, tMO-, or sMO-injected embryos at the 10-somite (10 s, dorsal view) stage and 24 hpf (24 hours, lateral view). The numbers indicated in the lower lefthand corner of each picture are the number (left) of affected embryos with phenotype similar to what is shown in the picture and the total number (right) of observed embryos. For gata1 expression in tMO-injected embryos at 24 hpf, the numbers from left to right were the number of embryos without expression and of embryos with reduced expression and the total number of observed embryos. The same number labeling was used thereafter. (D) Detection of hemoglobin in red blood cells staining with O-dianisidine at 48 hpf. Embryos in the top panel are shown in lateral view at lower magnification. Their anterior regions are shown in ventral view at higher magnification in the bottom panel, with indicating the red blood cells. (E-G) The expression of the myeloid marker pu.1 (E) and the pronephric duct markers pax2.1 (F) and cdh17 (G) in cMO- or tMO-injected embryos. Note that knockdown of mta3 resulted in reduction of pu.1 expression in anterior lateral mesoderm but in its loss in posterior lateral mesoderm at the 10-somite stage (dorsal views), and that mta3 knockdown had little effect on pu.1 expression at 24 hpf (dorsoanterior views). The expression of pax2.1 and cdh17 appeared normal in mta3 morphants.
Effect of mta3 knockdown on hematopoietic marker expression. (A-C) The expression of the erythroblast marker gata1 (A,C, in blue), the erythrocyte marker hbbe3 (B), and the paraxial mesoderm marker myod (C, in red) in cMO-, tMO-, or sMO-injected embryos at the 10-somite (10 s, dorsal view) stage and 24 hpf (24 hours, lateral view). The numbers indicated in the lower lefthand corner of each picture are the number (left) of affected embryos with phenotype similar to what is shown in the picture and the total number (right) of observed embryos. For gata1 expression in tMO-injected embryos at 24 hpf, the numbers from left to right were the number of embryos without expression and of embryos with reduced expression and the total number of observed embryos. The same number labeling was used thereafter. (D) Detection of hemoglobin in red blood cells staining with O-dianisidine at 48 hpf. Embryos in the top panel are shown in lateral view at lower magnification. Their anterior regions are shown in ventral view at higher magnification in the bottom panel, with indicating the red blood cells. (E-G) The expression of the myeloid marker pu.1 (E) and the pronephric duct markers pax2.1 (F) and cdh17 (G) in cMO- or tMO-injected embryos. Note that knockdown of mta3 resulted in reduction of pu.1 expression in anterior lateral mesoderm but in its loss in posterior lateral mesoderm at the 10-somite stage (dorsal views), and that mta3 knockdown had little effect on pu.1 expression at 24 hpf (dorsoanterior views). The expression of pax2.1 and cdh17 appeared normal in mta3 morphants.
To look into the effect of mta3 knockdown on primitive hematopoiesis, we examined the expression of the primitive erythropoietic marker gata1 and the erythroid-specific marker hbbe3 in the posterior lateral mesoderm at the 10-somite stage (14 hpf) and in the ICM at 24 hpf. The cMO (5 ng)–injected embryos exhibited normal expression of gata1 and hbbe3. In contrast, gata1 and hbbe3 expression was lost in almost all of the tMO (5 ng)–injected embryos at the 10-somite stage (14 hpf), and were diminished or remarkably reduced in the tMO-injected embryos at 24 hpf (Figure 1A-B). Similarly, injection with 10 ng of mta3 sMO resulted in elimination or dramatic decrease of gata1 expression of embryos at 14 hpf and 24 hpf (Figure 1C). Accordingly, tMO- or sMO-injected embryos at day 2 lacked red blood cells, as assayed by O-dianisidine heme staining (Figure 1D). The myeloid lineage marker pu.1 was reduced in anterior lateral mesoderm and eliminated in the posterior lateral plate in all of tMO-injected embryos (Figure 1E). At 24 hpf, pu.1 expression in the anterior region of tMO morphants was restored to a normal level, which was consistent with the previous finding that hematopoiesis in anterior lateral mesoderm and posterior lateral mesoderm may use different mechanisms.29 In contrast to the hematopoietic effects, knockdown of mta3 with tMO had no effect on the expression of the pronephric duct markers pax2.1 (Figure 1F) and cdh17 (Figure 1G), the axial mesoderm marker sonic hedgehog, and the paraxial mesoderm/myogenic marker myod (data not shown). Taken together, these data suggest that mta3 is essential for primitive hematopoiesis in zebrafish embryos.
mta3 is expendable for fate determination of endothelial precursors but required for angiogenesis
It is believed that primitive hematopoietic and vascular precursors may be derived from common hemangioblasts in zebrafish.2,30 We then examined the expression of several endothelial markers in mta3 tMO morphants at 24 hpf. We found that the VEGF receptor flk1 and the endothelial-specific transcription factor fli1a were expressed in the dorsal aorta and cardinal veins of the tMO morphants at levels comparable with those in the control (supplemental Figure 5A-B), and that the expression of the endothelial markers tie1 and tie2 appeared slightly reduced (supplemental Figure 5C-D). However, the expression of flk1, fli1a, and tie1 in the intersegmental vessels was lost or incomplete in tMO-injected embryos. Knockdown of mta3 in Tg(flk1:EGFP) transgenic embryos caused similar phenotypes as observed by fluorescent microscopy (supplemental Figure 5E). These observations suggest that mta3 may not be required for specification of angioblasts at earlier stages but it is needed for angiogenesis at later stages.
Overexpression of mta3 promotes primitive hematopoiesis
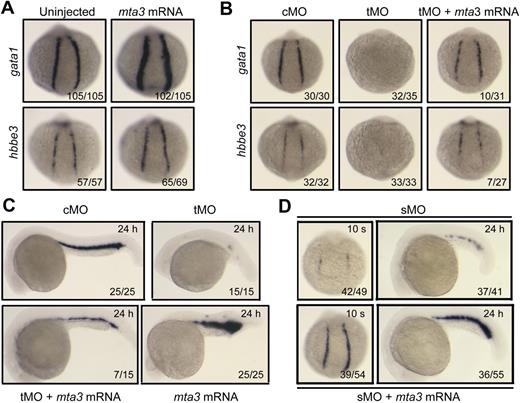
Next we asked whether overexpression of mta3 could promote primitive hematopoiesis. When injected with 350 pg of synthetic mta3 mRNA at the 1-cell stage, embryos showed enhanced expression of gata1 and hbbe3 in the posterior lateral mesoderm at 14 hpf (Figure 2A), indicating that mta3 plays a positive role in erythroid development. Importantly, coinjection of 350 pg of mta3 mRNA with 5 ng of mta3 tMO was able to restore gata1 and hbbe3 expression to normal levels in the posterior lateral mesoderm at 14 hpf (Figure 2B) in a proportion of embryos (> 26%), and to restore gata1 expression in the ICM of embryos (46.7%) at 24 hpf (Figure 2C), supporting the idea that the tMO specifically targets mta3. The sMO-induced reduction of gata1 expression at the 10-somite stage and 24 hpf was also efficiently restored by coinjection of mta3 mRNA (Figure 2D), which further confirms the role of mta3 in primitive hematopoiesis.
Effect of mta3 overexpression on primitive erythropoiesis. (A) The expression of gata1 and hbbe3 at the 10-somite stage was increased when 350 pg of mta3 mRNA was injected. Embryos were in posterodorsal view with anterior to the top. (B-C) Overexpression of mta3 mRNA partially rescued gata1 and hbbe3 expression in mta3 tMO-injected embryos at the 10-somite stage (B) or gata1 expression at 24 hpf (C). (D) The expression of gata1 was reduced in 10 ng of mta3 sMO-injected embryos at the 10-somite stage and at 24 hpf, and this effect was efficiently rescued by coinjection of 350 pg of mta3 mRNA.
Effect of mta3 overexpression on primitive erythropoiesis. (A) The expression of gata1 and hbbe3 at the 10-somite stage was increased when 350 pg of mta3 mRNA was injected. Embryos were in posterodorsal view with anterior to the top. (B-C) Overexpression of mta3 mRNA partially rescued gata1 and hbbe3 expression in mta3 tMO-injected embryos at the 10-somite stage (B) or gata1 expression at 24 hpf (C). (D) The expression of gata1 was reduced in 10 ng of mta3 sMO-injected embryos at the 10-somite stage and at 24 hpf, and this effect was efficiently rescued by coinjection of 350 pg of mta3 mRNA.
mta3 functions in primitive hematopoiesis independent of cell proliferation and apoptosis
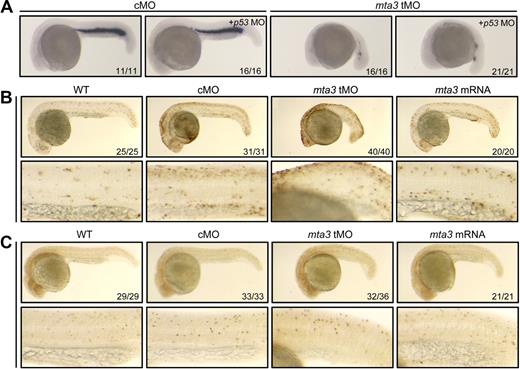
Loss of hematopoietic cells in mta3 morphants could be attributed to 3 mechanisms: first, extensive apoptosis of hematopoietic cells; second, aberrant proliferation of hematopoietic cells; and third, lack of hematopoietic precursors at the very beginning of the primitive hematopoiesis. A previous report demonstrated that off-target effects of morpholino injections in fish embryos, manifested mainly as extensive cell death, are induced through activation of p53 activation and can be ameliorated by coknockdown of p53 gene.31 We found that, like single injection with 5 ng of tMO, coinjection of 5 ng of tMO and 5 ng of p53 MO still caused loss of gata1 expression in the ICM (Figure 3A), which excludes the possibility that inhibition of gata1 expression by tMO injection is a p53-related off-target effect. Then, we exploited terminal transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) assay to examine DNA fragmentations in apoptotic cells. The number of apoptotic cells in the ICM of embryos injected with tMO or mta3 mRNA was comparable with that in cMO-injected embryos (Figure 3B). Thus, either knockdown or overexpression of mta3 had no effect on apoptosis of hematopoietic cells. To investigate the effect of mta3 on cell proliferation, we stained phosphohistone H3, which marks condensed chromosomes in metaphase and early anaphase cells, using a phospho-H3 antibody. As shown in Figure 3C, there was not much difference in the number of phosphohistone H3–positive cells in the ICM among uninjected, and cMO-, tMO- and mta3 mRNA-injected embryos. Furthermore, we failed to detect changes in apoptosis and cell proliferation in tMO- or mta3 mRNA-injected embryos at earlier stages (3-somite and 10-somite stages; data not shown). Taking together, we hypothesize that mta3 functions in the specification of primitive hematopoietic precursors rather than their survival or proliferation.
mta3 knockdown has no effect on hematopoietic cell apoptosis and proliferation. (A) gata1 expression was similarly affected by injection with 5 ng of tMO alone or in combination with 5 ng of p53 MO. It suggests that tMO-induced hematopoietic defects are not associated with off-target effect by MO-activated p53 signaling pathway. (B) Cell apoptosis was detected by TUNEL assay in 24 hpf embryos. The bottom panel showed an enlarged trunk region in which intermediate cell mass (ICM) resided. Injection with mta3 tMO or mRNA had no obvious effect on cell apoptosis within the ICM. (C) Cell proliferation was detected by whole-mount in situ immunohistochemistry using phospho-H3 antibody in 24 hpf embryos. The bottom panel showed ICM regions at higher magnification. Injection with mta3 tMO or mRNA had no obvious effect on cell proliferation within the ICM. All embryos were lateral views with anterior to the left.
mta3 knockdown has no effect on hematopoietic cell apoptosis and proliferation. (A) gata1 expression was similarly affected by injection with 5 ng of tMO alone or in combination with 5 ng of p53 MO. It suggests that tMO-induced hematopoietic defects are not associated with off-target effect by MO-activated p53 signaling pathway. (B) Cell apoptosis was detected by TUNEL assay in 24 hpf embryos. The bottom panel showed an enlarged trunk region in which intermediate cell mass (ICM) resided. Injection with mta3 tMO or mRNA had no obvious effect on cell apoptosis within the ICM. (C) Cell proliferation was detected by whole-mount in situ immunohistochemistry using phospho-H3 antibody in 24 hpf embryos. The bottom panel showed ICM regions at higher magnification. Injection with mta3 tMO or mRNA had no obvious effect on cell proliferation within the ICM. All embryos were lateral views with anterior to the left.
mta3 is required for specification of the earliest hematopoietic precursors
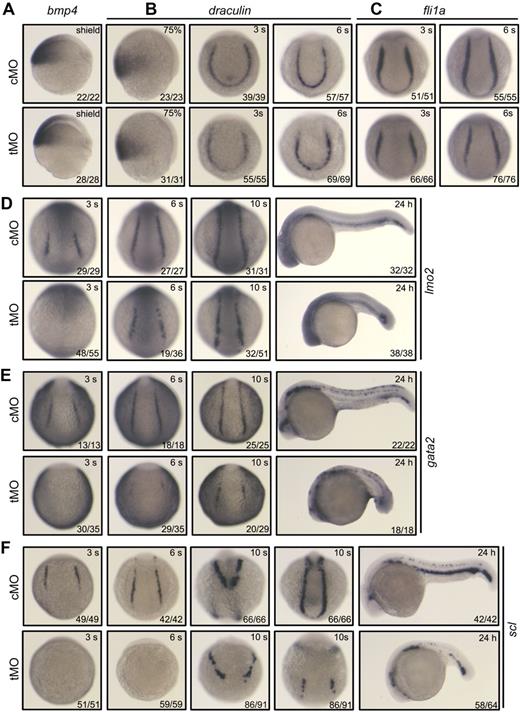
To verify the requirement of mta3 for specification of primitive hematopoietic precursors, we examined the expression of genes that establish the regulatory hierarchy of hemangioblasts and initiation of primitive hematopoiesis. Bmp signals are required for development of ventral mesoderm that gives rise to hemangioblasts,32,33 and knockdown of mta3 with tMO had no effect on the expression of bmp4 (Figure 4A). The expression of draculin marks ventral mesoderm during gastrulation and presumptive hematopoietic precursors during early segmentation, and it was not affected in mta3 morphants (Figure 4B). Knockdown of mta3 did not alter the expression of fli1a (Figure 4C), which is believed to be a marker for hemangioblasts.34,35 Lmo2, a LIM domain transcription factor, is expressed in both hematopoietic and endothelial precursors.36 lmo2 expression was not detected in mta3 morphants at the 3-somite stage, but it appeared almost recovered from the 6-somite stage onward (Figure 4D), which suggests that lmo2 expression at initial stages of primitive hematopoiesis requires mta3 functions but otherwise it could be resumed by mta3-independent signals at later stages.
Effect of mta3 knockdown on the expression of early mesoderm markers and hematopoietic precursor markers. (A-F) Embryos at the 1-cell stage were injected with 5 ng of cMO or tMO and examined for the expression of the indicated markers at indicated stages. Embryos at the shield stage or 75% epiboly stage are shown in lateral view; those at the 3-somite, 6-somite, or 10-somite stage were in posterodorsal view; and those at 24 hpf were in lateral view. Note that the expression of gata2 (E) and scl (F) was eliminated or dramatically decreased at all tested stages.
Effect of mta3 knockdown on the expression of early mesoderm markers and hematopoietic precursor markers. (A-F) Embryos at the 1-cell stage were injected with 5 ng of cMO or tMO and examined for the expression of the indicated markers at indicated stages. Embryos at the shield stage or 75% epiboly stage are shown in lateral view; those at the 3-somite, 6-somite, or 10-somite stage were in posterodorsal view; and those at 24 hpf were in lateral view. Note that the expression of gata2 (E) and scl (F) was eliminated or dramatically decreased at all tested stages.
The transcription factor Gata2 is also expressed in hemangioblasts/hematopoietic precursors.1 Interestingly, we found that gata2 expression was abolished at the 3- and 6-somite stages in the posterior lateral mesoderm, but it was detected at lower levels at the 10-somite stage and 24 hpf (Figure 4E).
The expression of the earliest hematopoietic marker scl, which is capable of converting mesoderm into hemangioblasts/hematopoietic precursors in zebrafish embryos,9,10 was eliminated in all of mta3 morphants at the 3- and 6-somite stages (Figure 4F). Later, around the 10-somite stage, scl expression appeared in the anterior lateral mesoderm with slightly reduced levels and occurred in the posterior lateral mesoderm at much lower levels; at 24 hpf, the number of scl-positive cells in the ICM was markedly reduced (Figure 4F). Considering that early scl-positive cells (hemangioblasts) can contribute to different hematopoietic lineages and endothelial precursors, we hypothesize that mta3 is essential for the initiation of scl expression in hemangioblasts, but it may not be necessary for scl expression in all lineages after differentiation of hemangioblasts.
To confirm that mta3 acts at the top of the regulatory hierarchy of primitive hematopoiesis, we injected 350 pg of mta3 mRNA at the 1-cell stage and examined the expression of scl and lmo2 during early somitogenesis. We found that expression of both scl and lmo2 was expanded in posterior lateral mesoderm in mta3-injected embryos (Figure 5A), whereas the expression of pronephric duct markers atp1b1a, cdh17, and slc12a3 was unaltered (supplemental Figure 6). It was reported that overexpression of scl or scl and lmo2 can expand gata1 expression.10 We found that injection of 375 pg of zebrafish scl rescued gata1 expression in mta3-depleted embryos at the 10-somite stage (Figure 5B), and that co-overexpression of 375 pg of scl and 50 pg of mouse Lmo2 (mLmo2) was still able to expand gata1 expression in mta3-depleted embryos (Figure 5C). These data suggest that Scl/Lmo2 act downstream of Mta3 for primitive hematopoiesis. However, gata2 overexpression failed to rescue gata1 expression in tMO-injected embryos. This is not surprising since gata2 overexpression alone did not enhance gata1 expression and even caused some embryos to reduce gata1 expression (Figure 5D), which is consistent with the finding that up-regulation of GATA2 in mammalian erythroid cell lines leads to decrease of GATA1 expression.37,38
mta3 acts upstream of scl and lmo2 during primitive hematopoiesis. (A) Injection of 350 pg of mta3 mRNA enhanced expression of the hematopoietic precursor markers scl and lmo2 in posterior lateral mesoderm. All embryos are shown in posterodorsal view. (B) Injection of 375 pg of scl mRNA enhanced gata1 expression in wild-type embryos and rescued gata1 expression in mta3 morphants at the 10-somite stage. Corresponding statistical data are shown on the right. (C) Coinjection of 375 pg of scl and 50 pg of mLmo2 mRNAs enhanced gata1 expression at the 10-somite stage, even if mta3 was knocked down simultaneously. The expression of the paraxial mesoderm marker myod was stained in red. Corresponding statistical data are shown on the right. (D) The majority (32/41) of embryos injected with 300 pg of gata2 mRNA showed normal expression of gata1 and the remaining portion (9/41) of the injected embryos showed slightly reduced expression of gata1. Coinjection of gata2 mRNA with mta3 tMO failed to restore gata1 expression.
mta3 acts upstream of scl and lmo2 during primitive hematopoiesis. (A) Injection of 350 pg of mta3 mRNA enhanced expression of the hematopoietic precursor markers scl and lmo2 in posterior lateral mesoderm. All embryos are shown in posterodorsal view. (B) Injection of 375 pg of scl mRNA enhanced gata1 expression in wild-type embryos and rescued gata1 expression in mta3 morphants at the 10-somite stage. Corresponding statistical data are shown on the right. (C) Coinjection of 375 pg of scl and 50 pg of mLmo2 mRNAs enhanced gata1 expression at the 10-somite stage, even if mta3 was knocked down simultaneously. The expression of the paraxial mesoderm marker myod was stained in red. Corresponding statistical data are shown on the right. (D) The majority (32/41) of embryos injected with 300 pg of gata2 mRNA showed normal expression of gata1 and the remaining portion (9/41) of the injected embryos showed slightly reduced expression of gata1. Coinjection of gata2 mRNA with mta3 tMO failed to restore gata1 expression.
Deacetylation activity of NuRD complexes is essential for primitive hematopoiesis
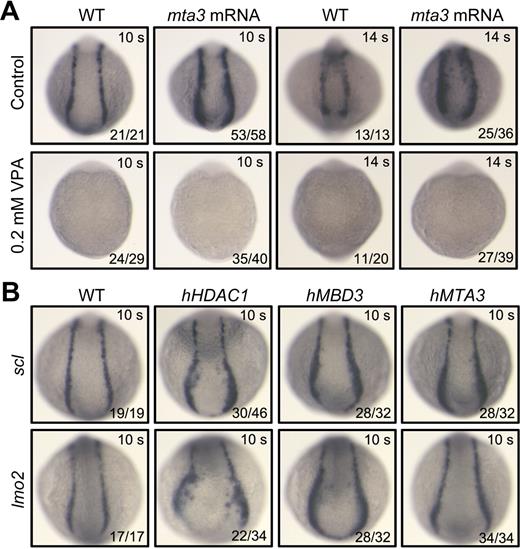
It has been found that Mta3/NuRD complexes repress transcription in an HDAC-dependent manner.39 We then asked whether the effect of mta3 on zebrafish primitive hematopoiesis is HDAC dependent. To address this issue, we used valproic acid (VPA), a drug that can inhibit deacetylase activity of HDACs in vitro and in vivo.40-43 Embryos injected at the 1-cell stage with 350 pg of mta3 mRNA were allowed to develop until 90% epiboly stage and then transferred to a media containing 0.2mM VPA for further development. As shown in Figure 6A, the expression of sc1 at the 10-somite or 14-somite stage was totally eliminated in most of uninjected embryos or in mta3-injected embryos in the presence of VPA. These results imply that overexpression of mta3 is unable to promote primitive hematopoiesis in the absence of HDAC activity, or in other words, that function of mta3 is HDAC dependent.
mta3 function is dependent on HDAC activity and other components of NuRD complex enhance primitive hematopoiesis. (A) Dependence of mta3 function on HDAC activity. Wild-type embryos or mta3 (350 pg)–injected embryos at 80 to 90 epiboly stages were incubated in Holtfreter solution (control) or in 0.2mM VPA (a deacetylase inhibitor) and fixed at the 10-somite or 14-somite stages for examining scl expression. Note that mta3 overexpression failed to restore scl expression in the presence of VPA, suggesting a requirement of HDAC activity for mta3 function. (B) scl and lmo2 expression in the posterior lateral mesoderm at the 10-somite stage was enhanced by overexpression of human HDAC1 (340 pg), MBD3 (500 pg), or MTA3 (500 pg). All embryos are shown in posterodorsal view.
mta3 function is dependent on HDAC activity and other components of NuRD complex enhance primitive hematopoiesis. (A) Dependence of mta3 function on HDAC activity. Wild-type embryos or mta3 (350 pg)–injected embryos at 80 to 90 epiboly stages were incubated in Holtfreter solution (control) or in 0.2mM VPA (a deacetylase inhibitor) and fixed at the 10-somite or 14-somite stages for examining scl expression. Note that mta3 overexpression failed to restore scl expression in the presence of VPA, suggesting a requirement of HDAC activity for mta3 function. (B) scl and lmo2 expression in the posterior lateral mesoderm at the 10-somite stage was enhanced by overexpression of human HDAC1 (340 pg), MBD3 (500 pg), or MTA3 (500 pg). All embryos are shown in posterodorsal view.
As Mta3 is only one of the components of NuRD complexes, we extended our investigation to the other 2 components, Hdac1 and Mbd3, by injecting human HDAC1 or MBD3 mRNA (Figure 6C). First, we found that, like overexpression of zebrafish mta3 mRNA, overexpression of human MTA3 mRNA (500 pg) in zebrafish embryos enhanced scl and lmo2 expression in the posterior lateral mesoderm of embryos at the 10-somite stage (Figure 6B). Then, we noted that injection with 340 pg of human HDAC1 mRNA or 500 pg of human MBD3 mRNA enhanced scl and lmo2 expression in the posterior lateral mesoderm in the majority of embryos at the 10-somite stage (Figure 6B). These observations suggest that the NuRD complex–mediated transcription regulation plays a positive role in primitive hematopoiesis.
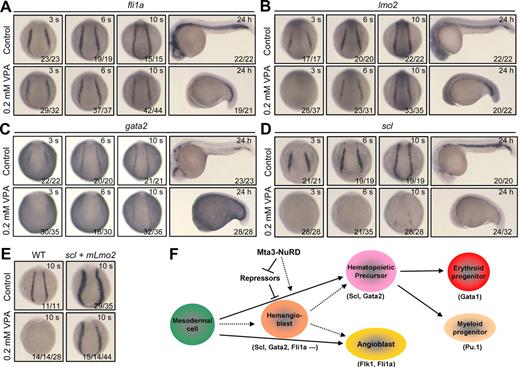
We set forth to further investigate the effect of histone deacetylation on primitive hematopoiesis by examining the expression of the hemangioblast/early hematopoietic markers after inhibition of deacetylase activity with the deacetylase inhibitors VPA or TSA. The expression of gata1 was also abolished at various stages when VPA or TSA was applied (supplemental Figure 7). However, VPA treatment had little effect on pax2.1 expression in the pronephric ducts (supplemental Figure 8), suggesting a specific effect of VPA treatment on primitive hematopoiesis. After treatment in 0.2mM VPA starting at the 80% to 90% epiboly stages, almost all of embryos showed normal expression of fli1a (Figure 7A), delay of lmo2 initiation followed by normal expression (Figure 7B), and absence or dramatic decrease of gata2 (Figure 7C) and scl (Figure 7D) expression. When the VPA concentration reduced to 0.1mM, similar changes in the markers' expression were observed (supplemental Figure 9). Furthermore, TSA treatments exerted similar effects on the expression of the same set of markers (supplemental Figure 10). The VPA- or TSA-induced phenotypes resemble those observed in mta3 morphants (Figures 1,4), implying that at least 2 components of NuRD complexes, Hdac and Mta3, are equally important for primitive hematopoiesis. Also similar to scl and lmo2 overexpression in mta3 morphants, co-overexpression of scl and mLmo2 was able to expand gata1 expression in the erythroid progenitors at the 10-somite stage in the presence of VPA (Figure 7E). Taken together, these data indicate that NuRD complexes act at the top of the regulatory hierarchy of primitive hematopoiesis in zebrafish embryos.
HDAC activity is required for initiation of primitive hematopoiesis. (A-D) Effect of VPA treatment on the expression of fli1a, lmo2, gata2, and scl. Wild-type embryos at 80% to 90% epiboly stages were incubated in 0.2mM VPA and fixed at indicated stages for examination of markers expression by whole-mount in situ hybridization. Embryos at segmentation stages are shown in posterodorsal view, whereas those at 24 hpf are shown in lateral view. (E) gata1 expression in wild-type embryos or embryos coinjected with 375 pg of scl and 50 pg of mLmo2 mRNAs at the 10-somite stage (posterodorsal view). Fifty percent (14/14) of VPA-treated embryos lacked gata1 expression and the other half showed very weak expression of gata1. In contrast, even in the presence of VPA, co-overexpression of scl and mLmo2 resulted in 15 of 44 of embryos with enhanced gata1 expression and 14 of 44 of embryos with normal expression of gata1, suggesting that scl and lmo2 act downstream of HDAC activity. (F) A model for Mta3-NuRD functions in primitive hematopoiesis. Hematopoietic precursors and angioblasts may be independently specified from the ventrolateral mesodermal cells or may be derived from the common ancestor cell population, hemangioblasts. Mta3-NuRD complex activates the expression of the master hematopoietic transcription factors Scl and Gata2, allowing the commitment of mesodermal cells to the hematopoietic fate. Mta3-NuRD complex may activate Scl and Gata2 expression indirectly by suppressing the expression of their repressors, or directly by remodeling their promoter chromatin or deacetylating repressor proteins.
HDAC activity is required for initiation of primitive hematopoiesis. (A-D) Effect of VPA treatment on the expression of fli1a, lmo2, gata2, and scl. Wild-type embryos at 80% to 90% epiboly stages were incubated in 0.2mM VPA and fixed at indicated stages for examination of markers expression by whole-mount in situ hybridization. Embryos at segmentation stages are shown in posterodorsal view, whereas those at 24 hpf are shown in lateral view. (E) gata1 expression in wild-type embryos or embryos coinjected with 375 pg of scl and 50 pg of mLmo2 mRNAs at the 10-somite stage (posterodorsal view). Fifty percent (14/14) of VPA-treated embryos lacked gata1 expression and the other half showed very weak expression of gata1. In contrast, even in the presence of VPA, co-overexpression of scl and mLmo2 resulted in 15 of 44 of embryos with enhanced gata1 expression and 14 of 44 of embryos with normal expression of gata1, suggesting that scl and lmo2 act downstream of HDAC activity. (F) A model for Mta3-NuRD functions in primitive hematopoiesis. Hematopoietic precursors and angioblasts may be independently specified from the ventrolateral mesodermal cells or may be derived from the common ancestor cell population, hemangioblasts. Mta3-NuRD complex activates the expression of the master hematopoietic transcription factors Scl and Gata2, allowing the commitment of mesodermal cells to the hematopoietic fate. Mta3-NuRD complex may activate Scl and Gata2 expression indirectly by suppressing the expression of their repressors, or directly by remodeling their promoter chromatin or deacetylating repressor proteins.
Discussion
Although NuRD complexes have been shown to be involved in megakaryocyte differentiation, T- and B-lymphocyte development, and erythroid differentiation, their involvements in primitive hematopoiesis in vertebrates have not been reported previously. In this study, we have found that mta3 is essential for primitive hematopoiesis in zebrafish embryos by positively regulating the expression of scl, a key player in hematopoiesis. We further demonstrate that the other components of NuRD complexes, in particular HDACs, are also involved in the initiation of primitive hematopoiesis. Our findings place the Mta3-containing NuRD complex at the top of the regulatory hierarchy of primitive hematopoiesis (Figure 7F).
Several lines of evidence support the conclusion that Mta3-containing NuRD complex is indispensable for the initiation of primitive hematopoiesis. First, knockdown of mta3 inhibited the expression of the hemangioblast/hematopoietic precursor markers scl and gata2 during early segmentation period, and as a result, the expression of the erythroid marker gata1, the hemoglobin gene hbbe3, and the myeloid marker pu.1 was blocked at later stages. The effect of mta3 knockdown was rescued by coinjection of mta3 mRNA. Second, embryos treated with the deacetylase inhibitor VPA failed to initiate primitive hematopoiesis and produced phenotypes almost identical to those observed in mta3 morphants. Third, overexpression of zebrafish mta3 or human MTA3, human MBD3, or human HDAC1 in zebrafish embryos enhanced the expression of the markers scl, lmo2, and gata1. Fourth, overexpression of scl or scl and lmo2 could overcome the inhibitory effect of mta3 knockdown or VPA treatment on hematopoiesis. Interestingly, a recent paper has reported that zebrafish hdac1−/− mutants lack definitive hematopoietic stem cells,44 but show normal primitive hematopoiesis. It is likely that multiple Hdac family members play redundant functions in zebrafish primitive hematopoiesis.
We found that unlike mta3, knocking down mta2 in zebrafish had no effect on primitive hematopoiesis. This observation is not surprising since distinct NuRD complexes containing different components may have disparate functions.14 For example, Mta3 overexpression in transgenic mice inhibits the ductal side branching in mammary glands due to reduced cell proliferation and differentiation by directly suppressing Wnt4 expression,39 whereas Mta1 overexpression in transgenic mice causes an excessive branching and precocious development of the mammary gland due to a higher degree of proliferation and differentiation.45
Primitive hemangioblasts/hematopoietic precursors are essentially derived from ventral mesoderm in fish and amphibians. In zebrafish, mta3 is expressed maternally and zygotically, which raises the possibility that Mta3-NuRD complex may regulate primitive hematopoiesis indirectly by controlling ventral mesoderm development. However, this possibility would be excluded by 2 lines of evidence. One is that in mta3 morphants dorsoventral patterning appeared unaffected and the ventral mesoderm–derived pronephric ducts were retained. Another piece of evidence is that VPA treatment starting at late gastrulation stages could abolish the initiation of scl expression. Thus, we speculate that Mta3-NuRD complex may function to regulate the specification of the hemangioblasts/earliest hematopoietic precursors at the onset of primitive hematopoiesis. The mechanisms underlying Mta3-NuRD function in primitive hematopoiesis remain unknown at this stage. Because NuRD complexes in most cases act as a transcription repressor, we hypothesize that Mta3-NuRD complex could derepress the transcription of unknown hematopoietic repressors by chromatin remodeling through deacetylation of chromatin histones during early primitive hematopoiesis (Figure 7F). But, we could not rule out the possibility that Mta3-RuRD complex functions to directly activate the transcription of genes required for primitive hematopoiesis (eg, Scl) by remodeling their chromatin or by deacetylating repressor proteins, because such mechanisms have been reported in a few cases for Mta1- or Mta2-containing NuRD complexes.46-49
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We are grateful to Drs Ming Li and Zilong Wen for critical reading of this manuscript; Yongfeng Shang, Jian Zhang, Zilong Wen, Feng Liu, and Shuo Lin for providing constructs; and members of the Meng Laboratory for helpful discussion and technical assistance.
This work was supported by grants from National Natural Science Foundation of China (30830068), from Major Science Programs of China (2006CB943401), from National Basic Research Program of China (2005CB522502), and from the 863 Program (2006AA02Z167).
Authorship
Contribution: X.L. designed and performed the experiments, analyzed data, and wrote the manuscript; S.J. identified mta3; S.W. and Y.W. performed the experiments; and A.M designed the experiments, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anming Meng, Department of Biological Sciences and Biotechnology, Tsinghua University, Beijing 100084, People's Republic of China; e-mail: mengam@mail.tsinghua.edu.cn or mengam@ioz.ac.cn.
References
Supplemental data
(A–D) The expression of the endothelial markers flk1 (A), fli1a (B), tie1(C) and tie2 (D) in cMO- or tMO-injected embryos at 24 hpf. In the lower panel were enlarged trunk regions to show intersegmental vessels (indicated by arrows). Note that the marker expression retained in the dorsal aorta and cardinal veins but was missing in intersegmental vessels when mta3 was knocked down. (E) Knockdown of mta3 only interrupted GFP expression in the intersegmental vessels (indicated by arrows) in Tg(flk1:EGFP) transgenic embryos at 26 hpf. Embryos were observed by fluorescence microscopy.Additional supplemental figures can be found here.