Abstract
The plasminogen system plays a crucial role in the repair of a variety of tissues, including skeletal muscle. We hypothesized that urokinase-type plasminogen activator (uPA) promotes muscle regeneration by activating hepatocyte growth factor (HGF), which, in turn, stimulates proliferation of myoblasts required for regeneration. In our studies, levels of active HGF and phosphorylation of the HGF receptor c-met were increased after muscle injury in wild-type mice. Compared with wild-type animals, mice deficient in uPA (uPA−/−) had markedly reduced HGF levels and c-met activation after muscle damage. This reduced HGF activity in uPA−/− animals was associated with decreased cell proliferation, myoblast accumulation, and new muscle fiber formation. On the other hand, HGF activity was enhanced at early time points in PAI-1−/− mice compared with wild-type mice and the PAI-1−/− animals exhibited accelerated muscle fiber regeneration. Furthermore, administration of exogenous uPA rescued HGF levels and muscle regeneration in uPA−/− mice, and an HGF-blocking antibody reduced HGF activity and muscle regeneration in wild-type mice. We also found that uPA promotes myoblast proliferation in vitro through its proteolytic activity, and this process was inhibited by an HGF-blocking antibody. Together, our findings demonstrate that uPA promotes muscle regeneration through HGF activation and subsequent myoblast proliferation.
Introduction
The plasminogen system plays a crucial role in the repair of a variety of tissues. This extracellular serine protease cascade includes the urokinase-type plasminogen activator (uPA) and its primary inhibitor, plasminogen activator inhibitor-1 (PAI-1). The classic function of uPA is to cleave plasminogen to form active plasmin, a broad-spectrum protease with activity toward a variety of extracellular matrix molecules.1,2 Genetic manipulations that inhibit plasminogen activation lead to impaired healing of lung, liver, kidney, and skin.3-6 Similarly, mice deficient in uPA or plasminogen demonstrate markedly impaired muscle regeneration.7-9 In contrast, mice lacking PAI-1 exhibit accelerated muscle regeneration.7
Despite its robust effect on skeletal muscle regeneration, the molecular mechanisms by which the plasminogen system regulates this process remain largely undefined. Clearance of the provisional fibrin matrix that is deposited after muscle injury may be one mechanism by which uPA promotes regeneration.8 However, uPA may also promote tissue repair through other pathways, such as the proteolytic activation of growth factors, including hepatocyte growth factor (HGF). HGF is synthesized and secreted as an inactive single-chain molecule, and proteolytic cleavage results in formation of the active 2-chain form, which has affinity for the c-met receptor.10-12 uPA is one of a select group of proteases known to activate HGF,11,12 the others being tissue-type plasminogen activator, factor XIa, factor XIIa, matriptase, and HGF activator.13-15 In addition, uPA appears to increase HGF bioactivity in the lung and liver after injury.5,16 Like uPA, HGF appears to play important roles in the repair of different tissues, including lung, liver, kidney, and skin,17-20 presumably by stimulating the proliferation and migration of cells involved in healing.
HGF has also been implicated in both skeletal muscle development and regeneration after injury. HGF and its receptor, c-met, are required for migration of myogenic cells into the limb buds and the development of limb skeletal muscle.21 HGF and c-met are also expressed in noninjured skeletal muscle of adult animals.22,23 Although HGF expression is increased at both the mRNA and protein levels after muscle injury,22,24 the precise role of this growth factor during muscle regeneration has not been established. Injection of exogenous HGF into noninjured muscle of rats activates quiescent satellite cells,23 which are muscle precursor cells required for regeneration. In addition, injection of HGF into damaged muscle of mice has been shown to stimulate myoblast proliferation.25 In addition to its in vivo effects, HGF also stimulates both proliferation and migration of myoblasts in vitro,26-28 and stretching myoblast cultures has been shown to produce an HGF-dependent increase in cell proliferation.29
The ability of uPA to both activate HGF and promote muscle repair, along with the capacity for HGF to activate myoblasts, led us to hypothesize that uPA promotes skeletal muscle regeneration by activating HGF. To test this hypothesis, we measured HGF and c-met activation along with cell proliferation after muscle injury in wild-type (WT), uPA-deficient (uPA−/−), and PAI-1–deficient (PAI-1−/−) mice. We also determined whether administering exogenous uPA would rescue HGF activation and muscle regeneration in uPA−/− mice, and whether an HGF-blocking antibody would impair muscle regeneration in WT mice. Furthermore, we tested whether uPA activation of HGF stimulated myoblast proliferation in vitro. The resulting data from these experiments indicate that uPA does indeed promote myoblast proliferation and muscle repair by activating HGF.
Methods
Mice
C57BL/6 (WT), uPA−/−, and PAI-1−/− mice were obtained from The Jackson Laboratory and bred in our animal facility. The uPA−/− and PAI-1−/− mice had been backcrossed onto the C57Bl/6 background for more than 8 generations. Mice were housed in a specific pathogen-free environment at a constant temperature and a 12:12 hour light-dark cycle. Experiments were performed on 10- to 14-week-old mice. All experimental procedures were approved by the Animal Care Committee at the University of Illinois at Chicago.
Muscle injury
Extensor digitorum longus (EDL) and tibialis anterior muscles were injured using cardiotoxin as previously described.7,30 Briefly, mice were anesthetized with an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (5 mg/kg), and a small incision (1 cm) was made to expose the muscles of the anterior leg. Cardiotoxin (10μM; Calbiochem) was administered with 2 intramuscular injections per muscle to ensure distribution throughout the muscle. The skin incision was closed with 7-0 nylon suture, and the procedure was repeated on the contralateral limb. At different times after injury, mice were killed by cervical dislocation while under anesthesia, and muscles were harvested. EDL muscles were mounted in tissue freezing medium and frozen in isopentane chilled with dry ice for histologic analysis. Tibialis anterior muscles were snap frozen in liquid nitrogen for immunoprecipitation or affinity purification and Western blot analysis.
Exogenous uPA and HGF-blocking antibody
To restore uPA to the muscles of uPA−/− mice, intramuscular injections of recombinant murine uPA (5 μg/muscle; Molecular Innovations) were administered daily on days 1 through 4 after injury. To inhibit HGF activity in damaged muscle of WT mice, 200 μg of an HGF-blocking antibody (R&D Systems)31 was administered intraperitoneally immediately after injury, and daily thereafter for 5 days. Control mice received an equal amount of nonspecific IgG.
Immunofluorescence
Cell proliferation was assessed in muscle cryosections using incorporation of bromodeoxyuridine (BrdU) into cell nuclei. One hour before death, mice were injected with 30 mg/kg BrdU. Cross-sections were cut from the mid-belly of each EDL muscle (10-μm thickness), fixed in cold acetone, washed in phosphate-buffered saline, and incubated in 2N HCl to denature DNA. Sections were neutralized in basic phosphate-buffered saline and then incubated in blocking buffer containing 0.2% gelatin and 3% bovine serum albumin. Proliferating cells were labeled with a BrdU antibody (Roche) for 1 hour and subsequently incubated with fluorescein isothiocyanate anti–mouse secondary antibody (Jackson ImmunoResearch). Alternatively, sections were stained for the myogenic transcription factor MyoD. These sections were fixed in cold acetone, incubated with blocking buffer, and then incubated with primary antibody (Santa Cruz Biotechnology) overnight. Sections were subsequently incubated with tetramethylrhodamine isothiocyanate anti–rabbit secondary antibody (Jackson ImmunoResearch Laboratories) and then mounted in Vectashield mounting medium containing 4,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). The number of DAPI+ nuclei that were also MyoD or BrdU+ was counted in 3 fields observed using a 40×/0.75 NA dry objective with a Nikon Labphot-2 microscope and SPOT digital camera and software (Diagnostic Instruments) for 2 sections per muscle and normalized to the volume of muscle sampled.
Morphology
Muscle cross-sections were stained with hematoxylin and eosin, and quantitative analysis was performed on 5 representative images of each muscle section obtained using a 40×/0.75 NA dry objective with a Nikon Labphot-2 microscope and SPOT camera and software. For each field, fibers were classified as normal, damaged, or regenerating as described.7 Regenerating fibers were identified as those containing centrally located nuclei without evidence of damage. The number and area of each type of fiber were recorded. The damaged area was then estimated in each muscle section by subtracting the summed area of normal and regenerating fibers from the total area of each field. Images were prepared for figures using Adobe Photoshop CS3.
HGF and c-met protein analysis
HGF protein levels were assessed by affinity purification followed by Western blotting. Muscles were homogenized in buffer A (20mM Tris, pH 7.5, 150mM NaCl, 0.1% Triton X-100) with protease and phosphatase inhibitors (1mM phenylmethylsulfonyl fluoride, 1μM leupeptin, 0.3μM aprotinin, 25mM β-glyerophosphate, 1mM Na3VO4, 5mM NaF). The full volume of muscle homogenate from each sample was incubated with heparin-agarose beads at 4°C overnight with rotation. Beads were washed and then boiled in 20 μL of sodium dodecyl sulfate (SDS) sample buffer to elute bound protein.
C-met protein levels and phosphorylation were assessed by immunoprecipitation followed by Western blotting. Muscles were homogenized in buffer B (20mM Tris, pH 7.5, 150mM NaCl, 0.1% SDS, 1% NP-40) with protease and phosphatase inhibitors. Homogenates (2 mg) were incubated with anti–c-met antibody (Santa Cruz Biotechnology) at 4°C overnight with rotation and then protein G-Sepharose beads for 1 hour. Beads were washed and then boiled in SDS sample buffer.
For both HGF and c-met analysis, one-half the total volume of each sample was separated on each of 2 SDS-polyacrylamide gel electrophoresis gels and transferred to nitrocellulose membranes. After transfer, membranes were blocked in 5% milk overnight. Membranes were incubated with primary antibody against HGF, c-met, or phosphotyrosine (all from Santa Cruz Biotechnology) and then incubated with secondary antibody conjugated to horseradish peroxidase (Pierce Chemical). Protein bands were detected using enhanced chemiluminescence (GE Healthcare), and band densities were determined by image analysis.
Cell isolation and real-time PCR
Cells were isolated from muscles in WT mice that were harvested 5 days after injury using a protocol modified from the literature.32 Briefly, muscles were dissected, minced, and then digested with 0.1% pronase (Calbiochem). After trituration to dissociate from fiber fragments, the suspension was filtered through 100-μm mesh, the filtrate was centrifuged, and cells were resuspended and counted. The total cell population was separated into macrophage and nonmacrophage cells using a Mac-1 antibody conjugated to microbeads and a magnetic column (Miltenyi Biotec). Total RNA was isolated from the Mac-1+ and Mac-1− cells using the RNeasy kit (QIAGEN) following the manufacturer's instructions, and then equal amounts of RNA were used to generate cDNA using the Thermoscript RT-PCR System (Invitrogen). Real-time polymerase chain reaction (PCR) was performed in a 7500Fast System (Applied Biosystems) using TaqMan Universal PCR Master Mix and TaqMan Gene Expression Assay primer/probe sets (Applied Biosystems) for mouse uPA, HGF, or GAPDH. Relative gene expression was determined using the 2−ΔΔCT method, with Mac-1− cells as baseline and GAPDH as endogenous control gene. Primer efficiency was verified using serial dilutions of sample cDNA.
Myoblast cultures
Primary myoblast cultures were derived from neonatal hind-limb muscles from WT and uPA−/− mice using an established method.32,33 Briefly, muscle was finely minced, digested in 1% pronase (Calbiochem), and cells were released by trituration. Pooled cells were filtered (100 μm), centrifuged, and resuspended. A preplating technique was used to separate fibroblasts from myoblasts; 1 hour after preplating on tissue-culture dishes, nonadherent cells (primarily myoblasts) were transferred to another dish coated with entactin/collagen/laminin (Upstate Biotechnology) and grown in selective proliferation medium (Ham F10, 20% fetal bovine serum [FBS], 5 ng/mL basic fibroblast growth factor, 1% penicillin/streptomycin) in a humidified 5% CO2 atmosphere at 37°C. Immunofluorescence analysis demonstrated that greater than 90% of cells cultured in this manner were positive for MyoD.
To test the influence of uPA on myoblast proliferation, WT and uPA−/− myoblasts were plated on entactin/collagen/laminin-coated coverslips and incubated in low serum medium overnight. Cells were then incubated in low serum (0.5% FBS) or high serum (20% FBS) medium and supplemented with recombinant mouse uPA and/or PAI-1 (Molecular Innovations), HGF or an HGF-blocking antibody (R&D Systems), the PI3K inhibitor LY294002 or the MEK1 inhibitor PD98059 (Calbiochem) for 24 hours. Cells were also incubated with 100μM BrdU, and incorporation of BrdU into cell nuclei was detected using a protocol similar to that described for muscle cryosections. The number of positively labeled cells was counted in 4 fields observed at 20× and normalized to the total number of cells present in each field.
Statistical analysis
Values are reported as mean plus or minus SE. Data were compared across time points, mouse genotypes, and/or treatment conditions using 2-way analysis of variance (analysis of BrdU and MyoD in muscle cryosections, densitometric analysis of HGF and c-met Western blots, myoblast proliferation experiments), 1-way analysis of variance (signaling inhibitor experiments), or t tests (in vivo HGF-blocking antibody experiments). The Student-Newman-Keuls test was used for post-hoc analysis of analysis of variance tests found to be statistically significant. The .05 level was taken to indicate statistical significance.
Results
HGF and c-met activation in uPA−/− and PAI-1−/− mice
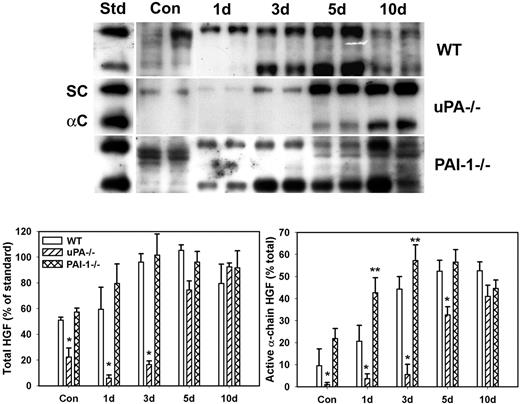
Because uPA is known to activate HGF and HGF is thought to play important roles in muscle regeneration, we assessed levels of total and active HGF after muscle injury in WT, uPA−/−, and PAI-1−/− mice. In uninjured control muscle, HGF was present in mainly its inactive single-chain form (∼ 90 kDa) with a greater amount of HGF isolated from WT and PAI-1−/− mice than uPA−/− mice (Figure 1). After muscle injury in WT mice, total HGF levels were increased by approximately 2-fold at 3 and 5 days after injury with approximately 50% present in the active α-chain form (∼ 60 kDa, Figure 1). In uPA−/− mice, very little total HGF was evident until 5 and 10 days after injury, and it was present predominantly in the inactive single-chain form until 10 days after injury. In PAI-1−/− mice, total HGF levels were similar to those in WT mice at 1 and 3 days after injury, but a greater percentage of the total was in the active α-chain form in the PAI-1−/− animals. These data indicate that levels of active HGF are down-regulated in the absence of uPA and up-regulated when uPA activity is not inhibited by PAI-1.
HGF protein levels after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected from uninjured control mice (Con) and on days 1, 3, 5, and 10 after cardiotoxin injury. (Top) Muscle homogenates subjected to heparin sulfate affinity purification and then Western blotting for HGF. An equal amount (2.5 ng) of an HGF standard (Std) was loaded onto each gel. SC indicates single chain; and αC: α-chain. (Bottom) Densitometric measurements performed for total HGF (single chain + α-chain) and active α-chain HGF. Total HGF normalized to HGF standard on each blot, and α-chain normalized to total HGF in each sample. Bars represent mean ± SE; n = 4 to 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
HGF protein levels after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected from uninjured control mice (Con) and on days 1, 3, 5, and 10 after cardiotoxin injury. (Top) Muscle homogenates subjected to heparin sulfate affinity purification and then Western blotting for HGF. An equal amount (2.5 ng) of an HGF standard (Std) was loaded onto each gel. SC indicates single chain; and αC: α-chain. (Bottom) Densitometric measurements performed for total HGF (single chain + α-chain) and active α-chain HGF. Total HGF normalized to HGF standard on each blot, and α-chain normalized to total HGF in each sample. Bars represent mean ± SE; n = 4 to 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
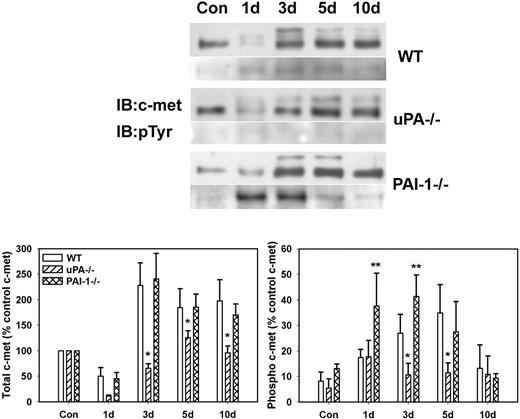
To determine whether differences in levels of active HGF corresponded to differences in activation of the HGF receptor c-met, we measured phosphorylation of c-met after muscle injury in WT, uPA−/−, and PAI-1−/− mice. The β-chain of c-met (∼ 145 kDa) was evident in uninjured control muscle of all mouse strains with very little, if any, phosphorylation (Figure 2). After muscle injury in WT and PAI-1−/− mice, total c-met was increased at 3 to 10 days after injury, with some of the immature c-met present (∼ 190 kDa; Figure 2). Although total c-met was up-regulated in uPA−/− mice, the increase was not as large as observed in the other 2 genotypes. After muscle injury in WT mice, there was a progressive increase in c-met phosphorylation with a peak at 5 days after injury. In contrast, c-met phosphorylation was significantly blunted in the injured muscles of the uPA−/− mice. Furthermore, the induction of c-met phosphorylation in PAI-1−/− mice was more rapid than that observed in WT animals.
c-met protein levels and phosphorylation after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected from uninjured control mice (Con) and on days 1, 3, 5, and 10 after cardiotoxin injury. (Top) Muscle homogenates subjected to c-met immunoprecipitation and then Western blotting for c-met and phosphotyrosine (pTyr). (Bottom) Densitometric measurements performed for c-met and pTyr and normalized to c-met level in control sample for each series of samples obtained for each strain. Bars represent ± SE; n = 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
c-met protein levels and phosphorylation after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected from uninjured control mice (Con) and on days 1, 3, 5, and 10 after cardiotoxin injury. (Top) Muscle homogenates subjected to c-met immunoprecipitation and then Western blotting for c-met and phosphotyrosine (pTyr). (Bottom) Densitometric measurements performed for c-met and pTyr and normalized to c-met level in control sample for each series of samples obtained for each strain. Bars represent ± SE; n = 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
Proliferation and myoblast accumulation in damaged muscle of uPA−/− and PAI-1−/− mice
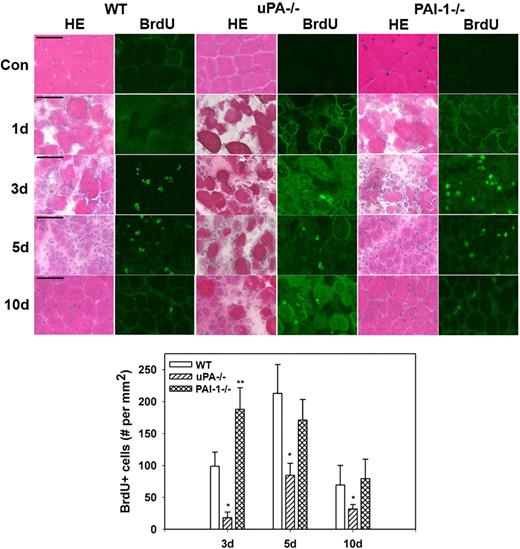
Morphologic observations from our current study confirmed the persistence of necrotic muscle fibers and lack of formation of regenerating fibers in injured muscles from uPA−/− mice and verified accelerated regeneration in muscles from PAI-1−/− mice (Figure 3). To assess cell proliferation after muscle injury, we counted cells that had incorporated BrdU into newly synthesized DNA. In WT, uPA−/−, and PAI-1−/− mice, there were very few BrdU+ cells in uninjured control muscles or at 1 day after cardiotoxin administration (Figure 3). In WT mice, BrdU+ cells were evident in damaged muscle at 3 days after injury, increased to a peak at 5 days, and then declined at 10 days. There were significantly fewer BrdU+ cells at all time points after injury in uPA−/− mice compared with WT animals, consistent with the morphologic observations of impaired regeneration in these mice. Furthermore, there were significantly more BrdU+ cells in PAI-1−/− mice at 3 days after injury compared with WT mice, consistent with the accelerated repair in these animals.
Muscle morphology and cell proliferation after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected from uninjured control mice (Con) and on days 1, 3, 5, and 10 after cardiotoxin injury. BrdU (30 mg/kg) injected intraperitoneally into mice 1 hour before muscle harvest. (Top) Cryosections stained with either hematoxylin and eosin for morphologic analysis or a BrdU antibody to detect incorporation of BrdU into newly synthesized DNA. Scale bar represents 50 μm. (Bottom) Number of nuclei staining positive for BrdU counted in 2 sections for each muscle, averaged, and expressed per square millimeter muscle area. Bars represent mean ± SE; n = 4 to 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
Muscle morphology and cell proliferation after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected from uninjured control mice (Con) and on days 1, 3, 5, and 10 after cardiotoxin injury. BrdU (30 mg/kg) injected intraperitoneally into mice 1 hour before muscle harvest. (Top) Cryosections stained with either hematoxylin and eosin for morphologic analysis or a BrdU antibody to detect incorporation of BrdU into newly synthesized DNA. Scale bar represents 50 μm. (Bottom) Number of nuclei staining positive for BrdU counted in 2 sections for each muscle, averaged, and expressed per square millimeter muscle area. Bars represent mean ± SE; n = 4 to 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
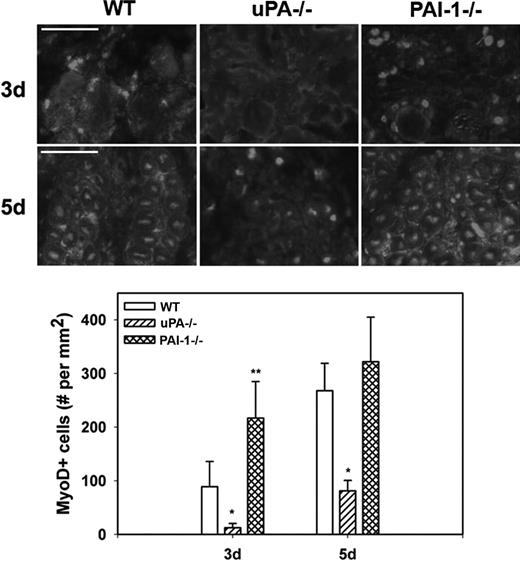
To assess myoblast accumulation and muscle fiber formation after muscle injury, we counted MyoD+ nuclei in muscle sections; nuclear staining was confirmed by costaining with DAPI (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). At 3 days after injury in WT mice, MyoD+ nuclei had accumulated at the periphery of damaged muscle fibers and in small cells within spaces between fibers (Figure 4). At 5 days, the number of MyoD+ nuclei increased and many of these were now within small regenerating (central nucleated) muscle fibers. There were significantly fewer MyoD+ nuclei in damaged muscle of uPA−/− mice compared with WT animals at both 3 and 5 days after injury, and none of the MyoD+ nuclei appeared to be regenerating fibers. On the other hand, the number of MyoD+ nuclei was significantly increased in PAI-1−/− mice compared with WT animals at 3 days after injury. Taken together, these data indicate that cell proliferation, myoblast accumulation, and muscle fiber formation are regulated by the balance of uPA and PAI-1 present in damaged muscle.
Myoblast accumulation after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected on days3 and 5 after cardiotoxin injury. (Top) Cryosections stained with antibody against the myoblast marker MyoD. Scale bar represents 50 μm. (Bottom) Number of nuclei staining positive for MyoD counted in 2 sections for each muscle, averaged, and expressed per square millimeter muscle area. Bars represent mean ± SE; n = 4 to 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
Myoblast accumulation after muscle injury in WT, uPA−/−, and PAI-1−/− mice. Muscles from WT, uPA−/−, and PAI-1−/− mice collected on days3 and 5 after cardiotoxin injury. (Top) Cryosections stained with antibody against the myoblast marker MyoD. Scale bar represents 50 μm. (Bottom) Number of nuclei staining positive for MyoD counted in 2 sections for each muscle, averaged, and expressed per square millimeter muscle area. Bars represent mean ± SE; n = 4 to 6 per time point. *Mean value significantly smaller than that for WT mice (P < .05). **Mean value significantly larger than that for WT mice (P < .05).
Exogenous uPA administration, HGF levels, and muscle regeneration in uPA−/− mice
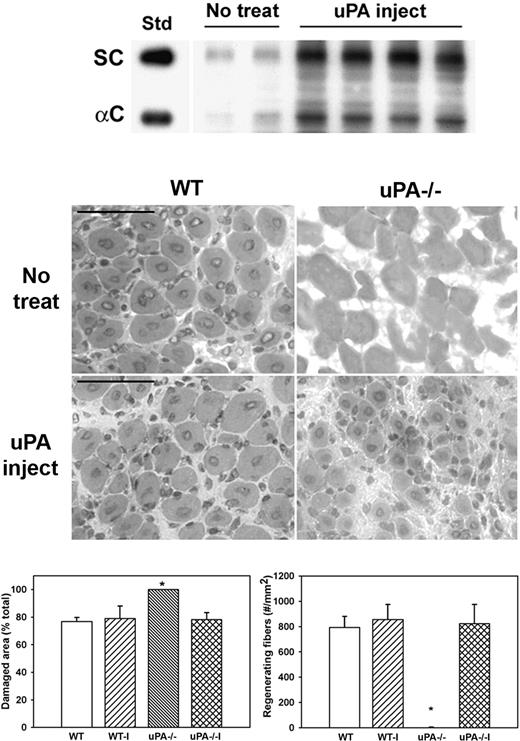
To determine whether administering exogenous uPA could rescue HGF activity and muscle regeneration in uPA−/− mice, we injected uPA into cardiotoxin-injured muscles of uPA−/− mice. Injured muscles from untreated uPA−/− mice contained relatively low levels of HGF (Figure 5). In contrast, injured muscles from mice treated with exogenous uPA exhibited both increased total and α-chain HGF levels (Figure 5). In addition, muscles from untreated uPA−/− mice were characterized by the persistence of damaged tissue and the absence of regenerating fibers (Figure 5). In conjunction with the increased levels of HGF, exogenous uPA increased the formation of regenerating fibers and enhanced the recovery of normal muscle morphology in uPA−/− mice. In contrast, treatment of injured muscles in WT mice with exogenous uPA did not alter the recovery of muscle morphology.
Administration of exogenous uPA to uPA−/− mice. uPA−/− mice were subjected to cardiotoxin muscle injury and either left untreated (No treat) or treated with intramuscular injection of exogenous uPA daily from 1 to 4 days after injury (uPA inject), and muscles collected at 5 days after injury. (Top) Muscle homogenates subjected to heparin sulfate affinity purification and then Western blotting for HGF. Note that treatment with exogenous uPA rescued HGF levels in muscle of uPA−/− mice. (Middle) Muscle cryosections stained with hematoxylin and eosin for morphologic analysis. Note the restoration of muscle regeneration in uPA−/− mice after treatment with uPA. Scale bar represents 50 μm. (Bottom) Quantitative analysis of morphology. WT indicates wild-type no treatment; WT-I, wild-type injected with uPA; uPA−/−, uPA−/− no treatment; and uPA−/−I, uPA−/− injected with uPA. Regenerating fibers identified as central nucleated fibers and counted in 2 sections per muscle and expressed as number per square millimeter muscle area. Damaged area estimated by subtracting summed area of normal and regenerating fibers from total muscle area. Bars represent mean ± SE; n = 4 to 6 per group. *Mean value significantly different from that for WT mice (P < .05).
Administration of exogenous uPA to uPA−/− mice. uPA−/− mice were subjected to cardiotoxin muscle injury and either left untreated (No treat) or treated with intramuscular injection of exogenous uPA daily from 1 to 4 days after injury (uPA inject), and muscles collected at 5 days after injury. (Top) Muscle homogenates subjected to heparin sulfate affinity purification and then Western blotting for HGF. Note that treatment with exogenous uPA rescued HGF levels in muscle of uPA−/− mice. (Middle) Muscle cryosections stained with hematoxylin and eosin for morphologic analysis. Note the restoration of muscle regeneration in uPA−/− mice after treatment with uPA. Scale bar represents 50 μm. (Bottom) Quantitative analysis of morphology. WT indicates wild-type no treatment; WT-I, wild-type injected with uPA; uPA−/−, uPA−/− no treatment; and uPA−/−I, uPA−/− injected with uPA. Regenerating fibers identified as central nucleated fibers and counted in 2 sections per muscle and expressed as number per square millimeter muscle area. Damaged area estimated by subtracting summed area of normal and regenerating fibers from total muscle area. Bars represent mean ± SE; n = 4 to 6 per group. *Mean value significantly different from that for WT mice (P < .05).
HGF-blocking antibody and muscle regeneration
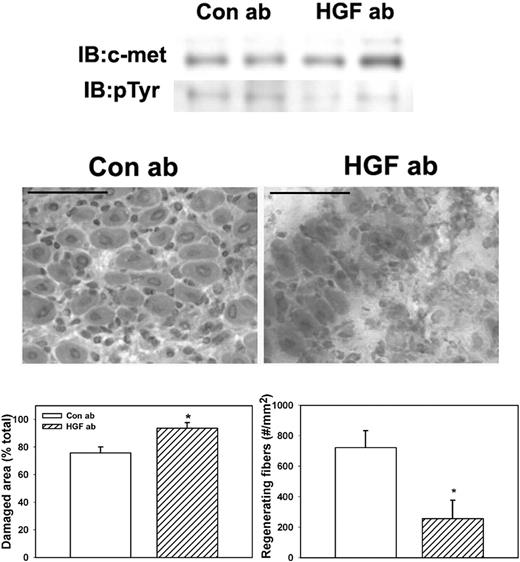
To determine whether HGF activity is required for efficient skeletal muscle regeneration in WT mice, we administered an HGF-blocking antibody daily after muscle injury. Treatment with the HGF-blocking antibody reduced phosphorylation of c-met compared with control goat IgG (Figure 6). Phosphorylated c-met normalized to total c-met was 37.2% (± 6.5%) for mice treated with control IgG versus 10.2% (± 3.6%) for mice treated with HGF-blocking antibody, verifying that the blocking antibody reduced HGF activity in damaged muscle. In addition, the HGF-blocking antibody significantly reduced the formation of regenerating fibers (Figure 6), indicating that blocking HGF activity after muscle injury impairs muscle regeneration.
Administration of HGF-blocking antibody to WT mice. WT mice were subjected to cardiotoxin muscle injury and treated with intraperitoneal injection of either HGF-blocking antibody or control IgG daily, and muscles collected at 5 days after injury. (Top) Muscle homogenates subjected to c-met immunoprecipitation and then Western blotting for c-met or phosphotyrosine (pTyr). Note that treatment with HGF-blocking antibody reduced c-met phosphorylation. (Middle) Muscle cryosections stained with hematoxylin and eosin for morphologic analysis. Note the impairment of muscle regeneration in WT mice after treatment with HGF-blocking antibody. Scale bar represents 50 μm. (Bottom) Quantitative analysis of morphology. Regenerating fibers identified as central nucleated fibers and counted in 2 sections per muscle and expressed as number per millimeter muscle area. Damaged area estimated by subtracting summed area of normal and regenerating fibers from total muscle area. Bars represent mean ± SE; n = 4 to 6 per group. *Mean value significantly different from that for WT mice (P < .05).
Administration of HGF-blocking antibody to WT mice. WT mice were subjected to cardiotoxin muscle injury and treated with intraperitoneal injection of either HGF-blocking antibody or control IgG daily, and muscles collected at 5 days after injury. (Top) Muscle homogenates subjected to c-met immunoprecipitation and then Western blotting for c-met or phosphotyrosine (pTyr). Note that treatment with HGF-blocking antibody reduced c-met phosphorylation. (Middle) Muscle cryosections stained with hematoxylin and eosin for morphologic analysis. Note the impairment of muscle regeneration in WT mice after treatment with HGF-blocking antibody. Scale bar represents 50 μm. (Bottom) Quantitative analysis of morphology. Regenerating fibers identified as central nucleated fibers and counted in 2 sections per muscle and expressed as number per millimeter muscle area. Damaged area estimated by subtracting summed area of normal and regenerating fibers from total muscle area. Bars represent mean ± SE; n = 4 to 6 per group. *Mean value significantly different from that for WT mice (P < .05).
Cellular sources of uPA and HGF during muscle regeneration
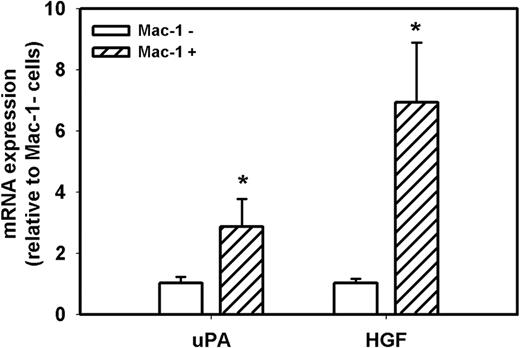
Because accumulation of macrophages in damaged muscle of uPA−/− mice is dramatically reduced compared with WT mice7,8,30 and macrophages can produce both uPA and HGF, we determined the level of expression of uPA and HGF in macrophages and nonmacrophage cells during muscle regeneration in WT mice. First, we isolated cells from muscle at day 5 after injury and separated the total population of cells present in the damaged muscle into Mac-1+ and Mac-1− cells; approximately 50% of these cells were Mac-1+. In a previous study, we verified the specificity of this cell sorting method, as Mac-1 mRNA was easily detected in Mac-1+ cells, but was not detectable in Mac-1− cells.34 In the present study, real-time PCR demonstrated that both Mac-1+ and Mac− cell populations expressed both uPA and HGF after muscle injury, but macrophages expressed 3- and 7-fold higher levels, respectively, than nonmacrophage cells (Figure 7). Thus, macrophages appear to be a primary source of these critical mediators of muscle regeneration after injury.
uPA and HGF expression in cells isolated from damaged muscle of WT mice. Cells were isolated from damaged muscle of WT mice and sorted into Mac-1+ and Mac-1− cell populations using a magnetic sorting method. Real-time PCR indicated that Mac-1+ cells expressed higher levels of uPA and HGF than Mac-1− cells. Bars represent mean ± SE; n = 3 per group. *Mean value significantly different from that for Mac-1− cells (P < .05).
uPA and HGF expression in cells isolated from damaged muscle of WT mice. Cells were isolated from damaged muscle of WT mice and sorted into Mac-1+ and Mac-1− cell populations using a magnetic sorting method. Real-time PCR indicated that Mac-1+ cells expressed higher levels of uPA and HGF than Mac-1− cells. Bars represent mean ± SE; n = 3 per group. *Mean value significantly different from that for Mac-1− cells (P < .05).
uPA and myoblast proliferation in vitro
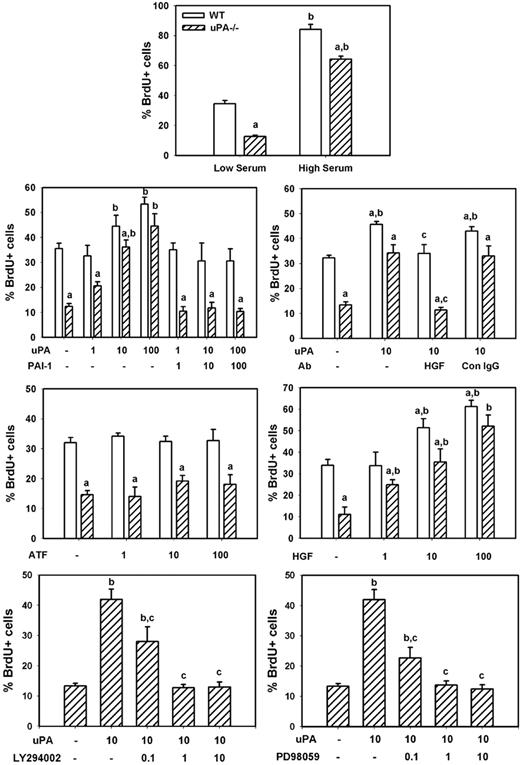
To determine whether uPA induces myoblast proliferation in vitro, we compared BrdU incorporation in isolated cells from uPA−/− and WT animals. We found that uPA−/− myoblasts exhibited an approximate 65% reduction in proliferation compared with WT cells when cultured in low serum medium, and a lesser but still significant impairment (∼ 25% reduction) when cultured in high serum medium (Figure 8). Subsequent experiments demonstrated that exogenously administered recombinant mouse uPA produced a dose-dependent increase in proliferation of both WT and uPA−/− myoblasts (Figure 8). This exogenous uPA had a greater stimulatory effect on uPA−/− than WT myoblasts, such that there was no difference in proliferation at the highest administered concentration of uPA (100 ng/mL). On the other hand, exogenously administered recombinant PAI-1, the proteolytic inhibitor of uPA, blocked the increase in proliferation induced by uPA, when added at the same concentration as uPA. Finally, the amino-terminal fragment of uPA, which lacks the proteolytic domain but retains the receptor-binding domains, did not stimulate proliferation when added to the cultures of either WT or uPA−/− myoblasts (Figure 8). These data indicate that the proteolytic activity of uPA stimulates myoblast proliferation.
uPA and myoblast proliferation in vitro. Myoblasts were isolated from neonatal WT and uPA−/− mice and cultured for proliferation experiments as described in “Myoblast cultures.” Cells were incubated in low serum medium without other factors overnight, and then incubated in experimental medium with designated factors added along with 100μM BrdU for 24 hours for assessment of cell proliferation. The number of BrdU+ cells was counted in 4 fields observed at 20× and normalized to the total number of cells present in each field. Bars represent mean ± SE; n = 6 to 12 per condition. aMean value for uPA−/− cells significantly smaller than that for WT cells (P < .05). bMean value for specific experimental condition significantly larger than that for control condition (low serum or no factors added; P < .05). cMean value for specific experimental condition significantly smaller than that for uPA treated condition (P < .05).
uPA and myoblast proliferation in vitro. Myoblasts were isolated from neonatal WT and uPA−/− mice and cultured for proliferation experiments as described in “Myoblast cultures.” Cells were incubated in low serum medium without other factors overnight, and then incubated in experimental medium with designated factors added along with 100μM BrdU for 24 hours for assessment of cell proliferation. The number of BrdU+ cells was counted in 4 fields observed at 20× and normalized to the total number of cells present in each field. Bars represent mean ± SE; n = 6 to 12 per condition. aMean value for uPA−/− cells significantly smaller than that for WT cells (P < .05). bMean value for specific experimental condition significantly larger than that for control condition (low serum or no factors added; P < .05). cMean value for specific experimental condition significantly smaller than that for uPA treated condition (P < .05).
To assess whether uPA-induced myoblast proliferation occurred as the result of HGF activation, we cultured myoblasts in the presence of both recombinant uPA and an HGF-blocking antibody. Again, uPA increased proliferation of both WT and uPA−/− myoblasts (Figure 8). The HGF-blocking antibody blocked the uPA-induced increase in proliferation, whereas control IgG had no effect. Furthermore, we cultured myoblasts with recombinant HGF and found a dose-dependent increase in proliferation of both WT and uPA−/− cells. Finally, we tested whether inhibiting HGF downstream signaling molecules PI3K or MEK1 reduced uPA-induced myoblast proliferation. Both the PI3K inhibitor LY294002 and the MEK1 inhibitor PD98059 produced a dose-dependent reduction in proliferation (Figure 8). Together, these data indicate that uPA activation of HGF promotes myoblast proliferation probably through PI3K and MEK1.
Discussion
The plasminogen system is required for efficient muscle regeneration, as mice deficient in either uPA or plasminogen exhibit markedly impaired regeneration.7-9 Existing evidence suggests that the plasminogen system regulates muscle regeneration in part through facilitation of fibrin removal from injured tissue.8 Whether other functions of uPA contribute to its profound effect on muscle regeneration remains unknown. Our findings elucidate a critical pathway by which uPA regulates muscle regeneration after injury. First, we demonstrated that uPA up-regulates HGF activity after muscle injury. Levels of active HGF and phosphorylation of the HGF receptor c-met were reduced in damaged muscles of uPA−/− mice compared with WT animals. In contrast, HGF and c-met activity were enhanced at early time points in mice deficient in the primary inhibitor of uPA, PAI-1. The effect of uPA on HGF activity was further established by treating injured uPA−/− animals with exogenous uPA protein. The resulting data show that such treatment restored both total and active HGF levels in damaged muscle. Second, our data demonstrate that HGF activity is required for efficient regeneration by stimulating myoblast activity. In each of our experimental models, we observed that increased levels of active HGF were positively associated with cell proliferation, myoblast accumulation, and new muscle fiber formation. Furthermore, an HGF-blocking antibody reduced formation of new muscle fibers in WT mice. Finally, in vitro experiments showed that uPA promotes myoblast proliferation through its proteolytic activity and that an HGF-blocking antibody inhibited this proliferative response.
The correlation of uPA activity with HGF levels after muscle injury was previously explored by Suelves et al.35 In contrast to our results, the previous study found that levels of active HGF were not different in injured muscles of WT and uPA−/− mice. Reasons for this discrepancy may have resulted from differences in the time points of analysis and/or the methods used for processing muscles for Western blots. On the other hand, our findings are consistent with data from a study on liver regeneration after massive hepatic apoptosis.5 In this liver study, mice deficient in uPA showed delayed production of active HGF and impaired liver regeneration compared with WT mice. In addition, PAI-1–deficient mice showed accelerated production of active HGF and liver regeneration. Furthermore, in vivo transfection of uPA into the liver of uPA−/− mice rescued production of active HGF and c-met activation and restored liver repair. Our findings are also consistent with in vitro findings demonstrating that uPA promotes HGF activation in fibroblast cultures.12,36 In these studies, uPA activated HGF both directly and indirectly, the latter via activation of plasminogen to plasmin.
The total levels of HGF were reduced at early time points in damaged muscle of uPA−/− mice compared with WT mice (Figure 1), which correlates with reduced accumulation of both macrophages7,8 and myoblasts (Figure 4) in damaged muscle of uPA−/− mice compared with WT mice. Because both macrophages and myoblasts are known to produce HGF in vitro,28,37 and our data show that both macrophages and nonmacrophage cells express HGF during muscle regeneration in vivo, with macrophages having 7-fold higher expression (Figure 7), the lower numbers of macrophages and myoblasts probably explain the lower levels of total HGF observed in damaged muscle of uPA−/− mice compared with WT mice. Another possibility is that uPA may directly influence HGF expression in any of these cell types during muscle repair.
In contrast to our findings that uPA promotes myoblast proliferation, Suelves et al found that uPA did not stimulate myoblast proliferation in vitro.35 One explanation for the differences in results is that Suelves et al35 performed their proliferation experiments in high serum medium (20% FBS), whereas most of our experiments were performed in low serum medium (0.5% FBS). Our data indicate a greater impairment in proliferation of uPA−/− cells in low serum medium than in high serum medium (eg, Figure 7), and a greater stimulatory effect of exogenous uPA in low serum compared with high serum medium (data not shown). Because HGF activator protein is present in serum,38 uPA may not be required for proteolytic activation of HGF in high serum medium. Our data are consistent with prior reports in which uPA was shown to stimulate proliferation of human myoblasts in a dose-dependent manner.26,39 Furthermore, a blocking antibody against uPA inhibited basic fibroblast growth factor–induced proliferation, indicating that uPA may mediate proliferation induced by fibroblast growth factor.26,39 Studies on vascular smooth muscle cells produced similar findings that uPA induces proliferation of these cells and may mediate growth factor–induced proliferation.40-42
Our data demonstrated that (1) intact uPA but not the amino-terminal fragment, which lacks the proteolytic domain, stimulated myoblast proliferation; (2) the specific proteolytic inhibitor PAI-1 blocked uPA-mediated myoblast proliferation; (3) the HGF-blocking antibody reduced uPA stimulation of myoblast proliferation; and (4) both the PI3K inhibitor LY and the MEK1 inhibitor PD98059 reduced uPA-mediated myoblast proliferation. Taken together, these data indicate that uPA stimulates myoblast proliferation through proteolytic activation of HGF and via signaling through PI3K and MEK1. These data are consistent with those of De Petro et al, who reported that uPA promotes proliferation of skin fibroblasts through a mechanism that required proteolytic activ-ity.43 These findings are also consistent with those of Tanski et al, who showed that proteolytic activity of uPA was required for stimulation of proliferation of smooth muscle cells, and that this occurred through an MEK1-dependent mechanism.42 HGF has been shown to induce phosphorylation of ERK1/2 in a PI3K-dependent manner in myogenic cells,44 which is consistent with the idea that uPA activates PI3K and ERK1/2 by activating HGF.
HGF and its receptor, c-met, are required for migration of myogenic cells into the limb buds and the development of limb skeletal muscle.21 However, the functions of HGF and c-met in adult muscle have been less clearly defined. HGF was found to be present in uninjured adult skeletal muscle and is a component of crushed muscle extract that activates satellite cells.23 In adult muscle as well as lung, liver, kidney, and spleen, HGF was found primarily in its inactive single-chain form, with the level of expression and activation increased after tissue injury.22,45 In contrast, other studies have reported that HGF is found primarily in its active α-chain form in uninjured skeletal muscle.46,47 The contrasting findings may be explained by differences in sample preparation. For studies in which single-chain HGF was observed, tissue samples were homogenized and HGF levels assayed by Western blotting. In studies reporting primarily α-chain HGF, muscles were incubated in buffer and HGF released into the buffer was assayed. Thus, freely soluble extracellular HGF may be primarily in the α-chain form, whereas HGF localized in the extracellular matrix and intracellularly may be primarily in the single-chain form. This idea is supported by findings that HGF recovered in lavage fluid from the lung is found only in the α-chain form.16 In our experiments, we assayed HGF in muscle homogenates and found HGF primarily in its single-chain form in uninjured muscle. Interestingly, the molecular size of this single-chain HGF appeared to be smaller in uninjured control muscle compared with damaged muscle and showed evidence of returning to this smaller size at 10 days after injury. The reason for this difference remains to be determined, but we speculate that it may be related to differential posttranslational modification of HGF in noninjured and injured muscle. Others have also observed different sizes of the single-chain HGF in lung, kidney, and spleen tissues.45
We and others have demonstrated that macrophage accumulation after cardiotoxin injury is almost completely abrogated in uPA−/− mice, associated with persistence of necrotic debris and the lack of muscle fiber regeneration.7,8 In addition, we recently showed that transferring WT bone marrow cells to uPA−/− mice rescues macrophage accumulation and muscle regeneration after injury, suggesting that impaired macrophage function in the absence of uPA plays an important role in the deficient healing.30 However, the mechanisms by which macrophages promote muscle fiber regeneration remain to be established. One possible mechanism is the production of uPA and/or HGF by macrophages that have accumulated in damaged muscle. Macrophages are known to express both uPA and HGF,37,48,49 both of which would promote satellite cell proliferation and migration required for muscle regeneration. We are currently investigating in further detail the mechanisms by which macrophages promote muscle regeneration and whether their effect on repair may be through the delivery of these key mediators to the site of damage.
In conclusion, we have shown that uPA activation of HGF plays an important role in stimulating the myoblast proliferation required for efficient muscle regeneration. These data extend previous findings of the critical role of the plasminogen system in muscle regeneration and provide a molecular mechanism by which uPA promotes regeneration. These data also indicate that the plasminogen system may provide an appealing target for interventions to improve muscle regeneration in muscle diseases that involve progressive degeneration (eg, muscular dystrophy) and other conditions in which muscle regeneration is impaired (eg, aging).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Department of Defense (USAMRMC #W81XWH-05-1-0159; T.J.K.) and the National Institutes of Health (R01 HL078871; T.H.S.).
National Institutes of Health
Authorship
Contribution: T.H.S. and T.J.K. designed the research, analyzed data, and wrote the paper; M.-H.N. performed the research, analyzed data, and wrote the paper; B.Y. and M.L.N. performed the research and analyzed data; and R.H.S. contributed vital tools, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Timothy J. Koh, Department of Movement Sciences, University of Illinois at Chicago, 1919 W Taylor, Rm 529 m/c 994, Chicago, IL 60612; e-mail: tjkoh@uic.edu.
References
Author notes
T.H.S. and M.-H.N. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal