To the editor:
Sepsis is one of the leading morbidity and mortality factors in newborns, occurring in more than 700 of every 100 000 live births.1 Newborns seem to have a unique susceptibility to early bacterial infections2 compared with adults, but the underlying pathomechanisms are still poorly defined. Neutrophils represent the first and most powerful cellular line of antibacterial host defense, as they are able to kill most bacteria within a few hours.3 These innate immune cells engulf and destroy pathogens intracellularly, a phenomenon known for more than 100 years as phagocytosis.4 In 2004, Brinkmann et al described for the first time a mechanism of how neutrophils kill bacteria extracellularly: neutrophil extracellular trap (NET) formation or NETosis.5,6 Upon stimulation, neutrophils undergo cytoplasmic and nuclear changes, the intracellular architecture is lost, and chromatin fibers are expelled that contain DNA, histones, and granular proteins to form NETs, a machinery used to trap and destroy bacteria surrounding the dying neutrophil.7
Recently, Yost and coworkers reported that neonatal neutrophils are impaired in NET formation, which may explain why newborns are prone to bacterial infections.8 Here, we present experimental data that complement and extend these findings and add to our understanding of how NETosis is regulated in newborns. Yost et al stimulated neonatal (cord blood–derived) and adult neutrophils for 1 hour with lipopolysaccharide (LPS) or platelet-activating factor (PAF) and analyzed NETosis. Their studies demonstrated an inability of neonatal neutrophils to form NETs at this early time point. We stimulated, in a similar manner, isolated neonatal and adult neutrophils with LPS, but (1) we extended the stimulation period to a maximum of 3 hours, and (2) we compared the Toll-like receptor 4 (TLR4) ligand LPS with an array of Gram-negative and Gram-positive TLR ligands. The ethical committee and the institutional review board of the Ludwig-Maximilians-Universität Munich have approved our study, which was conducted in accordance with the Declaration of Helsinki.
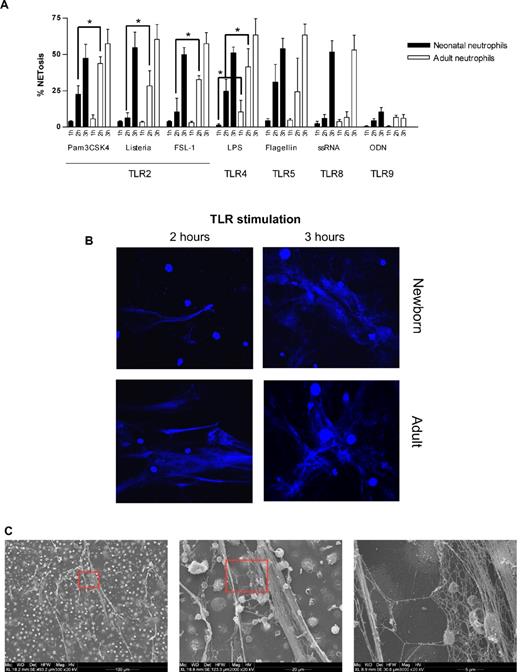
In line with Yost et al, we found that, upon stimulation with LPS for 1 hour, neonatal neutrophils showed no signs of NETosis compared with a moderate NET formation by adult neutrophils. However, around 2 hours of LPS stimulation, neonatal neutrophils started to form NETs and at 3 hours, neonatal neutrophils were almost equally potent in NET generation compared with adult neutrophils (Figure 1A-B).
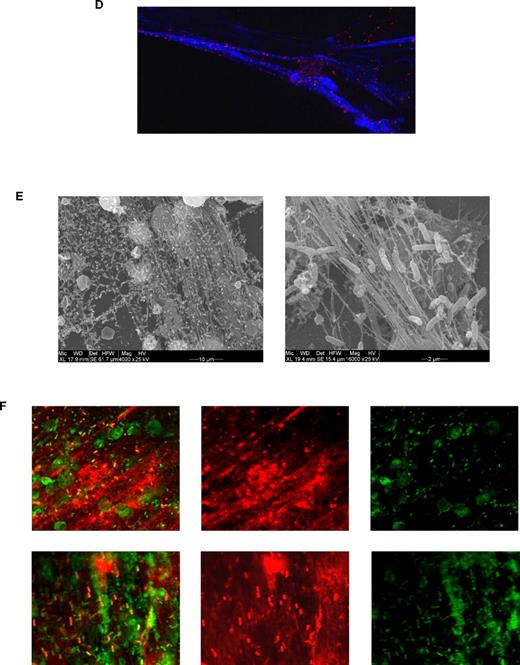
Neonatal NET formation. (A) Kinetics of NET formation by neonatal and adult neutrophils. Neutrophils were isolated from cord blood (n = 3) or peripheral adult blood (n = 3) by Percoll gradient centrifugation and erythrocyte lysis. Isolated neutrophils were stimulated with Pam3CSK4 (1 μg/mL), heat-killed Listeria monocytogenes (HKLM, 108/mL), FSL-1 (1 μg/mL), LPS (1 μg/mL), flagellin (1 μg/mL), ssRNA40 (1 μg/mL) or ODN2006 (2.5μM) for 1, 2, or 3 hours at 37°C. Afterward, NETosis was analyzed by DAPI (DNA staining) and citrullinated histone 3 staining and confocal microscopy. The percentage of NET-producing neutrophils was calculated from all neutrophils analyzed. *P < .05, neonatal versus adult NETosis. (B) Confocal laser scanning microscopy images of TLR-induced NETosis. Neutrophils were stimulated for 2 or 3 hours at 37°C with LPS (1 μg/mL). Blue channel indicates DNA (stained with DAPI) in stimulated neutrophils isolated from newborn and adult blood. (C) Scanning electron microscopy of neonatal NETosis. NETosis in neonatal neutrophils after LPS stimulation (1 μg/mL, 3 hours, 37°C) at 3 different magnifications. (D) Confocal laser scanning microscopy of histone citrullination. Neonatal neutrophils were stimulated with 1 μg/mL LPS for 3 hours at 37°C. Blue channel indicates DAPI; red channel, citrullinated histone 3; and green channel, F-actin. (E) Scanning electron microscopy of bacteria-induced NETosis. NET formation by neonatal neutrophils after coincubation with nonmucoid Pseudomonas aeruginosa (PAO.1) for 3 hours at 37°C. (F) Confocal light scanning microscopy of bacteria-induced NETosis. Neonatal neutrophils coincubated with nonmucoid P aeruginosa (PAO.1) for 3 hours at 37°C. Live/dead staining (BacLight bacterial viability kit, Invitrogen). Red channel indicates propidium iodide staining of extracellular DNA (NETs) and dead bacteria; green channel, Syto-9 stains live bacteria and intracellular DNA in the nuclei of living neutrophils; and yellow-red, overlay (dead bacteria). (B,D,F) Images were captured using an Olympus IX81 microscope (Fluoview 1000) and an Olympus XC30 camera, with Olympus Fluoview Software FV10-ASW 0.200, a 60×/1.35 oil objective, and Vectashield Mounting Medium. (C,E) Images were captured using an ESEM XL30 microscope (FEI, Philips; 20 kV).
Neonatal NET formation. (A) Kinetics of NET formation by neonatal and adult neutrophils. Neutrophils were isolated from cord blood (n = 3) or peripheral adult blood (n = 3) by Percoll gradient centrifugation and erythrocyte lysis. Isolated neutrophils were stimulated with Pam3CSK4 (1 μg/mL), heat-killed Listeria monocytogenes (HKLM, 108/mL), FSL-1 (1 μg/mL), LPS (1 μg/mL), flagellin (1 μg/mL), ssRNA40 (1 μg/mL) or ODN2006 (2.5μM) for 1, 2, or 3 hours at 37°C. Afterward, NETosis was analyzed by DAPI (DNA staining) and citrullinated histone 3 staining and confocal microscopy. The percentage of NET-producing neutrophils was calculated from all neutrophils analyzed. *P < .05, neonatal versus adult NETosis. (B) Confocal laser scanning microscopy images of TLR-induced NETosis. Neutrophils were stimulated for 2 or 3 hours at 37°C with LPS (1 μg/mL). Blue channel indicates DNA (stained with DAPI) in stimulated neutrophils isolated from newborn and adult blood. (C) Scanning electron microscopy of neonatal NETosis. NETosis in neonatal neutrophils after LPS stimulation (1 μg/mL, 3 hours, 37°C) at 3 different magnifications. (D) Confocal laser scanning microscopy of histone citrullination. Neonatal neutrophils were stimulated with 1 μg/mL LPS for 3 hours at 37°C. Blue channel indicates DAPI; red channel, citrullinated histone 3; and green channel, F-actin. (E) Scanning electron microscopy of bacteria-induced NETosis. NET formation by neonatal neutrophils after coincubation with nonmucoid Pseudomonas aeruginosa (PAO.1) for 3 hours at 37°C. (F) Confocal light scanning microscopy of bacteria-induced NETosis. Neonatal neutrophils coincubated with nonmucoid P aeruginosa (PAO.1) for 3 hours at 37°C. Live/dead staining (BacLight bacterial viability kit, Invitrogen). Red channel indicates propidium iodide staining of extracellular DNA (NETs) and dead bacteria; green channel, Syto-9 stains live bacteria and intracellular DNA in the nuclei of living neutrophils; and yellow-red, overlay (dead bacteria). (B,D,F) Images were captured using an Olympus IX81 microscope (Fluoview 1000) and an Olympus XC30 camera, with Olympus Fluoview Software FV10-ASW 0.200, a 60×/1.35 oil objective, and Vectashield Mounting Medium. (C,E) Images were captured using an ESEM XL30 microscope (FEI, Philips; 20 kV).
Neonatal neutrophils showed a similar delayed NETosis in response to synthetic or natural TLR2 agonists, whereas no delay was found upon TLR5, TLR8, or TLR9 activation. The identity of NETs was confirmed ultrastructurally (Figure 1C) and by immunostaining for citrullinated histone 3 (Figure 1D). Next, we challenged neonatal neutrophils with live bacteria, which elicited robust NET formation (Figure 1E). These NETs were functionally competent; they were capable of entangling and killing bacteria (Figure 1F). Consistent with TLR ligand stimulation, live bacteria-induced NETosis was also delayed in neonatal neutrophils when monitored over 3 hours (data not shown).
When viewed in combination, these findings demonstrate that neonatal neutrophils exhibit an intrinsic delay in TLR2/TLR4-mediated NET formation, but are capable of releasing functionally competent NETs. The underlying cellular mechanisms and the clinical implications of neonatal NETosis delay remain to be addressed in future studies.
Authorship
Contribution: V.M., L.V., A.H., and W.D.K. performed research and analyzed data; C.N. provided cord blood and designed research; E.V.M. performed research; D.R. and T.K. analyzed the data and wrote the paper; O.G.-B. provided cord blood; M.S. provided cord blood and wrote the paper; and D.H. designed and supervised research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dominik Hartl, Lindwurmstr 2a, 80337 Munich, Germany; e-mail: dominik.hartl@med.uni-muenchen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal