Abstract
We have established a model of leukemia immunotherapy using T cells expressing chimeric T-cell receptors (cTCRs) targeting the CD20 molecule expressed on normal and neoplastic B cells. After transfer into human CD20 (hCD20) transgenic mice, cTCR+ T cells showed antigen-specific delayed egress from the lungs, concomitant with T-cell deletion. Few cTCR+ T cells reached the bone marrow (BM) in hCD20 transgenic mice, precluding effectiveness against leukemia. Anti-hCD20 antibody-mediated B-cell depletion before adoptive T-cell therapy permitted egress of mouse CD20-specific cTCR+ T cells from the lungs, enhanced T-cell survival, and promoted cTCR+ T cell–dependent elimination of established mouse CD20+ leukemia. Furthermore, CD20-specific cTCR+ T cells eliminated residual B cells refractory to depletion with monoclonal antibodies. These findings suggest that combination of antibody therapy that depletes antigen-expressing normal tissues with adoptive T-cell immunotherapy enhances the ability of cTCR+ T cells to survive and control tumors.
Introduction
We and others have demonstrated both the promise and challenges of using adoptive T-cell immunotherapy for treatment of B-cell malignancies, using human T cells engineered to express chimeric T-cell receptor (cTCR) directed against the CD20 antigen.1-4 In vitro experimentation has shown that high expression density of CD20 on normal human B cells down-modulates cTCR molecules from the surface of CD20-specific cTCR+ T cells.5 Down-modulation of canonical TCR has been associated with reduced sensitivity and effector functions,6 suggesting cTCR down-modulation may limit target recognition. Persistent exposure to CD20 on B cells may also impair CD20-specific cTCR+ T-cell survival. T cells are anergized or deleted in environments characterized by abundant major histocompatibility complex–restricted antigen derived from neo-self antigens,7,8 tumor antigens,9 or chronic viral infections.10 Although B cells can exhibit tolerogenic properties when stimulating naive T cells, little is known about in vivo reactivation of effector T cells by antigen-expressing naive B cells.11-14 Clinical experience suggests cTCR+ T cells are diminished in the blood of patients with large antigen burdens,4,15 but it is unclear to what extent this rapid clearance represents deletion or retention at antigen rich sites. Global lymphodepletion has been shown to increase T-cell survival,16,17 but the effect of selective B-cell lymphodepletion before adoptive transfer of B-cell antigen-specific T cells has not been evaluated. Although several B cell–associated molecules have been targeted by cTCRs, including CD19,18,19 CD20,1-3 and CD22,5 no studies have addressed the in vivo function of cTCR+ T cells in a model system in which both normal and neoplastic cells express the same target molecule. In this study we have targeted CD20 on both normal and leukemic B cells in immunocompetent mice. Expression of CD20 on normal B cells profoundly impaired cTCR+ CD8+ T cell–mediated leukemia immunotherapy, resulting in T-cell deletion and limited T-cell accumulation in the bone marrow (BM). In mice lacking CD20 on B cells or in mice depleted of B cells with monoclonal antibodies, cTCR+ T cells trafficked to BM and eliminated leukemia cells. Our results suggest that B-cell depletion of patients before T-cell infusion may substantially improve the in vivo survival and function of B-cell antigen-specific cTCR+ T cells.
Methods
Mice
Human CD20 transgenic mice on the Balb/c background have been described previously.20 CL4 hemagglutinin-specific TCR transgenic mice21 were obtained from The Jackson Laboratory and bred at the Fred Hutchinson Cancer Research Center (FHCRC) animal facility. Thy1.1+ and Thy1.2+ Balb/cJ mice were obtained from The Jackson Laboratory or bred at the FHCRC animal facility. All experiments were performed with the approval of the FHCRC Institutional Animal Care and Use Committee.
Gene constructs
For the Leu16 and MB20-18 cTCR construction.
The mouse IgG1 sequence was cloned from the total RNA from the HD39 murine hybridoma with the use of reverse transcription–polymerase chain reaction. The CD3ζ chain was cloned from C57Bl/6 T cells. The IgG1 and CD3ζ gene sequences were combined with an intervening CD4 transmembrane domain with the use of overlapping oligonucleotides and PCR. The Leu16 scFv sequence was amplified from the previously described human Leu16 cTCR gene.22 The MB20-18 variable light and heavy gene sequences were combined with the use of overlapping oligonucleotides with an intervening peptide linker: VL-GSTSGGGSGGGSGGGGSS-VH. The click-beetle red luciferase gene was obtained from Promega and cloned 5′ of the cTCR genes, followed in-frame by the P2A self-cleaving peptide sequence, and a GSG linker.
Tumor-associated antigen constructs.
Human CD20 was cloned from the DOHH2 cell line obtained from David Maloney (FHCRC), and mCD20 was cloned from Balb/c B cells. The firefly luciferase gene (Promega) was cloned in-frame with the E2A self-cleaving peptide sequence, the Thy1.1 gene sequence (obtained from Thy1.1+ Balb/c T cells), a second T2A self-cleaving peptide sequence, and finally the Neo gene (obtained from the pcDNA3.1 vector). All constructs were cloned into the LZRS-pBMN vector obtained from Gary Nolan (Stanford University, Stanford, CA). 2A self-cleaving peptide sequences and nomenclature were derived from those described previously.23
Cell lines
A20 and EL4 were obtained from ATCC. BM18524 was a gift from Donald Kohn (University of Southern California, Los Angeles, CA). EL4-hCD20 was derived as a subclone from the parental line obtained from Josee Golay (Ospedali Riuniti di Bergamo, Bergamo, Italy).25 BM185-mCD20, BM185-hCD20, and EL4-mCD20 were generated by transduction with retrovirus supernatants obtained from Phoenix-E packaging cell lines transfected with LZRS constructs containing mouse and human CD20. BM185-hCD20 was sorted by flow cytometry for hCD20 expression 3 times with the use of a FACSVantage (BD Biosciences). BM185-mCD20 and EL4-mCD20 were cloned by limiting dilution after immunomagnetic CD20-positive selection with anti–CD20-PE antibodies and anti-PE beads (Miltenyi Biotec Inc). BM185-hCD20/firefly luciferase (ffLuc)–Thy1.1-Neo and BM185-mCD20/ffLuc-Thy1.1-Neo were generated by retroviral transduction of BM185-hCD20 and BM185-mCD20 with a retrovirus encoding the ffLuc-Thy1.1-Neo construct, followed by G418 selection. BM185-mCD20 and BM185-mCD20/ffLuc-Thy1.1-Neo were cloned by limiting dilution.
T-cell stimulation and retroviral transduction
Polyclonal Thy1.1+ splenocytes were stimulated in vitro with 1 μg/mL soluble anti-CD3/CD28 antibodies and 50 U/mL recombinant human interleukin-2 (rhIL-2) in IMDM 10% FCS with 0.1mM l-glutamine, 11 μg/mL sodium pyruvate, 1× nonessential amino acids (Sigma-Aldrich), 1000 U/mL penicillin, 1 mg/mL streptomycin, and 50μM β-mercaptoethanol (T-cell culture medium). Antibodies were removed after 24 hours, and retroviral transduction was performed. CL4 TCR transgenic T cells were obtained from spleens and peripheral lymph nodes (LNs) and incubated with 10 μg/mL IYSTVASSL peptide for 24 hours with 50 U/mL rhIL-2 in culture medium. T cells were depleted of B cells using anti-B220 magnetic beads and LD columns (Miltenyi Biotec Inc) before transduction with MB20-18 cTCR–producing retroviruses or directly transduced with Leu16 cTCR–producing retroviruses. T cells were expanded for 3 to 4 additional days in 50 U/mL rhIL-2 in T-cell culture medium before adoptive transfer. Retrovirus supernatants were concentrated with polyethylene glycol, and T cells or tumor cell lines were transduced as previously described.5
Antibodies and flow cytometry
Fluorochrome-conjugated antibodies to mCD4, mCD8, mCD19, hCD20, mCD21, mCD23, Thy1.1, and mCD62L and purified anti-CD3 and anti-CD28 were obtained from BD Biosciences. Anti–mCD20-PE was obtained from eBioscience. The depleting anti-hCD20 antibody 1F526 was produced in the FHCRC biologics facility from the hybridoma line HB-9645 obtained from ATCC. The blocking anti–LFA-1 (lymphocyte function–associated antigen-1) antibody FD441.8 was obtained from Bio X Cell. Flow cytometry was performed with the use of a FACSCanto (BD Biosciences). Blood was obtained from the retro-orbital plexus of mice. LNs and spleens were dissected away from mice after CO2 asphyxiation and were filtered through 40-μm screens to generate single-cell suspensions. BM was obtained by flushing the medullary cavities of femurs with RPMI 1640. After hypotonic lysis, blood, LN, BM, and spleen cells were analyzed by flow cytometry. Marginal zone (MZ) B cells27 were identified as CD19+ CD21hi CD23lo and follicular B cells as CD19+ CD21loCD23hi.
Adoptive transfers and B-cell depletion
Leukemia cells in exponential growth phase were injected intravenously through the tail vein in Hanks Balance Salt Solution after washing with PBS. T cells were injected intravenously through the tail vein on day 4 to 5 after stimulation. In LFA-1–blocking experiments, T cells were labeled at 2 × 107 cells/mL in PBS 2.5mM EDTA containing 50 μg/mL FD441.8 at 4°C for 30 minutes, followed by washing twice in PBS. T cells were immediately injected into mice. The effect of CD20-specific T cells was analyzed in mice depleted of B cells after intravenous injection with 200 μg of 1F5 4 days before T-cell injection.
In vitro T-cell analysis
For analysis of T-cell/B-cell conjugates, T cells were labeled with 1μM DDAO-SE (Invitrogen) and B cells or BM185-mCD20 was labeled with 0.1μM CFSE (Invitrogen). B cells were obtained by magnetic negative selection of Balb/c splenocytes (Miltenyi Biotec Inc). T cells were incubated with targets at a 1:2 effector-to-target ratio for 20 minutes at 37°C after pelleting in a 96-well round-bottom plate at 500g for 3 minutes. Conjugates were gently resuspended and analyzed by flow cytometry. The percentage of T cells in conjugates was calculated by dividing the percentage of CFSE+DDAO+ conjugates by the total percentage of DDAO+ events. Chromium release assays and 7AAD in vitro killing assays were performed as described previously.5
Bioluminescent imaging
Luciferase activity was analyzed in mice anesthetized with isoflurane 10 minutes after intraperitoneal injection of d-luciferin potassium salt (Caliper Life Sciences) at 150 mg/kg. Mice were imaged in a Xenogen IVIS Spectrum Imaging System (Caliper Life Sciences) using small binning and open emission filters. Mice were imaged for varying times as described in figure legends. For quantitation of signal, acquisition times were chosen that did not saturate the detector. Some images depicted represent saturated acquisitions for which quantitation was achieved with shortened acquisition times. Living Image software was used to analyze the luminescent image data. Total bioluminescent signal was obtained as photons/s/cm2/sr and regions of interest were used to calculate regional signals.
Statistics
Data are means plus or minus SE. Two-tailed t tests or 1-way analyses of variance (ANOVAs) were used as described in figure legends and in the text. For B-cell depletion experiments, the data were log-transformed and analyzed by 1-way ANOVA, followed by a Tukey post hoc test for multiple comparisons. Figure 5 was analyzed by 1-way ANOVA, followed by a Bonferroni selected comparison test. Survival analysis was compared with the use of the Kaplan-Meier log-rank test. P values less than .05 were considered statistically significant. Calculations were performed with the use of Prism (GraphPad Software Inc) or Excel (Microsoft) software.
Results
Expression of CD20 on normal B cells promotes deletion of CD20-specific cTCR+ T cells
We generated an hCD20-specific cTCR construct of entirely murine origin that was based on a previously described human Leu16 cTCR.22 Because a persisting CD20-specific T-cell population might lead to prolonged B-cell lymphopenia, we did not include costimulatory signaling domains in this cTCR, which promote survival of cTCR+ T cells.28,29 This murine Leu16-based cTCR, denoted Leu16, was expressed in polyclonal in vitro–generated effector T cells with the use of retroviral transduction (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). cTCR+ T cells promoted lysis proportional to the level of antigen expression by a panel of Jurkat T-cell clones expressing a range of hCD20 molecules (supplemental Figure 1B). Surface expression of the cTCR was down-modulated in an antigen dose-dependent fashion in response to Jurkat-hCD20 clones, or proportionally by hCD20+ B cells from hCD20 transgenic mice20 (supplemental Figure 1B) as previously described for cTCR+ human T cells.5 Interestingly, the murine and human Leu16 cTCR exhibited similar antigen sensitivities, with the murine Leu16 cTCR requiring 20 000 CD20 molecules/target cell (supplemental Figure 1B) and the human Leu16 cTCR requiring 15 000 CD20 molecules/target cell.5 Together, these data suggested that the murine Leu16 cTCR functions similarly to the previously described human Leu16 cTCR in vitro.5,22
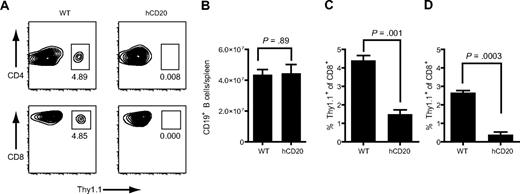
We next analyzed the in vivo behavior of Leu16+ T cells in wild-type (WT) or hCD20 mice. One week after injection of 107 Thy1.1+Leu16+ effector T cells into WT or hCD20 Thy1.2+ hosts, Thy1.1+ donor cells comprised 3.7% of CD4 and 4.5% of CD8 T cells in the spleens of WT mice, but only 0.01% (P = .002) and 0.02% (P = .001), respectively, in hCD20 mice (Figure 1A), indicating antigen-specific T-cell deletion. The numbers of B cells in the spleens of hCD20 and WT mice were similar, suggesting that T-cell deletion had limited the anti–B-cell activity of the transferred cells (Figure 1B). To confirm these findings with a better-defined population of T cells, we performed subsequent studies with in vitro–generated effector CD8+Thy1.1+ T cells derived from hemagglutinin-specific CL4 TCR transgenic mice21 (supplemental Figure 1C). To determine whether expression of hCD20 on the entire B-cell compartment was necessary to delete Leu16+ T cells, we injected WT mice with Leu16+ T cells and challenged them 24 hours later with 2 × 107 WT or hCD20 splenocytes. Three days after injection, Thy1.1+ donor cells were markedly reduced in the blood of hCD20 splenocyte-challenged mice compared with WT splenocyte-challenged mice (P < .001; Figure 1C). One week after injection, Thy1.1+ donor cells were also diminished in the spleens of hCD20-challenged mice compared with WT-challenged mice (0.4% vs 2.7% Thy1.1+ of CD8+; P = .003; Figure 1D). Transfer of 2 × 107 hCD20+ splenocytes into mice resulted in minimal population of the B-cell compartment, because only 1% of splenic B cells were of donor origin 4 hours after transfer (data not shown), suggesting a relatively small number of hCD20+ B cells can efficiently promote T-cell deletion or redistribution of T cells out of the blood and spleen.
Expression of CD20 on normal B cells promotes deletion of CD20-specific cTCR+ T cells. (A-B) Thy1.1+Leu16+ polyclonal effector T cells (107) were injected intravenously into Thy1.2+ WT or hCD20 mice. Spleens were obtained 7 days later and analyzed for donor T cells and endogenous B cells (B). Numbers under boxes in panel A represent the percentage of Thy1.1+ T cells of blood CD4 or CD8 T cells. (C-D) WT mice were injected with 107 Thy1.1+Leu16+CD8+ T cells. Twenty-four hours later, the mice were challenged with 2 × 107 WT or hCD20 splenocytes. (C) Three days later blood was analyzed for donor Thy1.1+ T cells. (D) Spleens were obtained 7 days after challenge and analyzed for donor T cells. Bar graphs represent mean ± SE. Data are representative of 2 experiments with 3 mice per group. P values were obtained with t tests.
Expression of CD20 on normal B cells promotes deletion of CD20-specific cTCR+ T cells. (A-B) Thy1.1+Leu16+ polyclonal effector T cells (107) were injected intravenously into Thy1.2+ WT or hCD20 mice. Spleens were obtained 7 days later and analyzed for donor T cells and endogenous B cells (B). Numbers under boxes in panel A represent the percentage of Thy1.1+ T cells of blood CD4 or CD8 T cells. (C-D) WT mice were injected with 107 Thy1.1+Leu16+CD8+ T cells. Twenty-four hours later, the mice were challenged with 2 × 107 WT or hCD20 splenocytes. (C) Three days later blood was analyzed for donor Thy1.1+ T cells. (D) Spleens were obtained 7 days after challenge and analyzed for donor T cells. Bar graphs represent mean ± SE. Data are representative of 2 experiments with 3 mice per group. P values were obtained with t tests.
Expression of CD20 on normal B cells severely impairs the in vivo antileukemic activity of CD20-specific cTCR+ T cells
To create an hCD20+ leukemia target that could be tracked in vivo, we modified the BM185 pre–B-cell leukemia cell line24 to express hCD20, Thy1.1, and ffLuc (supplemental Figure 2A). Leu16+ CD8+ T cells efficiently lysed this cell line (BM185-hCD20/Thy1.1-ffLuc-Neo) in vitro (supplemental Figure 1C). The leukemia cell line grew progressively in hCD20 hosts after intravenous injection, with leukemic cells detected in the blood and BM (supplemental Figure 2B-C) and dynamically observed by bioluminescent imaging in the femurs, sterna, humeral heads, and scapulae of leukemic mice (Figure 2B).
Expression of CD20 on normal B cells severely inhibits the in vivo antileukemic function of CD20-specific cTCR+ T cells. (A) WT or hCD20 mice were injected intravenously with 2 × 107 BM185-hCD20/Thy1.1-ffLuc-Neo leukemia cells and treated with 107 Leu16 cTCR or empty vector–transduced Thy1.1+CD8+ T cells 3 days later. Serial quantitative bioluminescent imaging was performed. (B) Bioluminescent images of mice. Two-minute acquisitions are shown, but shorter acquisitions were performed for signal quantitation of mice that saturated the detector. Images were assembled with the use of Illustrator (Adobe Systems). (C) Peripheral blood analysis of mice 2 days after T-cell injection. P value was obtained by 1-way ANOVA analysis. Panels A to C are representative of 2 experiments with 3 to 4 mice per group. Bar graphs and data points represent mean ± SE. (D) Comparison of leukemia bioluminescent signal and peripheral blood donor T-cell percentage of CD8 2 days after T-cell injection. Data points are individual mice from 2 experiments. In 1 of 2 experiments Leu16+ T cells were titrated into WT mice at 0.1, 0.3, and 107 T cells/mouse.
Expression of CD20 on normal B cells severely inhibits the in vivo antileukemic function of CD20-specific cTCR+ T cells. (A) WT or hCD20 mice were injected intravenously with 2 × 107 BM185-hCD20/Thy1.1-ffLuc-Neo leukemia cells and treated with 107 Leu16 cTCR or empty vector–transduced Thy1.1+CD8+ T cells 3 days later. Serial quantitative bioluminescent imaging was performed. (B) Bioluminescent images of mice. Two-minute acquisitions are shown, but shorter acquisitions were performed for signal quantitation of mice that saturated the detector. Images were assembled with the use of Illustrator (Adobe Systems). (C) Peripheral blood analysis of mice 2 days after T-cell injection. P value was obtained by 1-way ANOVA analysis. Panels A to C are representative of 2 experiments with 3 to 4 mice per group. Bar graphs and data points represent mean ± SE. (D) Comparison of leukemia bioluminescent signal and peripheral blood donor T-cell percentage of CD8 2 days after T-cell injection. Data points are individual mice from 2 experiments. In 1 of 2 experiments Leu16+ T cells were titrated into WT mice at 0.1, 0.3, and 107 T cells/mouse.
We administered 107 Leu16+ CD8+ T cells intravenously to WT and hCD20 mice 3 days after injection of 2 × 107 leukemia cells. The leukemia bioluminescent signal diminished to background levels within 3 days in WT mice, but the growth of leukemia in hCD20 mice was minimally impeded (Figure 2A-B). Thy1.1+ donor cells comprised only 1.5% of blood CD8 T cells in hCD20 hosts 2 days after transfer compared with 11.8% in WT hosts (P < .001; Figure 2C), consistent with T-cell deletion in hCD20 hosts. The percentage of donor T cells persisting in the blood 2 days after administration was correlated with reduction of the leukemia bioluminescent signal. Titrating Leu16+ T cells into WT leukemia-bearing hosts showed that 0.1 to 0.3 × 107 Leu16+ T cells were as effective in WT hosts as 107 Leu16+ T cells in hCD20 hosts (Figure 2D). These findings suggested that CD20-specific T cells were deleted in response to normal B cells, but not in response to leukemic B cells, and that this deletion abrogated the antileukemic efficacy of the T cells. This model could not be used to analyze the effect of Leu16+ cTCR+ T cells on long-term survival because WT mice developed an immune response that rejected the engineered BM185-hCD20/Thy1.1-ffLuc-Neo tumor cells.
Expression of CD20 on normal B cells alters the trafficking and survival of CD20-specific cTCR+ T cells
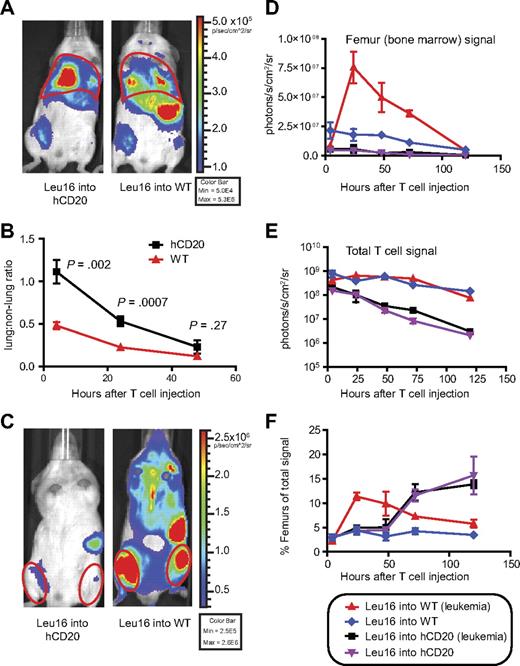
In addition to promoting the deletion of Leu16+ T cells, it was possible that hCD20+ B cells diminished antileukemic function by altering in vivo T-cell trafficking. Therefore, we adoptively transferred Leu16+ T cells expressing click-beetle red luciferase (CBR) into WT or hCD20 hosts 3 days after injection of BM185-hCD20 leukemia. Leu16+CBR+ T cells exhibited an increase in the lung-to-nonlung bioluminescent signal ratio in hCD20 hosts compared with WT hosts, suggesting antigen-specific T-cell sequestration in the lungs in response to interaction with antigen-expressing normal B cells (4 hours: 2.3-fold increase, P = .002; 24 hours: 2.4-fold increase, P < .001; Figure 3A-B). Stronger bioluminescent T-cell signals were emitted by the femurs, spleen, and sterna of WT versus hCD20 mice bearing established BM185-hCD20 leukemia (BM signal increased 13.4-fold in WT vs hCD20 at 24 hours; P = .002, t test; Figure 3C-D). Decay of the donor T-cell bioluminescent signal as evidence of deletion was more rapid in hCD20 versus WT hosts, resulting in a 40-fold reduced signal in hCD20 mice at 5 days (P < .001, t test; Figure 3E), suggesting that a combination of lung sequestration and deletion of T cells limited accumulation in the BM. The relative T-cell signal emitted from the BM reached its maximum value at 24 hours in WT mice bearing leukemia, compared with 72 hours in hCD20 mice (with or without leukemia), concomitant with delayed egress from the lungs (Figure 3F). This representation of the data, which allowed T-cell deletion to be unlinked from trafficking, showed that both deletion and impaired trafficking limited accumulation of Leu16+ T cells in the BM. The relative percentage of Leu16+ T cells in the BM of hCD20+ mice was higher after 72 hours than in nonleukemic WT mice, suggesting that hCD20 expression on normal B cells promoted eventual Leu16+ T-cell accumulation in the BM (Figure 3F), but only after significant deletion had occurred.
Expression of CD20 on normal B cells alters the trafficking and survival of CD20-specific cTCR+ T cells. (A) Bioluminescent imaging of 107 CBR+Leu16+Thy1.1+CD8+ T cells 4 hours after intravenous injection into WT or hCD20 mice. (B) The lung-to-nonlung bioluminescent ratio was calculated for mice injected with 107 CBR+Leu16+Thy1.1+CD8+ T cells after various time intervals. Data from 2 experiments were overlaid, comprising 7 to 10 mice per time point. Red outlines in panels A and C are representative of regions of interest for signal quantitation. (C) Bioluminescent image of mice 48 hours after T-cell injection. (E) Serial quantitation of T-cell bioluminescent signal from the femurs in panel D, and the entire mouse in panel E was performed over time. (F) The relative T-cell signal originating from the femurs was calculated by division of total signals from femurs by total signals from mice. The data are representative of 2 experiments with 3 to 5 mice per group each. Data points represent mean ± SE. P values were obtained by 2-tailed t tests. Bioluminescent images were assembled with the use of Illustrator (Adobe Systems).
Expression of CD20 on normal B cells alters the trafficking and survival of CD20-specific cTCR+ T cells. (A) Bioluminescent imaging of 107 CBR+Leu16+Thy1.1+CD8+ T cells 4 hours after intravenous injection into WT or hCD20 mice. (B) The lung-to-nonlung bioluminescent ratio was calculated for mice injected with 107 CBR+Leu16+Thy1.1+CD8+ T cells after various time intervals. Data from 2 experiments were overlaid, comprising 7 to 10 mice per time point. Red outlines in panels A and C are representative of regions of interest for signal quantitation. (C) Bioluminescent image of mice 48 hours after T-cell injection. (E) Serial quantitation of T-cell bioluminescent signal from the femurs in panel D, and the entire mouse in panel E was performed over time. (F) The relative T-cell signal originating from the femurs was calculated by division of total signals from femurs by total signals from mice. The data are representative of 2 experiments with 3 to 5 mice per group each. Data points represent mean ± SE. P values were obtained by 2-tailed t tests. Bioluminescent images were assembled with the use of Illustrator (Adobe Systems).
Antigen-specific lung retention of CD20-specific cTCR+ T cells depends on CD3ζ signaling and LFA-1
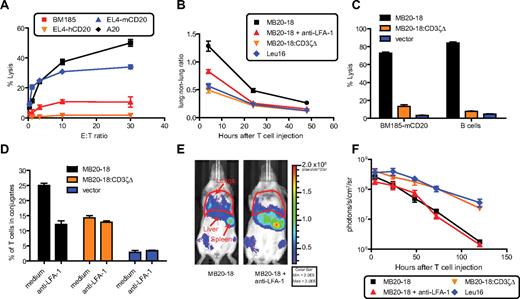
To allow use of an anti-hCD20 antibody to selectively deplete hCD20+ normal B cells in transgenic mice, but not induce antibody-induced antitumor effects against the leukemia being treated, we generated a cTCR based on the MB20-18 monoclonal antibody (mAb)30 specific for murine CD20 (mCD20) to target an mCD20+ B-cell leukemia that grows progressively in WT and hCD20 mice. This cTCR, denoted MB20-18, promoted lysis of the mCD20+ cell lines A20 and EL4-mCD20, but not BM185 or EL4-hCD20 (Figure 4A). After injection of 107 MB20-18+ T cells labeled with CBR into WT mice, we observed increased lung retention compared with irrelevant Leu16+CBR+ T cells (4 hours: 2.3-fold increase, P < .001; 24 hours: 2.1-fold increase, P < .001; 48 hours: 2.2-fold increase, P < .001, 1-way ANOVA; Figure 4B). By contrast, T cells expressing an MB20-18–based cTCR lacking the CD3ζ chain, denoted MB20-18:CD3ζΔ, did not exhibit retention in the lung (Figure 4B), suggesting that lung retention required cTCR signaling. Unlike the MB20-18 cTCR, MB20-18:CD3ζΔ was unable to promote lysis of BM185-mCD20 or WT B cells (Figure 4C), confirming the absence of an intact CD3ζ signal.
Antigen-specific retention of CD20-specific cTCR+ T cells depends on CD3ζ signaling and LFA-1. (A) Murine CD8+ T cells expressing the MB20-18+ cTCR exhibit antigen-specific cytolytic activity toward mCD20 expressing cell lines (A20, EL4-CD20), but not control cells (EL4-hCD20, BM185). Standard 5-hour 51Cr release assay analysis of MB20-18+cTCR+CD8+ T-cell lytic activity. Data are representative of 2 experiments. (B) Calculation of lung to non-lung bioluminescent signal ratio from mice injected intravenously with 107 CBR+cTCR+Thy1.1+CD8+ T cells. Data from 2 experiments were overlaid comprising 7 to 8 mice per time point. (C) Lytic activity of cTCR− or cTCR+ T cells toward WT B cells and BM185-mCD20. T cells were incubated with targets for 3 hours, and target lysis was determined by a 7AAD assay as described in “In vitro T-cell analysis.” Data are representative of 2 experiments. (D) cTCR− or cTCR+ T cells were conjugated with freshly isolated WT B cells, and the percentage of T cells in conjugates was determined by flow cytometry as described in “In vitro T-cell analysis.” Data are representative of 3 experiments. (E) Bioluminescent image of mice 24 hours after receiving 107 CBR+MB20-18+CD8+ T cells that had been treated with anti–LFA-1 or PBS before injection. (F) Serial quantitation of T-cell bioluminescent signal from mice injected with CBR+cTCR+Thy1.1+CD8+ T cells. Data are representative of 2 experiments with 3 to 4 mice per group. Bar graphs and data points represent mean ± SE. Bioluminescent images were assembled with the use of Illustrator (Adobe Systems).
Antigen-specific retention of CD20-specific cTCR+ T cells depends on CD3ζ signaling and LFA-1. (A) Murine CD8+ T cells expressing the MB20-18+ cTCR exhibit antigen-specific cytolytic activity toward mCD20 expressing cell lines (A20, EL4-CD20), but not control cells (EL4-hCD20, BM185). Standard 5-hour 51Cr release assay analysis of MB20-18+cTCR+CD8+ T-cell lytic activity. Data are representative of 2 experiments. (B) Calculation of lung to non-lung bioluminescent signal ratio from mice injected intravenously with 107 CBR+cTCR+Thy1.1+CD8+ T cells. Data from 2 experiments were overlaid comprising 7 to 8 mice per time point. (C) Lytic activity of cTCR− or cTCR+ T cells toward WT B cells and BM185-mCD20. T cells were incubated with targets for 3 hours, and target lysis was determined by a 7AAD assay as described in “In vitro T-cell analysis.” Data are representative of 2 experiments. (D) cTCR− or cTCR+ T cells were conjugated with freshly isolated WT B cells, and the percentage of T cells in conjugates was determined by flow cytometry as described in “In vitro T-cell analysis.” Data are representative of 3 experiments. (E) Bioluminescent image of mice 24 hours after receiving 107 CBR+MB20-18+CD8+ T cells that had been treated with anti–LFA-1 or PBS before injection. (F) Serial quantitation of T-cell bioluminescent signal from mice injected with CBR+cTCR+Thy1.1+CD8+ T cells. Data are representative of 2 experiments with 3 to 4 mice per group. Bar graphs and data points represent mean ± SE. Bioluminescent images were assembled with the use of Illustrator (Adobe Systems).
Because formation and retention of T-cell/B-cell conjugates in the pulmonary vasculature could potentially explain the observed antigen-dependent sequestration of CD20-specific T cells in the lungs, we analyzed the efficiency of cTCR-induced T-cell conjugation with normal B cells. Analysis of conjugate formation between WT B cells and T cells expressing the MB20-18, MB20-18:CD3ζΔ, or Leu16 cTCR showed that CD3ζ signaling nearly doubled the antigen-specific conjugation achieved with a CD3ζ-deficient receptor, which promoted basal conjugation in the absence of signaling (Figure 4D). This suggested that CD3ζ signaling was necessary for optimal T-cell/B-cell conjugation, potentially through activation of adhesion molecules. Treatment of MB20-18+ T cells with anti–LFA-1 reduced conjugation to the level seen with MB20-18:CD3ζΔ+ cells, suggesting that cTCR signaling increases conjugation largely through LFA-1 activation (Figure 4D).
To determine the role of cTCR signal–induced LFA-1 avidity maturation in lung retention of CD20-specific cTCR+ T cells, CBR+MB20-18+ T cells were incubated with blocking anti–LFA-1 mAb before injection into WT mice. Four hours after injection, anti–LFA-1 pretreated MB20-18+ T cells showed a 36% decrease in lung retention relative to MB20-18+ T cells (P < .001, t test) and at 24 hours were indistinguishable from Leu16+ irrelevant T cells (Figure 4B,E). This suggested CD20-specific cTCR+ T cells activated LFA-1 in response to B cells in the blood, which promoted enhanced T-cell/B-cell conjugate formation, and subsequent lung retention. MB20-18:CD3ζΔ+ T cells and Leu16+ T cells showed similar survival, whereas MB20-18+ cells were deleted regardless of LFA-1 blockade, suggesting that cTCR signaling was necessary for antigen-dependent T-cell deletion and that accelerating lung egress was insufficient to improve survival (Figure 4F). The enhanced lung egress of MB20-18+ T cells treated with anti–LFA-1 occurred concomitantly with increased trafficking to the BM compared with untreated MB20-18+ T cells (Figure 4B,E and Figure 5A,C), showing that sequestration in the lungs limited trafficking of CD20-specific T cells to the BM. Anti–LFA-1 did not increase LN trafficking of MB20-18+ T cells, which exhibited minimal accumulation in this tissue. In contrast, antigen nonspecific Leu16+ T cells showed progressive accumulation in the LNs (Figure 5B-C).
Anti–LFA-1 enhances accumulation of CD20-specific T cells in the BM but not LNs. (A-B) A total of 107 CBR+Thy1.1+cTCR+CD8+ T cells pretreated with either anti–LFA-1 or PBS were injected intravenously into WT mice. Serial bioluminescent imaging of T cells was performed, and the relative signals emitted were calculated by dividing the signal from the femurs in panel A or cervical LN (cLN) in panel B by the total bioluminescent signal. Data points represent the mean ± SE. Figures contain data from 2 experiments with 7 to 8 mice per group total. P values were obtained by 1-way ANOVAs, followed by a Bonferroni selected comparison test. (C) Depiction of cervical LN and femur gates from representative mice.
Anti–LFA-1 enhances accumulation of CD20-specific T cells in the BM but not LNs. (A-B) A total of 107 CBR+Thy1.1+cTCR+CD8+ T cells pretreated with either anti–LFA-1 or PBS were injected intravenously into WT mice. Serial bioluminescent imaging of T cells was performed, and the relative signals emitted were calculated by dividing the signal from the femurs in panel A or cervical LN (cLN) in panel B by the total bioluminescent signal. Data points represent the mean ± SE. Figures contain data from 2 experiments with 7 to 8 mice per group total. P values were obtained by 1-way ANOVAs, followed by a Bonferroni selected comparison test. (C) Depiction of cervical LN and femur gates from representative mice.
B-cell depletion with anti-hCD20 antibodies enhances CD20-specific cTCR+ T-cell survival
Previous reports have shown that treatment of hCD20 mice with anti-hCD20 mAbs promotes elimination of more than 95% of LN and mature BM B cells and 70% to 95% of spleen B cells.20,31,32 We injected hCD20 mice intravenously with 200 μg of anti-hCD20 antibody 1F5 and noted 91% depletion of blood B cells after 24 hours (data not shown). Seven days after mAb injection, greater than 95% reduction of mature B cells was observed in the LN, BM, and spleen (supplemental Figure 3).
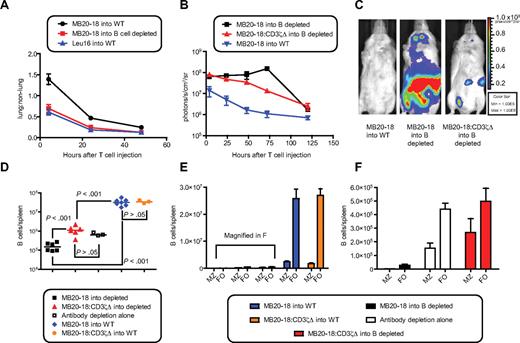
To determine the effect of mAb-mediated B-cell depletion on mCD20-specific cTCR+ T-cell survival and function, we injected 2 × 107 MB20-18+CBR+ T cells into WT or hCD20 mice treated with 1F5. Irrelevant Leu16+ T cells were injected into WT mice as a control. Depletion of hCD20+mCD20+ B cells from the blood of hCD20 mice permitted egress of MB20-18+ cells from the lungs identical to that observed with irrelevant Leu16+ cells (Figure 6A). In contrast to the previously observed deletion, MB20-18+ T cells showed a modest 2.5-fold increase in the total bioluminescent signal in B cell–depleted mice (signal at 72 vs 4 hours; P = .02, t test), peaking at 3 days in the BM (8.0-fold increase; P = .003) and cervical LN (6.5-fold increase; P = .01; Figure 6B-C; supplemental Figure 4). This expansion was dependent on cTCR signaling, because MB20-18:CD3ζΔ+ T cells did not expand in B cell–depleted mice (Figure 6B-C). Five days after T-cell injection, Thy1.1+ MB20-18+ T cells showed enhanced survival in the BM, LNs, and spleens of B cell–depleted versus nondepleted mice (supplemental Figure 5). MB20-18+ T cells showed a reduction of CD62L expression in the spleens and BM of B cell–depleted mice compared with MB20-18:CD3ζΔ+ T cells, but not deletion, probably reflecting limited cTCR-triggering in response to the small number of remaining B cells33,34 (supplemental Figure 5). In nondepleted mice, MB20-18+ T cells were unable to accumulate in the LN, probably reflecting multiple cTCR-triggering events in response to numerous B cells, resulting in decreased survival, reduced CD62L expression, and altered trafficking (Figure 5B-C; supplemental Figures 4-5). These findings showed that depletion of normal B cells before adoptive transfer promoted the survival of CD20-specific cTCR+ cells and enhanced the trafficking of these cells into the LN and BM, sites in which leukemias and lymphomas accumulate.
B-cell depletion with anti-hCD20 antibodies promotes CD20-specific cTCR+ T-cell survival and function. (A) Calculation of lung-to-nonlung bioluminescent signal ratio from mice injected intravenously with 2 × 107 CBR+cTCR+Thy1.1+CD8+ T cells. Data from 2 experiments were overlaid, comprising 7 to 8 mice per time point. (B) Serial quantitation of T-cell bioluminescent signal from mice injected with cTCR+ T cells. Data are representative of 2 experiments with 3 to 4 mice per group. Data points represent mean ± SE. (C) Bioluminescent image of mice from panel B 72 hours after injection. (D-F) Five days after CBR+cTCR+Thy1.1+CD8+ T cells were injected into untreated or B cell–depleted mice, spleens were harvested, and the remaining CD19+ B cells were enumerated. (D) 1F5 (200 μg) was injected into hCD20 mice 4 days before T-cell injection. Horizontal lines represent the mean of the data. (E) Quantitation of MZ and follicular (FO) B cells. Bar graphs represent mean ± SE. Combined data from 2 experiments with 3 to 4 mice per group are shown (D-E). Bar graphs and data points represent mean ± SE. (F) Magnification of panel E. P values were obtained by 1-way ANOVA with a Tukey post hoc test. Bioluminescent images were assembled with the use of Illustrator (Adobe Systems).
B-cell depletion with anti-hCD20 antibodies promotes CD20-specific cTCR+ T-cell survival and function. (A) Calculation of lung-to-nonlung bioluminescent signal ratio from mice injected intravenously with 2 × 107 CBR+cTCR+Thy1.1+CD8+ T cells. Data from 2 experiments were overlaid, comprising 7 to 8 mice per time point. (B) Serial quantitation of T-cell bioluminescent signal from mice injected with cTCR+ T cells. Data are representative of 2 experiments with 3 to 4 mice per group. Data points represent mean ± SE. (C) Bioluminescent image of mice from panel B 72 hours after injection. (D-F) Five days after CBR+cTCR+Thy1.1+CD8+ T cells were injected into untreated or B cell–depleted mice, spleens were harvested, and the remaining CD19+ B cells were enumerated. (D) 1F5 (200 μg) was injected into hCD20 mice 4 days before T-cell injection. Horizontal lines represent the mean of the data. (E) Quantitation of MZ and follicular (FO) B cells. Bar graphs represent mean ± SE. Combined data from 2 experiments with 3 to 4 mice per group are shown (D-E). Bar graphs and data points represent mean ± SE. (F) Magnification of panel E. P values were obtained by 1-way ANOVA with a Tukey post hoc test. Bioluminescent images were assembled with the use of Illustrator (Adobe Systems).
To determine whether B-cell depletion increased the anti–B-cell activity of MB20-18+ T cells, the remaining B cells in the spleens of mice were enumerated 5 days after T-cell injection. In mice pretreated with 1F5, MB20-18+ T cells depleted 86% of the remaining splenic B cells (P < .001), resulting in depletion of 99.6% of splenic B cells compared with nondepleted mice (P < .001; Figure 6D). As previously reported, MZ B cells were relatively resistant to anti-hCD20 antibody-mediated depletion,20,32 with the follicular-to-MZ B-cell ratio dropping from 11.0 in nondepleted mice to 2.4 in antibody-depleted mice (P < .001, t test; Figure 6E-F). In contrast, MB20-18+ T cells efficiently depleted residual antibody-resistant MZ B cells, showing a 240-fold reduction of MZ B cells compared with MB20-18:CD3ζΔ+ T-cell/1F5–treated mice (P < .001, t test; Figure 6F). Additional B-cell depletion also occurred in the BM, but not the peripheral LNs (supplemental Figure 6). These results showed that the reduction of the number of normal B cells by antibody-mediated depletion revealed anti–B-cell activity of CD20-specific T cells, which was capable of eliminating antibody-resistant cells.
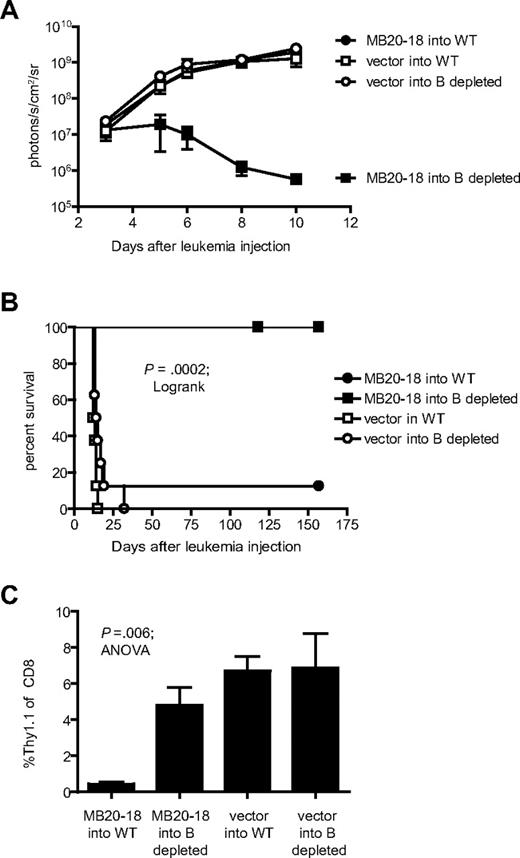
B-cell depletion enhances the antileukemic function of CD20-specific cTCR+ T cells
To determine whether B-cell depletion could enhance the survival and antileukemic function of CD20-specific cTCR+ T cells in mice bearing an established leukemia, we injected 107 CD8+ MB20-18 cTCR+ or empty vector-transduced T cells into WT or B cell–depleted hCD20 mice 3 days after injection of 2 × 107 BM185-mCD20/Thy1.1-ffLuc-Neo leukemia cells. The hCD20 mice were depleted of B cells 4 days before the T-cell therapy by injection of 200 μg of 1F5. Although MB20-18+ T cells were ineffective at preventing leukemia progression in non–B cell–depleted mice, the therapy cured 100% of mice depleted of B cells before T-cell injection (P < .001; Figure 7A-B). Analysis of blood on day 6 after injection of leukemia cells showed a dramatic decrease in the percentage of MB20-18+ donor T cells in WT mice but not in B cell–depleted mice (0.45 vs 4.8% Thy1.1+ of CD8+; P = .005), suggesting that increased T-cell persistence in B cell–depleted hosts probably contributes to the observed enhanced antileukemic activity (Figure 7C). Analysis of blood after 3 months from mice cured by combined therapy with anti-hCD20 mAb and MB20-18+cTCR+ T cells showed complete restoration of B cells with nearly total loss of Thy1.1+ donor T cells (data not shown), showing that this approach did not result in persistent suppression of B-cell numbers.
B-cell depletion enhances the antileukemic function of CD20-specific cTCR+ T cells. (A) WT or B cell–depleted mice received 2 × 107 BM185-mCD20/Thy1.1-ffLuc-Neo intravenously and were treated 3 days later with 107 MB20-18 or vector-transduced Thy1.1+CD8+ T cells. 1F5 (200 μg) was injected 4 days before T-cell injection. Serial quantitation of leukemia bioluminescent signal was performed after T-cell injection. Data are representative of 2 experiments with 4 mice per group. (B) Survival of mice after treatment. Cumulative data from 2 separate experiments with 8 mice per group total are depicted. The first experiment was censored at 154 days and the second at 118 days, when data analysis was performed. P value was obtained with the log-rank test. (C) Peripheral blood analysis of mice 3 days after T-cell injection. P value was obtained by 1-way ANOVA analysis. Data are representative of 2 experiments with 4 mice per group. Data points and bar graphs represent mean ± SE.
B-cell depletion enhances the antileukemic function of CD20-specific cTCR+ T cells. (A) WT or B cell–depleted mice received 2 × 107 BM185-mCD20/Thy1.1-ffLuc-Neo intravenously and were treated 3 days later with 107 MB20-18 or vector-transduced Thy1.1+CD8+ T cells. 1F5 (200 μg) was injected 4 days before T-cell injection. Serial quantitation of leukemia bioluminescent signal was performed after T-cell injection. Data are representative of 2 experiments with 4 mice per group. (B) Survival of mice after treatment. Cumulative data from 2 separate experiments with 8 mice per group total are depicted. The first experiment was censored at 154 days and the second at 118 days, when data analysis was performed. P value was obtained with the log-rank test. (C) Peripheral blood analysis of mice 3 days after T-cell injection. P value was obtained by 1-way ANOVA analysis. Data are representative of 2 experiments with 4 mice per group. Data points and bar graphs represent mean ± SE.
Discussion
Persistence of infused effector T cells has been identified as a determinant of the efficacy of adoptive T-cell immunotherapy of cancer.16 Clinical trials investigating adoptive transfer of cTCR+ T cells have shown such T cells persist only briefly in patients with large numbers of antigen-bearing cells.4,15 Consistent with these clinical studies, our results have shown that expression of CD20 on normal B cells profoundly attenuates the survival and function of CD20-specific cTCR+ T cells and suggest that antibody-mediated B-cell depletion may be an effective strategy to enhance the therapeutic efficacy of CD20-specific T cells. Normal B cells have been shown to promote anergy or deletion when presenting antigens to naive T cells.11-14 In contrast, memory T cells were shown to expand in response to B cell–expressed antigens.14 CD20-specific cTCR+ effector T cells were deleted in response to normal B cells, showing that the tolerogenic potential of B cell–expressed antigen is not abrogated by preactivation of T cells. Although T-cell deletion represents a major mechanism by which the antileukemic function of CD20-specific cTCR+ T cells is limited after encounter with normal B cells expressing CD20, other mechanisms may limit T-cell function before deletion. High density expression of target antigens on normal tissue could interfere with antitumor function of T cells by promoting cTCR down-modulation and T-cell desensitization5 and by decreasing the in vivo effector-to-target ratio by increasing the total number of antigen-expressing cells. Therefore, the presence of antigen on normal tissues might impair antitumor function of antigen-specific T cells even if T-cell survival were maintained.
Lymphodepletion and hematopoietic stem cell transplantation before adoptive T-cell transfer have been shown to promote enhanced survival and function of the antigen-specific T-cell population.16,35-37 However, significant toxicities can be associated with global lymphodepletion-conditioning regimens and autologous stem cell transplantation.38-41 Our results show that B-cell depletion alone, which produces few toxicities in patients,42 is sufficient to allow T cells to eliminate a modest established leukemia burden without producing long-term T cell–mediated B-cell lymphopenia. In contrast to global lymphodepletion,35 B-cell depletion did not induce homeostatic proliferation of T cells, but it did promote modest antigen-specific proliferation that may contribute to the antileukemic response. In the setting of minimal residual disease, infusing B-cell tumor–associated antigen-specific T cells into B cell–depleted patients may be sufficient to eliminate anti-CD20 mAb-resistant disease without requiring global lymphodepletion. However, established B-cell malignancies in the clinical setting may require greater persistence of cTCR+ T cells than was achieved in our model with B-cell depletion alone. Combining B-cell depletion with multiple T-cell infusions or interventions to increase T-cell survival such as inclusion of costimulatory domains43,44 in cTCR may be needed to affect the elimination of established disease burden in patients.
T cells must traffic to and persist in tumor sites for immunotherapy to be successful.45 We found that cTCR+ T cells were sequestered in the lungs of mice in a CD3ζ and LFA-1–dependent mechanism after recognition of normal B cells. This lung retention and concomitant T-cell deletion resulted in dramatic reduction of the number of CD20-specific T cells in the BM and treatment failure. Analysis of T-cell/B-cell conjugate formation suggested that LFA-1 activation after cTCR signaling stabilizes the conjugates, which might be trapped in the pulmonary vasculature. In addition, adhesion of recently activated T cells to intercellular adhesion molecule-1 expressed on the pulmonary endothelium and possible transmigration into the interstitium of the lung in an LFA-1–dependent mechanism46,47 may also contribute to the lung retention. The presence of large numbers of circulating antigen-expressing target cells at the time of administration of antigen-specific effector T cells may not only interfere with therapeutic efficacy but could also result in pulmonary or generalized toxicity, especially when infusing cTCR-containing costimulatory domains that promote enhanced inflammatory cytokine production, and further investigation of this process is warranted.43,44 CD20-specific T cells were deficient in their ability to traffic to LNs, but depletion of B cells before adoptive transfer promoted accumulation of CD20-specific T cells at this site (Figure 5B-C; supplemental Figures 4-5). The bioluminescent signal from MB20-18+ T cells peaked in the LNs of B cell–depleted mice 2 days later than nonsignaling MB20-18:CD3ζΔ+ T cells, which occurred concomitantly with the peak in the BM (supplemental Figure 4). This suggests that, in response to the remaining B cells in B cell–depleted mice, CD20-specific T cells undergo an antigen-dependent expansion, followed by a subsequent accumulation of these cells in the LNs and BM. This accumulation of antigen-specific T cells in primary and secondary lymphoid tissues is probably required for elimination of leukemias and lymphomas, which reside in these tissues. Depletion of B cells before adoptive transfer of T cells specific for B-cell tumor–associated antigens may therefore be necessary to allow T cells to efficiently traffic to malignant B cells, persist at the site of disease, and eliminate the transformed cells.
Rituximab-refractory B-cell malignancies develop despite retention of CD20 expression, suggesting resistance to the antibody effector mechanisms.48-50 Previous studies have shown that MZ B cells are resistant to B-cell depletion with anti-hCD20 antibodies.20,32 We found that CD20-specific T cells depleted more than 99% of the remaining MZ B cells in mice that had been treated with anti-hCD20 antibodies, showing that T cells possess the capacity to eliminate cells that are ineffectively depleted by antibody-mediated therapy. In patients with rituximab-refractory B-cell malignancies, CD20-specific cTCR+ T cells may therefore be capable of eliminating leukemia or lymphoma cells that have escaped anti-CD20 mAb therapy but which still express CD20. The effector function of cTCR+ T cells was only evident in B cell–depleted mice, however, suggesting that removal of the large antigen burden of a normal B-cell compartment is required to promote CD20-specific T cell–mediated antileukemic activity. In conclusion, we have demonstrated that target antigen expression on normal host tissues negatively affects the trafficking, survival, and function of CD20-specific cTCR+ T cells and that this impediment to immunotherapy can be overcome by antibody-mediated depletion of antigen-expressing B cells before T-cell infusion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Institutes of Health (grant R21 CA-117131, O.W.P.; grants P01 CA18029, and R01 CA33084, P.D.G.; grant R01-AR44077, M.J.S.; and grants CA105001 and AI56363, T.F.T.); the Lymphoma Research Foundation (grant MCLI-07-012, O.W.P.); gifts from David and Patricia Giuliani, Geary and Mary Britton-Simmons, the Hext Family Foundation, and the Edson Foundation (O.W.P.); and the Leukemia & Lymphoma Society (grant 7008-08, P.D.G.). S.E.J. was supported by a Poncin fellowship, a Cancer Research Institute fellowship, and an Academic Rewards for College Scientists (ARCS) fellowship.
National Institutes of Health
Authorship
Contribution: S.E.J, P.D.G., and O.W.P. designed the study; S.E.J., N.N.O., and Y.L. performed experiments; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: M.C.J. has received licensing agreement revenues from his COH CD20-CAR construct patent and has ownership interests in a start-up company covering the CD20-CAR. The remaining authors declare no competing financial interests.
Correspondence: Oliver W. Press, Clinical Research, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, M/S D3-190, Seattle, WA 98109; e-mail: press@u.washington.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal