Abstract
During graft-versus-host disease (GVHD), donor T cells become activated and migrate to tissue sites. Previously, we demonstrated a crucial role for the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) in GVHD regulation. Here, we show that upon arrival in the colon, activated donor T cells produced interferon-γ that up-regulated IDO, causing T-cell anergy and apoptosis. IDO induces GCN2 kinase, up-regulating a T-cell stress response implicated in IDO immunosuppression. Donor T cells did not require GCN2 kinase to respond to IDO, suggesting toxic IDO metabolites, and not tryptophan depletion, were responsible for suppression. When exogenous metabolites were administered, GVHD lethality was reduced. To determine whether IDO could be induced before transplantation for enhanced GVHD suppression, we first determined whether antigen-presenting cells (APCs) or epithelial cells were primarily responsible for IDO expression and subsequent GVHD suppression. Recipients with wild-type versus IDO−/− APCs had increased survival, regardless of epithelial-cell expression of IDO, suggesting that APCs were suitable targets for inducing IDO. Administration of an agonist to toll-like receptor-7/8, a receptor expressed primarily on APCs, induced IDO and reduced injury in the colon and ameliorated lethality. We conclude that IDO up-regulation may have therapeutic potential for preventing GVHD in the clinic.
Introduction
Graft-versus-host disease (GVHD) is a complication of hematopoietic stem cell transplantation that occurs as the result of recognition of host antigens as foreign by donor T cells. Prevention and treatment is accompanied by immunosuppression, further endangering patients with weakened immune systems. GVHD is initiated when conditioning damages gut epithelial cells, allowing translocation of bacterial products from the lumen into the tissue. These products induce innate immune cells to produce inflammatory cytokines and chemokines, attracting alloreactive donor T cells that arrive and mediate more tissue damage. Primacy of the gut in GVHD is demonstrated by the fact that gnotobiotic (germ-free) mice are resistant to GVHD1 and that interventions to spare the gut lead to increased survival.2 We recently published that indoleamine 2,3-dioxygenase (IDO) is highly up-regulated in the colon after allogeneic transplantation and that lack of IDO leads to increased colon GVHD injury and accelerated lethality.3 The seemingly localized action of IDO makes it an intriguing target for GVHD therapies. We sought to better understand IDO's induction, location, and mechanism to identify ways in which it could be manipulated to suppress GVHD.
IDO is an intracellular enzyme that catalyzes the first and rate-limiting step in tryptophan catabolism. It has immunosuppressive properties that have been implicated in maternal-fetal acceptance,4 tumor immunity,5 chronic infection,6 and autoimmunity.7,8 IDO's mechanism of action is still somewhat controversial, but it is thought to result from tryptophan depletion and/or accumulation of the tryptophan breakdown products called kynurenines. Tryptophan depletion leads to an increase in the intracellular concentration of uncharged tryptophanyl-tRNA. This accumulation activates GCN2 kinase, a member of a family of kinases that phosphorylate the translation initiation factor eukaryotic initiation factor 2-α and depress general protein translation while activating a stress pathway that leads to anergy and/or apoptosis. GCN2−/− T cells cannot respond to IDO, as shown in vitro9,10 and some in vivo studies.11 However, kynurenines cause T-cell apoptosis in vitro12,13 and suppress inflammation in vivo.14-16 Fallarino et al10 found both tryptophan depletion and kynurenine production in vitro were required for immunosuppression. Which of these mechanisms is operative during GVHD is unknown.
IDO, induced by inflammatory signals including type I and type II interferons and Toll-like receptor (TLR) signaling, down-regulates immune responses. This ability of interferon γ (IFN-γ), the signature cytokine of highly inflammatory Th1 responses, to also trigger suppressive mechanisms may explain its paradoxical role in GVHD. Although Th1 cytokines are highly expressed during GVHD and associated with increased disease,17,18 the lack of IFN-γ through knockout donors,19 neutralizing antibody,19 or IFN-γR−/− recipients20 leads to dramatically accelerated GVHD in lethally irradiated hosts. The actions of IFN-γ in GVHD are complex, causing different effects on different organs and varying with conditioning.20-22 Understanding how IDO and IFN-γ interact during GVHD may help to parse out these effects. Identifying the signals for IDO induction and the cell types capable of expressing IDO and modulating GVHD will suggest strategies for increasing IDO production as a means of prevention or therapy.
The authors of a recent report23 indicated that plasmacytoid dendritic cells (pDCs), a prominent IDO-expressing antigen-presenting cell (APC) subtype, are capable of antigen presentation in GVHD. Endowed with the capacity to produce enormous amounts of type I interferons in response to pathogens, pDCs are considered a crucial part of the innate antiviral immune response. IFN production is triggered by activation of pattern recognition receptors, which include TLRs. The antiviral activity of pDCs depends in part on their expression of TLR7 and 9, which recognize single-stranded RNA and unmethylated CpG-containing oligonucleotides, respectively. Although secretion of inflammatory cytokines aids the immune response, ligation of TLRs on pDCs also up-regulates the immunosuppressive enzyme IDO.24,25 Therefore, pDCs have suppressive properties under some conditions. Because GVHD is critically dependent on host APCs,26 it is likely the propensity of pDCs to produce IDO could be manipulated to increase suppression.
Here we show that donor T cells up-regulate colon IDO by IFN-γ production, contributing to the known suppressive effects of IFN-γ. IDO does not mediate suppression through GCN2 kinase, suggesting its effects are not caused by tryptophan depletion but to the production of tryptophan catabolites, that is, kynurenines. Consistent with this result, we found exogenous kynurenines were capable of suppressing GVHD. We further determined that APCs are the crucial IDO-expressing cells and therefore targeted APCs for IDO up-regulation by using a TLR7/8 agonist. This intervention led to decreased GVHD tissue injury and lethality. These results both have increased our understanding of the mechanism of GVHD suppression by IDO and led to 2 novel therapeutic approaches with clinical relevance for the prevention of GVHD.
Methods
Mice
IFN-γ−/−, IFN-γR−/−, and recipient C57BL/6 (H2b) (termed B6) and BALB/c (H2d) were purchased from The Jackson Laboratory. Donor B6 and BALB/c mice were purchased from the National Institutes of Health. B6 IDO−/−, BALB/c IDO−/−, and GCN2−/− mice were generated as previously described.11,27 Mice were bred and housed in a specific pathogen–free facility in microisolator cages and used at 6 to 16 weeks of age, and all mouse experiments were approved by the institutional animal care and use committee of the University of Minnesota.
Bone marrow transplantation
B6 or B6 IDO−/− mice, or B6.Ly5.1 for chimeras, were irradiated with 8.0- to 9.0-Gy total body irradiation and BALB/c or BALB/c IDO−/− mice with 6.0 to 7.0 Gy by an x-ray or cesium source on day −1. A total of 10 million bone marrow (BM) cells with or without purified T cells were infused on day 0. To deplete BM of T cells, BM was incubated with anti-CD4 and -CD8 on ice for 20 minutes followed by the addition of rabbit complement at 37°C for 30 minutes. For GVHD induction, T cells were isolated from lymph nodes and purified by incubation with phycoerythrin-labeled antibodies to CD19, γδ TCR, and DX5 or NK1.1 (eBioscience), followed by incubation with antiphycoerythrin beads and depletion on a magnetic column (Miltenyi Biotec). Flow cytometric phenotyping demonstrated more than 99% of the desired phenotype. Mice were monitored daily for survival, weighed twice weekly for the first month, then weekly thereafter and examined for clinical GVHD. In some experiments, recipients were killed, and cryosections of liver, lung, colon, small intestine, and spleen were histologically assessed by the use of a semiquantitative GVHD scoring system (0 to 4.0 grades in increments of 0.5) as published.28 Coded sections were graded by one of us (A.P.-M.) without knowledge of the treatment.
Frozen tissue preparation
Tissues, including colon, small intestine, liver, lung, and spleen, were taken at indicated days after transplantation, embedded in optimal cutting temperature compound (Miles), snap-frozen in liquid nitrogen, and stored at −80°C.
Immunohistochemistry
After acetone fixation, cryosections (6 μm) were stained with immunoperoxidase by the use of avidin-biotin blocking reagents, ABC-peroxidase conjugate, and DAB chromogenic substrate, all purchased from Vector Laboratories. Antibodies used were biotinylated mAbs to CD4 (clone GK1.5) and CD8 (clone 2.43), purchased from BD Biosciences. Sections were counterstained with methyl green.
For IDO staining, tissues were fixed in formalin and paraffin embedded. Sections underwent high-temperature antigen retrieval with the use of Target Retrieval Solution (Dako Corporation) and were stained by the use of rabbit anti-IDO polyclonal antibody (prepared by Southern Biotech, based on the peptide sequence previously described by Mellor et al29 ). Biotinylated anti-rabbit secondary antibody was incubated for 1 hour, followed by avidin-biotin complex (ABC)–peroxidase conjugate and 3-Amino-9-ethylcarbazole (AEC) chromogenic substrate, all from Biogenex. Sections were counterstained with hematoxylin.
Images were obtained on an Olympus BX51 microscope by use of a 20×/0.70 numeric aperture objective lens and acquired with a Spot CCD digital camera and Spot software (Diagnostic Instruments Inc) and processed with Adobe Photoshop CS2 Version 9.0.2 (Adobe Systems).
T-cell isolation
Livers were harvested and mashed into a single-cell suspension. Colons from cecum to rectum were harvested, cut into pieces, and incubated 3 times with 5mM ethylenediaminetetraacetic acid (EDTA) in RPMI with 10% serum for 15 minutes at 37°C. Supernatants containing intraepithelial lymphocytes were discarded. Colons were then incubated twice with 0.5 mg/mL and once with 1 mg/mL Collagenase D (Roche) for 1 hour at 37°C with shaking. Colon pieces were mashed, and liver and colon cells were isolated on a 40/80 Percoll (Sigma-Aldrich) gradient. Some cells were stained with antibodies for CD4 and CD8 (eBioscience) and annexin V or Ki-67 (BD Biosciences) according to manufacturer's instructions.
Reagents
L-Kynurenine, 3-hydroxyanthranilic acid, and 3-hydroxykynurenine (Sigma-Aldrich) were dissolved in 1M NaOH and brought to a final concentration of 1.5 mg/mL in phosphate-buffered saline (PBS) after adjusting pH to 7.2 to 7.4. 3M-011 was obtained from 3M Corp, dissolved in dimethyl sulfoxide, and diluted in PBS to 400 μg/mL.
Quantitative polymerase chain reaction
cDNA was synthesized from TRIzol-isolated total RNA by use of the SuperScript III First Strand Synthesis SuperMix for quantitative reverse-transcribed polymerase chain reaction (qRT-PCR; Invitrogen) according to manufacturer's instructions. For qRT-PCR experiments, reactions containing the TaqMan Universal PCR Master Mix and probes for IDO (Mm00492586_m1) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Mm99999915_g1) as the control gene were run on the Applied Biosystems 7300 Real Time PCR System and analyzed with ABI Relative Quantification Study software (Applied Biosystems). The relative quantification of IDO in experimental animals compared to unmanipulated mice was done using the 2−ΔΔCt method following normalization to GAPHD.
Statistics
Survival data were analyzed by life-table methods, and actuarial survival rates are shown. Group comparisons were made by log-rank test statistics. Group comparisons of cell counts, pathology scores, and flow cytometric data were analyzed by Student t test. P values less than .05 were considered significant.
Results
IFN-γ response is required for IDO induction during GVHD
We have published that IDO is highly up-regulated in the colon after allogeneic transplantation, but we did not identify the signal for IDO induction.3 IFN-γ was a reasonable candidate because it is a potent stimulus for IDO induction. We examined the involvement of IFN-γ in IDO's role in GVHD by transplanting wild-type (wt) and IFN-γR−/− mice, incapable of receiving IFN-γ signals from donor T cells, with allogeneic BM with or without T cells. Figure 1A shows recipients that cannot respond to IFN-γ were far more susceptible to GVHD-induced lethality. We asked whether this protective effect of IFN-γ was attributable to its ability to induce IDO. Colons from wt and IFN-γR−/− recipients were harvested 7 days after transplantation, and IDO expression was assessed by qPCR and immunohistochemistry (IHC). As expected, wt mice receiving allogeneic T cells highly up-regulated IDO mRNA and protein (Figure 1B). In contrast IFN-γR−/− recipients expressed less IDO than even untransplanted wt mice, indicating IFN-γ is required for IDO up-regulation in the colon. To exclude the possibility that fewer T cells traffic to IFN-γR−/− colons, leading to less production of the IDO-inducing factor, we performed qPCR for CD4 and found equivalent levels, indicating similar numbers of T cells were available in both colons for inducing IDO.
Donor T cell–produced IFN-γ is required for colon IDO induction and effects on T-cell proliferation and survival. (A) Wt (filled symbols) or IFN-γR−/− (open symbols) B6 mice were lethally irradiated and infused with allogeneic BALB/c BM with (squares) or without (circles) 3 × 106 whole T cells. n = 8 mice/group. Survival is shown. P < .001. (B) Mice from transplant in panel A were killed 7 days after transplantation, and colons were examined for IDO protein by IHC and IDO and CD4 mRNA by qPCR. For IDO in wt versus IFN-γR−/− GVHD groups, n = 4 mice/group, P < .001. (C) Wt B6 mice were lethally irradiated and received BALB/c BM with or without 3 × 106 wt or IFN-γ−/− BALB/c T cells. At 7 days later, colons were harvested and assessed for IDO protein and mRNA. n = 4 mice/group, P = .03. (D) Wt (filled symbols) or IDO−/− (open symbols) B6 mice were transplanted with BALB/c BM with (squares) or without (circles) 3 × 106 wt (left) or IFN-γ−/− (right) BALB/c T cells. Survival is shown. n = 8 mice/group. For wt donors, P = .04, for IFN-γ−/− donors, P = .78. (E) Wt or IDO−/− BALB/c mice were lethally irradiated and received B6 BM and 106 wt or IFN-γ−/− B6 T cells. Sixteen days after transplantation, T cells were isolated from colons and assessed by flow cytometry for annexin V and Ki-67. Data are pooled from 2 identical experiments with n = 3-4 mice/group; *P < .05. Only statistically significant differences are noted.
Donor T cell–produced IFN-γ is required for colon IDO induction and effects on T-cell proliferation and survival. (A) Wt (filled symbols) or IFN-γR−/− (open symbols) B6 mice were lethally irradiated and infused with allogeneic BALB/c BM with (squares) or without (circles) 3 × 106 whole T cells. n = 8 mice/group. Survival is shown. P < .001. (B) Mice from transplant in panel A were killed 7 days after transplantation, and colons were examined for IDO protein by IHC and IDO and CD4 mRNA by qPCR. For IDO in wt versus IFN-γR−/− GVHD groups, n = 4 mice/group, P < .001. (C) Wt B6 mice were lethally irradiated and received BALB/c BM with or without 3 × 106 wt or IFN-γ−/− BALB/c T cells. At 7 days later, colons were harvested and assessed for IDO protein and mRNA. n = 4 mice/group, P = .03. (D) Wt (filled symbols) or IDO−/− (open symbols) B6 mice were transplanted with BALB/c BM with (squares) or without (circles) 3 × 106 wt (left) or IFN-γ−/− (right) BALB/c T cells. Survival is shown. n = 8 mice/group. For wt donors, P = .04, for IFN-γ−/− donors, P = .78. (E) Wt or IDO−/− BALB/c mice were lethally irradiated and received B6 BM and 106 wt or IFN-γ−/− B6 T cells. Sixteen days after transplantation, T cells were isolated from colons and assessed by flow cytometry for annexin V and Ki-67. Data are pooled from 2 identical experiments with n = 3-4 mice/group; *P < .05. Only statistically significant differences are noted.
We did not detect IDO induction in colons of mice receiving BM alone, leading us to hypothesize that donor T cells were the source of IFN-γ. Therefore, we transplanted BM with or without wt or IFN-γ−/− T cells into lethally irradiated allogeneic recipients and 7 days later harvested colons to determine IDO induction by IHC and qPCR. Figure 1C confirms that donor T cells were required for IDO up-regulation and shows that although wt allogeneic donor T cells induced substantial IDO mRNA and protein, donor T cells unable to make IFN-γ could not induce IDO above that observed in BM-only controls.
Having shown that IFN-γ is an IDO-inducing factor, we hypothesized that GVHD acceleration in the absence of IFN-γ was caused, at least in part, by the lack of IDO up-regulation. Therefore, we transplanted wt or IDO−/− mice with wt or IFN-γ−/− T cells. As has been shown previously, IFN-γ−/− T cells caused accelerated GVHD in wt hosts (Figure 1D). Whereas wt T cells caused increased lethality in IDO−/− versus wt recipients, IFN-γ−/− T cells caused equivalent lethality in wt and IDO−/− recipients. Thus, in the absence of IFN-γ and its ability to up-regulate IDO, the survival advantage of wt over IDO−/− recipients was abrogated.
Our previous work showed IDO-based suppression of GVHD correlated with decreased proliferation and survival of donor T cells in the colon. We now examined these IDO effects in mice receiving wt or IFN-γ−/− T cells. As shown in Figure 1E and supplemental Figure 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article), a greater proportion of wt colon CD4+ and CD8+ T cells from IDO−/− recipients were positive for Ki-67 and negative for annexin V compared with wt recipients, consistent with our previous results. However, when donor T cells were IFN-γ−/−, there was no difference in annexin V staining in CD4+ or CD8+ donor T cells and only a slight, albeit significant, decrease in Ki-67 staining in CD4+ T cells in wt versus IDO−/− recipients of IFN-γ−/− T cells. Here, again, the absence of IFN-γ lessened or abrogated the differences in wt and IDO−/− recipients. The remaining difference in Ki-67 suggests that basal levels of IDO in wt mice are capable of restraining T-cell proliferation but to a lesser degree than when IDO is strongly up-regulated.
BM-derived rather than epithelial-cell expression of IDO is more critically important for GVHD suppression
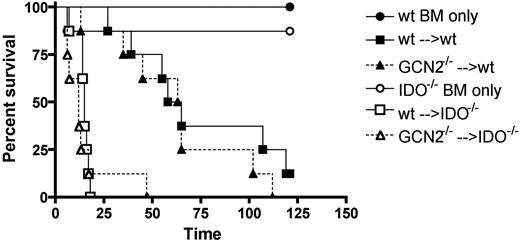
Both epithelial cells and BM-derived APCs are capable of IDO expression. To determine which cell type is critical for IDO-mediated GVHD suppression, we created BM chimeras in which the tissue cells, including epithelial cells, were IDO−/−, and BM-derived cells, including most APCs, were IDO+/+. These mice (wt→IDO−/−) could then be used to determine whether replacing IDO−/− with wt APCs could ameliorate disease. Conversely, we reconstituted wt mice with IDO−/− BM, creating IDO−/−→wt chimeras that could not express IDO in BM-derived cells but did express IDO in epithelial cells. We also created 2 groups of control chimeras (wt→wt and IDO−/−→IDO−/−). After waiting 3 months to ensure turnover of host BM-derived cells, we analyzed peripheral blood for engraftment. Nearly 100% of CD19+ cells were of BM donor origin (not shown). We then reirradiated the chimeras and infused BM and allogeneic T cells to induce GVHD. As expected, IDO−/−→IDO−/− chimeras died significantly faster than wt→wt chimeras (Figure 2A). Compared with IDO−/−→IDO−/− chimeras, survival was significantly prolonged in wt→IDO−/− chimeras (IDO only expressed in APCs), supporting an important role of IDO expression in host APCs and GVHD prevention. Survival also was prolonged in wt→wt versus IDO−/−→wt chimeras (IDO only expressed in tissues), although this finding did not quite reach statistical significance, further supporting a role for APC IDO. Epithelial cell expression of IDO had a smaller effect because survival was modestly reduced in IDO−/−→IDO−/− versus IDO−/−→wt chimeras and even less so in wt→IDO−/− versus wt→wt chimeras. Taken together, these data suggest that wt APCs provide a physiologically important source of IDO that contributes to GVHD prevention.
BM-derived host cells are more critical than epithelial cells in suppressing donor T cells via the IDO pathway. (A) WT or IDO−/− B6 mice were lethally irradiated and reconstituted with wt or IDO−/− B6 BM. After 3 months, the chimeras were reirradiated and infused with allogeneic BALB/c BM and 3 × 106 T cells. Survival is shown. For wt→wt (■) versus IDO−/−→IDO−/− (□) chimeras, P = .02. For wt→IDO−/− (▲) versus IDO−/−→IDO−/− chimeras, P = .04. For wt→wt versus IDO−/−→wt (△), P = .07. Representative of 2 experiments with n = 8-10 mice/group. (B) Chimeras were generated and transplanted as above. At 18 to 20 days later, T cells were isolated from colons and assessed by flow cytometry for Ki-67, annexin V, and PD-1. Data are pooled from 2 similar experiments with n = 3-5 mice/group. *P < .05.
BM-derived host cells are more critical than epithelial cells in suppressing donor T cells via the IDO pathway. (A) WT or IDO−/− B6 mice were lethally irradiated and reconstituted with wt or IDO−/− B6 BM. After 3 months, the chimeras were reirradiated and infused with allogeneic BALB/c BM and 3 × 106 T cells. Survival is shown. For wt→wt (■) versus IDO−/−→IDO−/− (□) chimeras, P = .02. For wt→IDO−/− (▲) versus IDO−/−→IDO−/− chimeras, P = .04. For wt→wt versus IDO−/−→wt (△), P = .07. Representative of 2 experiments with n = 8-10 mice/group. (B) Chimeras were generated and transplanted as above. At 18 to 20 days later, T cells were isolated from colons and assessed by flow cytometry for Ki-67, annexin V, and PD-1. Data are pooled from 2 similar experiments with n = 3-5 mice/group. *P < .05.
We next used these chimeras to examine the effects of cell type–restricted IDO expression on colon T cells. In line with the survival data, Figure 2B shows replacing IDO−/− with wt APCs resulted in significantly less proliferation and more apoptosis in colon CD4+ T cells. Conversely, replacing wt APCs with IDO−/− APCs led to less apoptosis and increased proliferation in colon CD4+ T cells. Therefore, APCs express IDO and suppress T-cell proliferation and survival in the colon. Because there was no difference in Ki-67 expression or annexin V staining in colon T cells between wt→IDO−/− chimeras and wt→wt chimeras or between IDO−/−→IDO−/− chimeras and IDO−/−→wt chimeras, these data suggest that epithelial cell expression of IDO alone is not sufficient to control T-cell proliferation or apoptosis.
Given the link we have previously established between IDO and another regulatory pathway, the PD-1/PD-L1, -L2 system,9 we examined colon T cells from the chimeras for PD-1, a marker of “exhausted” T cells. Intriguingly, CD4+ T cells in IDO−/−→IDO−/− colons expressed lower levels of PD-1 than those from wt→wt, and replacing IDO−/− APCs or IDO−/− epithelial cells with wt cells increased PD-1 expression. These data suggest that in the complete absence of host IDO expression, colonic T cells are less “exhausted” than when IDO is expressed, despite their greater proliferation rate. However, replacing host wt APCs or tissue with IDO−/− cells did not change PD-1 expression compared with wt→wt chimeras, suggesting PD-1 is up-regulated as long as some IDO is present, regardless of which cell expresses it. Notably, no differences were observed in Ki-67, annexin V, or PD-1 in T cells from spleen or liver, confirming the colon-specific actions of IDO (not shown).
Kynurenines and not donor T-cell GCN2 kinase influence GVHD suppression by the IDO pathway
IDO metabolizes tryptophan, resulting in 2 effects: decreased tryptophan and increased tryptophan metabolites. Both have been cited as the mechanism of IDO-based immunosuppression. T cells require GCN2 kinase to respond to low tryptophan. To determine whether this pathway is operative during GVHD, we compared survival in wt hosts receiving wt or GCN2−/− T cells. In the context of GVHD, if GCN2 activation leads to cell-cycle arrest and apoptosis, then GCN2−/− donor T cells should cause increased GVHD. However, this hypothesis proved incorrect because GCN2−/− versus wt T cells did not accelerate GVHD in wt hosts (Figure 3). If donor T cells require GCN2 to respond to IDO, then the survival advantage of wt over IDO−/− hosts should disappear if donor T cells cannot express GCN2. However, Figure 3 shows that IDO−/− recipients of GCN2−/− T cells died more quickly than wt recipients of GCN2−/− T cells. Together, these data indicated that IDO suppresses GVHD through a GCN2-independent mechanism.
IDO-based GVHD suppression is GCN2 independent. Wt (filled symbols) or IDO−/− (open symbols) BALB/c mice were lethally irradiated and infused with B6 BM with or without (circles) 2 × 106 wt (squares) or GCN2−/− (triangles) T cells. Survival is shown. For wt versus IDO−/− given wt cells, P < .001; for wt versus IDO−/− given GCN2−/− T cells, P = .001; for wt versus GCN2−/− in wt recipients, P = .38; for wt versus GCN2−/− in IDO−/− recipients, P = .53. Data are representative of 4 independent experiments with n = 7-8 mice/group.
IDO-based GVHD suppression is GCN2 independent. Wt (filled symbols) or IDO−/− (open symbols) BALB/c mice were lethally irradiated and infused with B6 BM with or without (circles) 2 × 106 wt (squares) or GCN2−/− (triangles) T cells. Survival is shown. For wt versus IDO−/− given wt cells, P < .001; for wt versus IDO−/− given GCN2−/− T cells, P = .001; for wt versus GCN2−/− in wt recipients, P = .38; for wt versus GCN2−/− in IDO−/− recipients, P = .53. Data are representative of 4 independent experiments with n = 7-8 mice/group.
Because survival is not a sensitive measure of GVHD, we also examined the effects of GCN2 deficiency on T-cell phenotype and organ pathology. GCN2−/− versus wt T cells underwent similar rates of proliferation and apoptosis and expressed similar amounts of IFN-γ early after transplantation. Colons of mice receiving wt or GCN2−/− T cells showed similar CD4+ T-cell infiltration 3 weeks after transplantation (not shown). Therefore, the lack of GCN2 did not affect the proliferative or IFN-γ–secreting behavior of donor T cells during the priming or effector phases of GVHD.
The donor T-cell GCN2-independence of the influence of IDO expression on GVHD suggested that IDO suppression of GVHD occurred via kynurenine production rather than tryptophan depletion. To determine whether exogenous kynurenines could suppress GVHD, we treated recipients with PBS or 75 mg/kg/day of a mixture of L-kynurenine, 3-hydroxykynurenine, and 3-hydroxyanthranillic acid by intraperitoneal injection starting 17 days after transplantation. As shown in Figure 4A, kynurenine injection significantly ameliorated GVHD lethality. When kynurenine administration was stopped at day 60, previously treated mice succumbed to GVHD, indicating that continuous exposure to kynurenines was required for GVHD inhibition even at this later time after BM transplantation (Figure 4B). Mice treated with kynurenines had less weight loss than the placebo group (Figure 4C), which is consistent with decreased gastrointestinal pathology as would be expected based upon our previous results demonstrating that a major beneficial effect of tryptophan catabolism after BM transplantation is to protect the intestine against GVHD injury.
Exogenous kynurenines inhibit GVHD lethality. (A) Wt B6 mice were lethally irradiated and infused with BALB/c BM with or without 3 × 106 T cells. On day 7, when weight loss was apparent, recipients were started on daily intraperitoneal injections of PBS (■) or 75 mg/kg of a mixture of L-kynurenine, 3-hydroxyanthranilic acid, and 3-hydroxykynurenine (□). Day 60 survival is shown, n = 5-6 mice/group. P = .03. (B) Treatment of mice from panel A was discontinued after 60 days. Long-term survival of mice surviving the first 60 days is shown. (C) Weight curves from the mice in panel A.
Exogenous kynurenines inhibit GVHD lethality. (A) Wt B6 mice were lethally irradiated and infused with BALB/c BM with or without 3 × 106 T cells. On day 7, when weight loss was apparent, recipients were started on daily intraperitoneal injections of PBS (■) or 75 mg/kg of a mixture of L-kynurenine, 3-hydroxyanthranilic acid, and 3-hydroxykynurenine (□). Day 60 survival is shown, n = 5-6 mice/group. P = .03. (B) Treatment of mice from panel A was discontinued after 60 days. Long-term survival of mice surviving the first 60 days is shown. (C) Weight curves from the mice in panel A.
Pre-BM transplantation IDO up-regulation suppresses GVHD
A second strategy for suppressing GVHD through IDO is to induce IDO expression before transplantation. In this way, T cells will be exposed to IDO activity immediately upon arrival in the colon, before they are able to proliferate and cause significant damage. Figure 2 highlights the importance of APCs for IDO-based suppression; these data suggested targeting APCs for IDO up-regulation may be successful for GVHD suppression. Studies have determined pDCs are crucial IDO expressers.30-32 In response to TLR ligation, pDCs release large amounts of type I IFNs, leading to IDO up-regulation. Therefore, we examined whether TLR ligation could induce IDO in recipient mice. Mice were lethally irradiated and 4 to 5 hours later were injected intravenously with PBS or 200 μg of a TLR7/8 agonist, 3M-011. Forty-eight hours later, we observed significant up-regulation of IDO in colons but not spleens of 3M-011–treated mice (Figure 5A). We then transplanted BM and T cells. We performed this step 2 days after irradiation, as opposed to the usual 24 hours, to minimize any direct effects that this small molecule agonist might have on the transferred cells. We assessed IDO expression 2 and 4 days after transplantation. On day 2, IDO expression had fallen to baseline. On day 4, IDO expression in the colon and spleen began to rise in PBS-treated animals, consistent with increased inflammation and IFN-γ production, whereas IDO remained low in the agonist-treated animals, suggestive of a reduced GVHD reaction.
Ligation of TLR7/8 induces transient expression of IDO in the colon and reduces GVHD lethality. (A) B6 Mice were lethally irradiated and 5 hours later injected intravenous with PBS or 200 μg of 3M-011. On day 0, 48 hours later, some mice were killed and colons, and spleens were assessed for IDO mRNA. The remaining mice were infused with BALB/c BM and 3 × 106 T cells. On days 2 and 4 after BM transplantation, IDO mRNA was again assessed from colons and spleens. For colon IDO on D0, *P = .05; for spleen on D4, *P = .02. n = 4 mice/group, D0 colon data are representative of 2 identical experiments. (B) Wt (left) or IDO−/− (right) mice were pretreated with PBS (■) or 3M-011 (□) and transplanted 48 hours later as in panel A. Survival is shown. Data are pooled from 2 identical experiments, n = 7-8 mice/group. P = .03. (C) Mice were treated and transplanted as in panels A and B. At 37 days after transplantation, colons were harvested, sectioned, and stained by hematoxylin and eosin. Sections were scored for pathology. n = 5 mice/group. *P < .05. (D) Sections taken from day 37 tissues in panel C were stained for CD4 by IHC. Representative sections are on the left, quantification of CD4+ cells is shown on the right. n = 5 mice/group. *P = .01.
Ligation of TLR7/8 induces transient expression of IDO in the colon and reduces GVHD lethality. (A) B6 Mice were lethally irradiated and 5 hours later injected intravenous with PBS or 200 μg of 3M-011. On day 0, 48 hours later, some mice were killed and colons, and spleens were assessed for IDO mRNA. The remaining mice were infused with BALB/c BM and 3 × 106 T cells. On days 2 and 4 after BM transplantation, IDO mRNA was again assessed from colons and spleens. For colon IDO on D0, *P = .05; for spleen on D4, *P = .02. n = 4 mice/group, D0 colon data are representative of 2 identical experiments. (B) Wt (left) or IDO−/− (right) mice were pretreated with PBS (■) or 3M-011 (□) and transplanted 48 hours later as in panel A. Survival is shown. Data are pooled from 2 identical experiments, n = 7-8 mice/group. P = .03. (C) Mice were treated and transplanted as in panels A and B. At 37 days after transplantation, colons were harvested, sectioned, and stained by hematoxylin and eosin. Sections were scored for pathology. n = 5 mice/group. *P < .05. (D) Sections taken from day 37 tissues in panel C were stained for CD4 by IHC. Representative sections are on the left, quantification of CD4+ cells is shown on the right. n = 5 mice/group. *P = .01.
We then tested whether this treatment could delay GVHD. Figure 5B shows that wt mice injected with TLR7/8 agonist before transplantation had significantly delayed GVHD lethality. IDO−/− recipients did not benefit from TLR7/8 agonist injection, proving the effect was IDO dependent. We then examined the colons of treated and untreated mice 37 days after transplantation. Figure 5C shows mice treated with 3M-011 had significantly lower pathology scores compared with PBS-treated controls (average score 2.7 vs 3.6, P < .05). In addition, IHC for CD4 revealed that 3M-011–treated recipients had fewer colon-infiltrating CD4+ cells than mice treated with PBS (Figure 5D), which is consistent with the protective effect of increased colonic IDO expression.
Discussion
Several major findings were uncovered by the present study. First, IFN-γ proved critical for host IDO induction during the GVHD response. Second, host APCs were a biologically more important source of IDO for suppressing GVHD than epithelial cells. Third, GCN2 kinase, which senses tryptophan depletion, was not involved in regulating GVHD lethality in wt or IDO−/− hosts. Fourth, kynurenines, tryptophan catabolic products, reduced GVHD lethality but only while administration continued. Fifth and finally, the purposeful induction of IDO pre-BM transplantation by a TLR7/8 agonist reduced subsequent GVHD lethality.
This work further characterizes the biology of IDO during GVHD. The complete lack of IDO up-regulation in the absence of donor IFN-γ is somewhat surprising, given the ability of multiple inflammatory signals, including type I IFNs, to induce IDO. Gut damage caused by conditioning leads to translocation of bacterial products from the lumen to the tissue, ligating TLRs and causing the release of cytokines including IFNs. It is possible these signals do not reach the threshold required for IDO up-regulation in this setting.
Although IFN-γ produced by donor T cells leads to massive IDO up-regulation in epithelial cells as observed by IHC, our chimera studies indicate APCs are the more important cells for IDO expression and control of GVHD. Examination of the phenotype of colon T cells implicates APC expression of IDO as the regulator of T-cell survival and proliferation. Most of the parameters examined were more prominently affected in the CD4+ T-cell subset. Because CD4+ T cells are the predominant infiltrating cells in the colon, this may simply be the result of low numbers of CD8+ T cells recovered. In support of the importance of APC-derived IDO in GVHD, the authors of a recent study33 found that the amelioration of GVHD by histone deacetylase inhibitors was caused by the induction of IDO in APCs. Injection of wt but not IDO−/− DCs treated with histone deacetylase inhibitor delayed GVHD, and treatment of recipients with the inhibitor was only efficacious in mice with wt APCs.33 In these studies, chimeras were used but no survival difference between wt→wt and IDO−/−→wt chimeras was found.33 Our chimera experiments using larger group sizes uncovered a trend toward decreased survival in IDO−/−→wt chimeras. At the tissue level, the consequence of IDO−/− APCs was more evident, with significant effects on T cell survival and proliferation. These conclusions beg the question of the importance of IDO expression in epithelial cells. It is possible epithelial cell expression of IDO dampens GVHD through T cell–independent means or by affecting T cells in ways not related to survival and proliferation. This contribution may have a beneficial effect not detectable by the survival rate, a relatively crude measurement.
Our data further suggest epithelial cell, as well as APC-derived, IDO is associated with PD-1 expression, although the differences determined by fluorescence-activated cell sorting analysis did not perfectly match the survival differences. The PD-1 effect is especially relevant in our system, as blockade of the PD-1 pathway dramatically accelerates GVHD.34 Thus, greater PD-1 expression in wt versus IDO−/− mice may contribute to the reduced GVHD lethality observed in the former. Only a few links between IDO and PD-1 have been reported. Increased PD-1 expression was observed in association with increased IDO expression and decreased viral clearance in an HCV model,35 and IDO and PD-1 were up-regulated in tumors that did not respond to cytokine treatment, along with several other negative immune regulators.36 In neither study did authors look specifically at T-cell expression of PD-1. IDO may regulate PD-1 expression, or the 2 pathways may be simply activated by the same immune modulatory signals. It is interesting that cells stimulated by alloantigen for weeks and continuing to proliferate are also less “exhausted” because of IDO expression. Whether this is a direct effect of IDO exposure and regulators of the PD-1 pathway on donor T cells or is an indirect effect is not known. A stronger association exists between IDO and the PD-1 ligands, PD-L1, and PD-L2. Expression of these ligands in tolerogenic environments, such as placenta37 and tumor microenvironments,38 sites that express IDO, as well as their up-regulation by IFN-γ,38 the main IDO inducer, suggests a possible link between the 2. In addition, we have previously reported that the function of IDO-activated Tregs required up-regulation of PD-L1 and PD-L2 on DCs, rendering such DCs suppressive.9 The exact mechanistic link between IDO and the PD-1 pathway requires further study.
Although GCN2 is not the exclusive sensor for amino acid deprivation, it is the only mechanism implicated in IDO-based immunosuppression. The GCN2-independence of GVHD suppression suggests tryptophan metabolites are the primary mechanism for IDO in this system. The ability of IDO to act as a free-radical scavenger could contribute to its anti-inflammatory properties and gut protection, and this action would be GCN2-independent as well.
The in vitro and in vivo suppressive effects of exogenous kynurenines are in accord with other murine studies on autoimmunity, allergen immunotherapy, and alloresponses.14-16 Furthermore, in a murine model of chronic granulomatous disease, Romani et al39 showed defective IDO activation led to the inability to produce tryptophan metabolites. By providing kynurenine, the first breakdown product, they could bypass the IDO block and reestablish immune suppression. This strategy required coinjection of IFN-γ to induce the tryptophan metabolism enzymes downstream of IDO, including kynurenine 3-monooxygenase. Presumably the high levels of IFN-γ observed in GVHD would ensure the expression of these enzymes in the tryptophan catabolic pathway, so that even in the absence of IDO, providing kynurenine would lead to production of each of these potentially suppressive metabolites. Differing reports have found 3-hydroxykynurenine16 or 3-hydroxyanthranilic acid12 (and not the other) or both13,14 to be suppressive. Whether they are further metabolized in vivo to other more or less suppressive compounds and what equilibrium is reached between concentrations of these compounds is not known. Future studies are needed to determine whether GVHD inhibition by kynurenines can override host IDO deficiency. Finally, it is notable that discontinuing kynurenines was followed by GVHD lethality. These data suggest that kynurenines held donor T-cell number below a threshold needed to generate lethality; discontinuing kynurenines released this brake, indicating that kynurenines did not induce tolerance in donor T cells but rather controlled the size of the alloreactive T-cell population. Whether kynurenine treatment affects antimicrobial immunity will require future study.
The striking ability of TLR ligation to lessen GVHD is even more interesting considering the early and transient up-regulation of IDO. The 5- to 10-fold induction 2 days after TLR agonist injection is far less than the hundreds- to thousands-fold induction observed on day 7 after BM transplantation as the result of donor T-cell IFN-γ production, and yet it controls GVHD better than the later, more intense IDO up-regulation. Because donor T cells expand 50- to 100-fold and are highly activated by day 5 after BM transplantation,40 early heightened IDO expression may be particularly effective at reducing the overall T-cell burden during the most intense inflammatory phase of pre-BM transplantation conditioning. The chimera experiments highlighted the importance of APCs in suppressing GVHD through IDO, despite strong IDO expression in colonic epithelial cells. Thus, the efficacy of lower levels of TLR-induced IDO could be caused in part by the greater importance of IDO expression in APCs versus epithelial cells. Because TLR7/8 agonists target APCs and colons contain fewer APCs than epithelial cells, the up-regulation of IDO in this small population of cells likely results in a physiologically more effective mechanism to suppress GVHD, perhaps because of the special interactions between T cells and APCs. Early IDO expression in APCs may be capable of suppressing the first T cells that arrive in the colon, stopping one of the primary events in the cycle of inflammation and destruction. Moreover, early IDO induction may have tissue-protective effects as mentioned previously, and it is possible that decreasing epithelial cell damage dampens another step in the destructive cycle. Given the minor component of APCs in the colon, it is not surprising that a massive increase in IDO expression in the colon was not observed after TLR agonist administration, as colonic APCs and epithelial cells were not separately analyzed.
GVHD inhibition was achieved by administering TLR7/8 agonist before BM transplantation, in contrast to the GVHD acceleration we observed with post-BM transplantation TLR agonist administration, the latter likely caused by overwhelming proinflammatory cytokine release with inadequate control by IDO.41 These results are especially encouraging given the local and temporary nature of IDO induction, suggesting immunity to pathogens in the gastrointestinal tract would not be affected by such a treatment. Because regulation of the immune response by IDO during GVHD appears confined to colon T cells,3 and because TLR7 agonists given to patients have antitumor effects,42 this approach may prove to be especially efficacious in preserving a graft-versus-malignancy effect as will be addressed in future studies.
In conclusion, these data increase our understanding of the mechanism of IDO induction and action during a relevant disease process and, most importantly, have led to the development of 2 novel and clinically applicable therapeutic strategies to prevent and treat GVHD. Because TLR7/8 agonists have been, and kynurenines are being, developed for use in the clinic, we believe these results are an exciting first step toward potent new GVHD therapies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank David Ron for his generous gift of the GCN2−/− mice and Lawrence Steinman for kindly providing 3-hydroxykynurenine. We also thank Emily Goren and Luna Liu for excellent animal husbandry, Christine Vogtenhuber and Andy Price for technical assistance, and Chris Lees and Mark Osborn for expert assistance with qPCR.
This work was supported by National Institutes of Health grants F30 AG032170 (to L.K.J.), R01 AI34495 (to B.R.B.), R01 CA 72 669 (to B.R.B), R37 HL56067 (to B.R.B.), CA103320 (to D.H.M.), and AI063402 (to A.L.M.).
National Institutes of Health
Authorship
L.K.J., B.R.B., and D.H.M. designed research; L.K.J. and C.B. performed research; A.L.M. provided reagents; D.H.M. provided reagents and advice; A.P-M contributed data; L.K.J. analyzed the data and wrote the paper; and C.B., A.P-M., D.H.M., and B.R.B. edited the paper.
Conflict-of-interest disclosure: D.H.M. and A.L.M. have intellectual property interests in the therapeutic use of IDO and IDO inhibitors and receive consulting income and research support from NewLink Genetics Inc. The remaining authors of this manuscript declare no competing financial interests.
Correspondence: Bruce R. Blazar, MD, University of Minnesota Cancer Center and Department of Pediatrics, Division of BMT, MMC 109, 420 Delaware St, Minneapolis, MN 55455; e-mail: blaza001@umn.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal