Abstract
In this issue of Blood, Yost and colleagues demonstrate that neutrophils from newborn infants lack the ability to produce so-called extracellular traps and exhibit impaired extracellular killing of bacteria.
Newborn babies present with various immaturities of their humoral and cellular immune system, particularly during the first days of life. This has important and deleterious consequences. Bacterial infection is a major cause of death and long-term morbidity in preterm newborn infants.1 Therefore, despite the origin of the word (from innatus, existing at the time of birth), the innate immune responses against microbial invasion are apparently impaired in neonates. Defective antibacterial function of neutrophils of neonates has thus been noted for some time, and circumstantial evidence for an immaturity of granulopoiesis—which might explain the frequent development of neutropenia in neonates in response to bacterial sepsis—has also been documented.1 However, the syndrome of neonatal neutrophil deficiency remains incompletely understood.
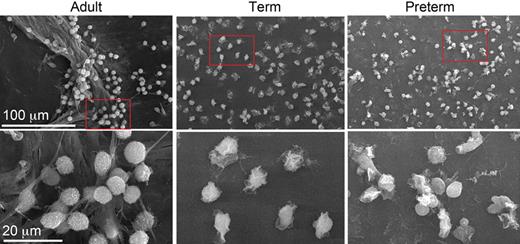
No NET formation in neutrophils from newborn babies. Yost et al examine NET formation by scanning electron microscopy following stimulation of isolated neutrophils with bacterial lipopolysaccharide (LPS). LPS-stimulated neutrophils from an adult donor were found to generate extensive extracellular lattices (left panels). In contrast, NET formation could not be detected when control or LPS-stimulated neonatal neutrophils were examined (middle panels: term; right panels: preterm). The net result of this novel defect was shown to be impaired extracellular killing of bacteria. See the complete figure in the article beginning on page 6419.
No NET formation in neutrophils from newborn babies. Yost et al examine NET formation by scanning electron microscopy following stimulation of isolated neutrophils with bacterial lipopolysaccharide (LPS). LPS-stimulated neutrophils from an adult donor were found to generate extensive extracellular lattices (left panels). In contrast, NET formation could not be detected when control or LPS-stimulated neonatal neutrophils were examined (middle panels: term; right panels: preterm). The net result of this novel defect was shown to be impaired extracellular killing of bacteria. See the complete figure in the article beginning on page 6419.
Neutrophils are essential effector cells of the innate immune system and play a crucial role in the killing of microorganisms. Neutrophils kill pathogens through reactive oxygen species (ROS)–dependent and –independent mechanisms. The former pathway is associated with activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase at the phagosomal membrane. Patients suffering from chronic granulomatous disease (CGD) harbor mutations in genes encoding subunits of NADPH oxidase and are at risk for life-threatening bacterial and fungal infections, thus underscoring the importance of ROS-dependent antimicrobial defenses. The ROS-independent mechanisms include the release of cationic peptides that disrupt the pathogen membrane, and an assortment of proteases that contribute to the degradation of invading microorganisms.
Stimulated neutrophils also produce extracellular structures called neutrophil extracellular traps (NETs) that capture and kill microorganisms.2 NETs consist of an extracellular web of fibers composed of DNA, histones, and granule proteins such as elastase. It was suggested that NETs may enhance the efficiency of bacterial killing by providing a high local concentration of antimicrobial molecules. Moreover, these extracellular structures are apparently active even when the neutrophil itself has died,3 thus serving to prolong the tour of duty for these cells. In a series of elegant experiments in this issue of Blood, Yost et al now reveal a novel innate immune deficiency of neonates: impaired NET formation.4 Neutrophils from term and preterm neonates thus fail to form NETs upon stimulation ex vivo with inflammatory agonists (see figure). Importantly, the authors also show that neutrophils isolated from term infants were defective for extracellular killing of bacteria.
Previous studies have indicated that NET formation is dependent on the generation of ROS by NADPH oxidase.3 Neutrophils derived from CGD patients thus failed to produce NETs when stimulated with Staphylococcus aureus or PMA, a potent activator of the oxidative burst.5 In contrast, Yost et al report that generation of ROS alone is not sufficient to induce NET formation in neonatal neutrophils. Indeed, they show that the impaired NET formation in neutrophils from newborn babies is not due to a deficiency of NADPH oxidase activation in those cells.4 Thus, the signaling events that govern the formation of NETs remain to be elucidated. However, the discovery that neonatal neutrophils lack the ability to cast NETs suggests a potentially useful model system to further dissect the underlying signaling cascades.
The prevailing view is that neutrophils undergoing apoptosis are recognized and removed by neighboring macrophages before their disintegration, and it is thought that this process also contributes to the resolution of inflammation.5 In this context, the discovery that activated neutrophils may burst and extrude extracellular lattices of chromatin and other intracellular constituents begs this question: How are NETs dismantled after an infection has been cleared? A related question is, of course, whether the prolonged presence of NETs in the extracellular milieu may induce autoimmune responses. It is not inconceivable that inadvertent immune responses to vast amounts of DNA and associated histones may occur.
Finally, an important question is whether NET formation could also contribute to tissue injury during severe infection. A recent report showed that platelets can activate neutrophils to produce NETs, which retain their integrity under flow conditions and are able to ensnare bacteria in the vasculature.6 In fact, these events occurred primarily in the narrowest of vessels, the sinusoidal capillaries where NETs would have the greatest capacity for bacterial trapping. However, this antimicrobial mechanism occurred at the expense of tissue injury to endothelium and liver tissue in a mouse model of endotoxemia.
Finding new strategies to enhance the neonatal immune defense against infection is an important goal. The novel observation that NET formation is reduced in neutrophils obtained from neonates draws our attention to the importance of extracellular mechanisms of antimicrobial defense, and could also inspire new therapeutic approaches.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal