Abstract
Neutrophils are highly specialized innate effector cells that have evolved for killing of pathogens. Human neonates have a common multifactorial syndrome of neutrophil dysfunction that is incompletely characterized and contributes to sepsis and other severe infectious complications. We identified a novel defect in the antibacterial defenses of neonates: inability to form neutrophil extracellular traps (NETs). NETs are lattices of extracellular DNA, chromatin, and antibacterial proteins that mediate extracellular killing of microorganisms and are thought to form via a unique death pathway signaled by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase–generated reactive oxygen species (ROS). We found that neutrophils from term and preterm infants fail to form NETs when activated by inflammatory agonists—in contrast to leukocytes from healthy adults. The deficiency in NET formation is paralleled by a previously unrecognized deficit in extracellular bacterial killing. Generation of ROSs did not complement the defect in NET formation by neonatal neutrophils, as it did in adult cells with inactivated NADPH oxidase, demonstrating that ROSs are necessary but not sufficient signaling intermediaries and identifying a deficiency in linked or downstream pathways in neonatal leukocytes. Impaired NET formation may be a critical facet of a common developmental immunodeficiency that predisposes newborn infants to infection.
Introduction
Polymorphonuclear leukocytes (PMNs, neutrophils) are highly specialized cellular effectors in host defense and immune surveillance. Mature human PMNs from healthy adults have a unique repertoire of activities, including phagocytosis, degranulation of antimicrobial enzymes and peptides, and generation of oxygen radicals with antimicrobial properties.1-6 Synthesis of inflammatory and regulatory lipids and proteins complements these innate mechanisms.1,4,5 PMNs have evolved for capture, containment, and destruction of bacteria and fungi and also have activity against intracellular pathogens and viruses.2,3 PMNs have additional important roles in tissue repair and integration of innate and adaptive immune responses.6 If, however, these specialized defensive mechanisms become dysregulated or unregulated, PMNs can paradoxically be mediators of inflammatory tissue injury.1,6
Consistent with their requisite activities in host defense, defects in PMN functions cause immune deficiency syndromes.2,7 Neutrophil defects can be hereditary, developmental, or acquired in nature. Specific genetic deficiencies in PMN function cause significant morbidity in subsets of children and adults and, in parallel, provide unique insights into molecular mechanisms that regulate leukocyte activities.7,8 Nevertheless, these disorders are rare and arcane. In contrast, the developmental syndrome of neonatal neutrophil dysfunction, which is particularly important in premature infants, is common and contributes to infections in infants worldwide. As an example, neonatal PMN dysfunction is thought to be a pivotal feature of sepsis in the newborn.9-11 The incidence of neonatal sepsis is estimated to be 1 to 5 cases per 1000 live births in the United States and to be even higher after very low birth weight premature deliveries (15-19/1000); in contrast, the incidence of sepsis is much lower in children older than 1 year of age and in young adults.12-15 Furthermore, the incidence of neonatal sepsis is as high as 25% in some areas of the developing world.16,17 Thus, neonatal PMN dysfunction is a contributor to a public health problem of significant proportions, and also may precipitate other severe infectious syndromes.10,11
Neonatal PMN dysfunction is multifactorial, in part depending on the degree of prematurity, and is only partially characterized.10,11,18 There is no established surrogate model of neonatal neutrophil dysfunction, although altered PMN responses to noninfectious stimuli have been reported in neonatal mice.19 PMNs isolated from premature human neonates have impaired phagocytosis, decreased capacity to generate oxygen radicals, and deficient intracellular bacterial killing compared with neutrophils from healthy adults when studied in the apparent absence of physiologic stress or infectious challenge to the infant.11,18,20 Whereas neutrophils from full-term infants have normal phagocytosis, oxidant generation, and intracellular bacterial killing when examined under basal conditions, antibacterial functions are dramatically impaired when PMNs isolated from babies with suspected sepsis or respiratory distress are studied.21 Thus, defects in antimicrobial capacity are basally present in neutrophils from premature neonates and are detected in PMNs from term neonates when the infants are infected or subjected to physiologic stress or inflammatory challenge. Additional defects in adhesion and expression of leukocyte integrins, again in part related to the degree of maturity of the infant,22-25 and developmentally regulated deficiency in selectin display by endothelial cells26 compound deficiencies in neonatal PMN antimicrobial defenses.
Recently a newly recognized activity of PMNs from adult subjects was reported in which the leukocytes generate extracellular traps, or “NETs,” for bacteria.27 NETs are lattices of DNA, histones, granule enzymes, and antimicrobial proteins that are released by the PMNs in parallel with extrusion of nuclear material.27 These DNA complexes accomplish both capture and extracellular killing of bacteria27 and fungi,28 antimicrobial activities that can be blunted or subverted by endonucleases and DNases expressed by some microorganisms.29,30 NETs are also implicated in vascular injury associated with platelet-neutrophil interactions in human and experimental sepsis.31 Although the exact molecular mechanisms contributing to NET formation remain incompletely determined, generation of reactive oxygen species (ROSs) via NADPH oxidase is reported to trigger a unique cell death pathway distinct from both apoptosis and necrosis; in PMNs from adults, this results in intracellular mixing of PMN chromatin and granular antibacterial proteins followed by rupture of the leukocyte plasma membrane with subsequent extracellular NET formation.32 The ability of stimulated adult PMNs to form NETs appears to depend on the degree of cellular differentiation.33
Because neonatal neutrophils have defective antibacterial functions, we explored the possibility that NET formation is altered, or deficient, in neutrophils from infants. We found that stimulated PMNs isolated from the blood of term and preterm babies fail to form NETs when examined in parallel with PMNs from healthy adults and that this is associated with impaired extracellular bacterial killing. Complementation with exogenous ROSs fails to rescue this deficiency, indicating that oxygen radical generation is not sufficient to mediate NET formation by human PMNs and that additional molecular pathways or mechanisms are deficient in neonatal neutrophils. Failure of NET formation and consequent impaired extracellular bacterial killing are previously unrecognized neonatal neutrophil deficiencies that may contribute to the pathogenesis of severe infections early in life.
Methods
Reagents
Lipopolysaccharide (Escherichia coli serotype 0111:134), poly-l-lysine, cytochalasin B, cytochalasin D, N-(methoxysuccinyl)-Ala-Ala-Pro-Val 4 nitroanilide, glutaraldehyde, paraformaldehyde, glucose oxidase, nitroblue tetrazolium, phorbol myristate acetate, fibrinogen, and sodium cacodylate were from Sigma-Aldrich (St Louis, MO). Additional reagents were as follows: TOPRO-3, wheat germ agglutinin (WGA), DNA standard (Invitrogen, Carlsbad, CA); Fluo-4, λ DNA standard, fluorescein isothiocyanate (FITC)–labeled LPS, Syto Green, and Sytox Orange (Molecular Probes, Carlsbad, CA); diphenylene iodonium (DPI; Calbiochem, San Diego, CA); DNase (Promega, Madison, WI); Anti-CD15+–impregnated microbeads (Miltenyi Biotec, Auburn, CA); medium 199 (Lonza Biologics, Basel, Switzerland); osmium tetroxide (Stevens Metallurgical, New York, NY); hexamethyldisalizane (Ted Pella, Redding, CA); and platelet activating factor (PAF; Avanti Polar Lipids, Alabaster, AL).
PMN isolation
PMNs were isolated from anticoagulated (ACD or EDTA) umbilical cord blood from preterm or healthy term infants and from venous blood of healthy adults under protocols approved by the University of Utah Institutional Review Board and informed consent was obtained in accordance with the Declaration of Helsinki. Clinical characteristics and causes of delivery of preterm infants are summarized in Table S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article). PMN suspensions (> 95% pure) were prepared by immunoselection using anti–CD15-coated microbeads and an auto-MACS sorter (Miltenyi Biotec) and were resuspended at indicated concentrations in M-199 (37°C).
Signaling pathway analysis
Expression of messenger RNAs (mRNA) coding for TLR4 and the PAF receptor (PAF-R) was determined using reverse-transcription–polymerase chain reaction (RT-PCR) and the following primer sets: TLR4 sense 5′-AACCTGGACCTGAGCTTTAATCC-3′, antisense 5′-AGCCACCAGCTTCTGTAAACTTG-3′; PAF-R sense 5′-GGCTGGGGCCAGGACCCAGA-3′, antisense 5′-CTGCCCTTCTCGTAATGCTC-3′. In functional studies, PMNs from each subject group were incubated with various concentrations of FITC-LPS at 37°C for 1 hour while adherent to poly-l-lysine (1 mg/mL) coated coverslips. They were then imaged by confocal microscopy with nuclear staining (Topro-3) and cytoplasmic counterstaining using WGA. To examine PMN responsiveness to PAF, intracellular calcium release assays were performed. PMNs were preincubated with Fluo-4 (1 ng/mL), a Ca++-sensitive fluorescent dye. One minute after stimulation with PAF (10−10-10−6 M) calcium transients were determined by fluorometry (Biotek Synergy HT, Winooski, VT) using filter settings of 405 excitation/528 emission and procedures similar to those in a previously described method.34 To examine the functionality of toll-like receptor 4 (TLR4) and PAF receptor signaling pathways, supernatants from activated PMNs were assayed for interleukin 8 (IL-8) following stimulation with PAF or LPS via enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).5
Live cell imaging
PMNs (2 × 106 cells/mL) from adults or neonates were incubated with control buffer or stimulated with LPS (0.1-100 ng/mL) or PAF (10−10-10−7 M) for 1 hour at 37°C in 5% CO2/95% air on glass coverslips coated with poly-l-lysine or fibrinogen. In selected studies, PMNs were treated with phorbol myristate acetate (PMA) or incubated with bacteria for live cell imaging and/or scanning electron microscopy. In other experiments, PMNs were incubated with glucose oxidase (200-1000 mU/mL) together with glucose (1 mM) to generate exogenous ROSs alone or following pretreatment with DPI (20 μM) for 30 minutes to inhibit endogenous ROS generation. After preincubation and/or stimulation, PMNs were gently washed with PBS and incubated with a mixture of cell-permeable (Syto Green) and impermeable (Sytox Orange) DNA fluorescent dyes. Confocal microscopy was accomplished using a FV300 1 × 81 microscope and FluoView software (Olympus, Center Valley, PA) maintained by the Fluorescent Microscopy Core Facility at the University of Utah. Both 20× and 60× objectives were used. Z-series images were obtained at a step size 0.5 μm over a range of 20 μm for each field. Adobe Photoshop CS2 was used for image processing (Adobe Systems, San Jose, CA).
Scanning electron microscopy
LPS- and PAF-stimulated PMNs were fixed with 2.5% glutaraldehyde and 1% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 24 hours. In some experiments PMNs were incubated with E coli or Staphylococcus aureus bacteria prior to fixation. The fixed cells were then rinsed with sodium cacodylate and postfixed with 1% osmium tetroxide and 1% tannic acid, dehydrated with a graded series of ethanol, and then dried in hexamethyldisalizane. The samples were subsequently mounted on colloidal graphite, sputter-coated, and visualized using a scanning electron microscope (S2460 N; Hitachi, San Jose, CA).
DNA quantification
LPS-stimulated PMNs (2 × 106/mL; 100 ng/mL) were allowed to settle on surfaces coated with poly-l-lysine in 24-well tissue culture plates for 2 hours at 37°C in 5% CO2/95% air. The resultant supernatants were gently removed from the wells and replaced with warmed M-199 media followed by continued incubation for 15 minutes with gentle agitation to break down and release NETs formed in response to LPS stimulation. Supernatant DNA concentrations were quantified by incubating the samples with Sytox Orange (1 μM) and comparing the values against a concentration curve constructed with various concentrations of a known DNA standard33 using fluorometry with filter settings of 530 (excitation)/645 (emission) (BioTek Synergy HT). Statistical comparisons were made via one-way ANOVA with posthoc pairwise multiple comparisons made via the Holm-Sidak method.
Quantification of NE activity
PMNs (4 × 106 cells /mL) in M-199 were incubated at 37°C in 5% CO2/95% air with control buffer, LPS (100 ng/mL), or PAF (10 nM) for 15 to 120 minutes in a 24-well poly-l-lysine–coated tissue culture plate. NET-associated neutrophil elastase (NE) activity was determined at selected time points as described.29 Statistical comparisons were made via one-way ANOVA with posthoc pairwise multiple comparisons made via the Holm-Sidak method.
Bacterial killing assay
Bacterial killing efficiency of PMNs freshly isolated from adult subjects and term infants using an assay modified from the literature29,32 was determined. PMNs were incubated for 30 minutes at 37°C in 5% CO2/95% air alone or with the phagocytosis inhibitors cytochalasins B and D (10 μm).27,30 Cytochalasin treatment inhibits total bacterial killing by inhibiting phagocytosis without blocking PMN degranulation.35 The leukocytes were then stimulated with LPS (100 ng/mL), placed in poly-l-lysine–coated wells of a 24-well tissue culture plate, and incubated at 37°C for 1 hour to induce cellular activation and formation of NETs. To inhibit NET-mediated bacterial killing, we incubated selected wells with DNase (40 U/mL) for 15 minutes prior to addition of bacteria. E coli (1 colony-forming unit [CFU]/100 PMNs) were then added to the PMNs, followed by continued incubation for 2 hours. The PMNs were then permeabilized with 0.1% Triton-X 100 for 10 minutes and each well was scraped to free all cells. Serial dilutions were performed and cultures were grown on 5% sheep blood agar plates (Hardy Diagnostics, Santa Maria, CA). After a 24-hour incubation, bacterial counts were determined. Total and fractional NET-mediated bacterial killing were determined as described.29,32 Statistical comparisons between adult and term infant PMN bacterial killing were made using the Student t test.
Results
PMNs isolated from newborn infants fail to form NETs following stimulation with inflammatory agonists
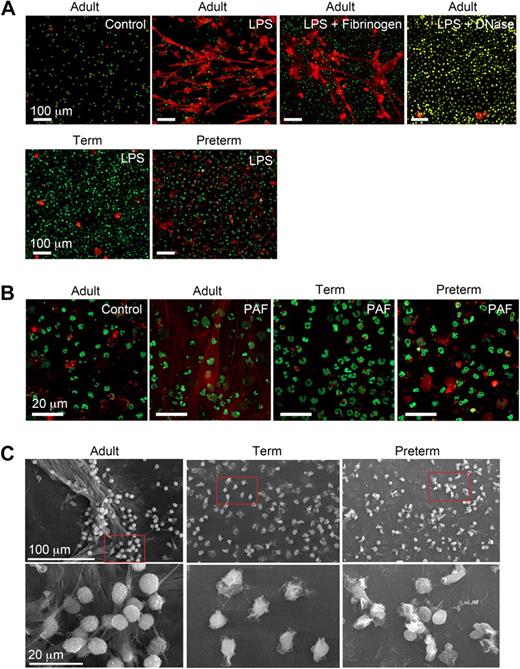
We characterized NET formation by PMNs isolated from term and premature infants and healthy adult subjects using conditions based on and adapted from descriptions of NET generation in the field.27-30,32 LPS and PAF were chosen as inflammatory agonists because there is evidence that these mediators contribute to the pathophysiology of sepsis and other infectious and inflammatory disorders in both neonates and adults.36-38 Adult PMNs stimulated with LPS or PAF formed NETs after a 60-minute incubation period, with no NET generation by quiescent control PMNs studied in parallel (Figure 1A,B). Treatment of LPS-stimulated adult PMNs with DNase (2.5 U/mL) degraded NETs generated by stimulated PMNs (Figure 1A). This is consistent with evidence that adult PMNs activated with interleukin 8 (IL-8) or the nonphysiologic agonist PMA generated NETs.27 In contrast, in a previous report adult PMNs failed to generate NETs in response to the complement fragment C5a, which is considered to be a relatively potent PMN agonist,39 in the absence of a priming stimulus.33 Thus, there may be differential regulation of the response by specific neutrophil receptors. We also found that PMNs adherent to fibrinogen (Figure 1A) and other immobilized proteins (C.C.Y., M.J.C., N.L.T., unpublished data, July 2008) extrude NETs when stimulated, consistent with evidence that NETs form in inflamed vessels and tissues.27,29-32
Stimulated PMNs isolated from newborn term or preterm human infants fail to form NETs. (A) NET formation was detected by live cell imaging with confocal microscopy using 2 DNA dyes, one cell impermeable (Sytox Orange, which stains extracellular DNA red in these images) and the other cell permeable (Syto Green, which identifies nuclear DNA) after activation of PMNs with LPS (100 ng/mL) for 60 minutes. Experiments were performed on poly-l-lysine– or fibrinogen-coated (top row, third panel) glass coverslips. DNase (2.5 Units/mL) was added following LPS stimulation (60 minutes) to dismantle extracellular NETs (top row, far right panel). The images are representative of experiments performed with LPS-stimulated PMNs isolated from 6 different donors from each subject group (adult, term, and preterm). Incubations on immobilized fibrinogen and treatment of LPS-stimulated PMNs with DNase were performed with neutrophils from 3 different adult subjects in each case. Control and LPS stimulation, 20× objective. (B) NET formation was detected as in panel A following stimulation of adult, term, and preterm PMNs with PAF (10 nM) for 60 minutes. These images are representative of separate experiments using PMNs isolated from 6 different donors in each subject group (adult, term, and preterm). Control and PAF stimulation, 60× objective. (C) NET formation was examined by scanning electron microscopy following stimulation of PMNs with LPS (100 ng/mL) for 60 minutes. These images are representative of 3 different experiments with PMNs from different donors in each subject group.
Stimulated PMNs isolated from newborn term or preterm human infants fail to form NETs. (A) NET formation was detected by live cell imaging with confocal microscopy using 2 DNA dyes, one cell impermeable (Sytox Orange, which stains extracellular DNA red in these images) and the other cell permeable (Syto Green, which identifies nuclear DNA) after activation of PMNs with LPS (100 ng/mL) for 60 minutes. Experiments were performed on poly-l-lysine– or fibrinogen-coated (top row, third panel) glass coverslips. DNase (2.5 Units/mL) was added following LPS stimulation (60 minutes) to dismantle extracellular NETs (top row, far right panel). The images are representative of experiments performed with LPS-stimulated PMNs isolated from 6 different donors from each subject group (adult, term, and preterm). Incubations on immobilized fibrinogen and treatment of LPS-stimulated PMNs with DNase were performed with neutrophils from 3 different adult subjects in each case. Control and LPS stimulation, 20× objective. (B) NET formation was detected as in panel A following stimulation of adult, term, and preterm PMNs with PAF (10 nM) for 60 minutes. These images are representative of separate experiments using PMNs isolated from 6 different donors in each subject group (adult, term, and preterm). Control and PAF stimulation, 60× objective. (C) NET formation was examined by scanning electron microscopy following stimulation of PMNs with LPS (100 ng/mL) for 60 minutes. These images are representative of 3 different experiments with PMNs from different donors in each subject group.
In sharp contrast to PMNs isolated from adults, PMNs from preterm or term infants failed to robustly form NETs following stimulation with LPS or PAF. Organized NETs were not detected by live cell imaging at any time point in incubations of activated preterm PMNs and were rarely seen in studies of activated term neutrophils, even though there was consistent formation of extracellular DNA lattices by adult PMNs examined as controls in side-by-side fashion (Figure 1A and data not shown). In some studies there were bright, focal “hot spots” of staining by the cell-impermeable DNA dye suggesting local pericellular DNA extrusion, which is thought to be an initial step in complex extracellular NET formation.30,32 Whether these foci of staining represent pericellular DNA release by a subset of neonatal PMNs that are more developmentally advanced in the spectrum of cellular maturity remains to be determined. Similar focal areas of extracellular DNA staining were seen in some incubations of control PMNs from adults (Figure 1A and data not shown).
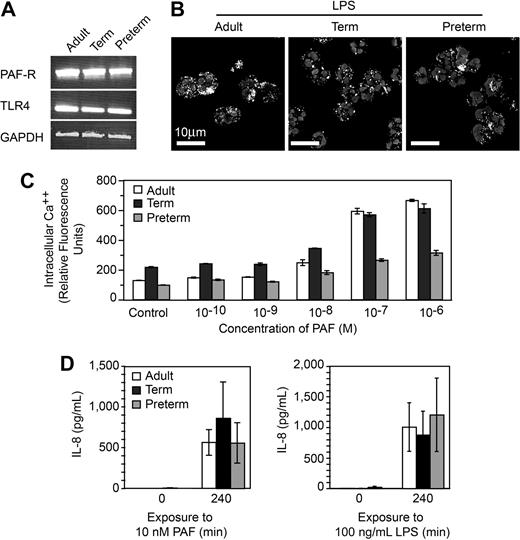
One possible explanation for defective NET formation is inability of neonatal PMNs to respond to the inflammatory agonists because of developmental delays in expression of receptors or recognition mechanisms for LPS and PAF and/or downstream intracellular signaling systems.10,11 For example, preterm PMNs are reported to have impaired responses to LPS compared with PMNs isolated from term infants and adults,38 although LPS responsiveness was recently demonstrated in a murine model of neonatal sepsis.40 To examine this issue, we first probed PMNs from preterm and term infants for mRNA for TLR4, which recognizes LPS, and the receptor for PAF and found that these transcripts are present, as they are in adult PMNs (Figure 2A). In functional assays, we found that PMNs from each group internalize fluorescently labeled LPS (Figure 2B). Neonatal and adult PMNs also responded to PAF in a concentration-dependent fashion by releasing intracellular calcium (Ca++; Figure 2C). The concentration-response relationship for Ca++ transients in preterm PMNs was clearly different from that of full-term and adult PMNs (Figure 2C), however, was consistent with previous evidence that neonatal PMNs have impaired Ca++ mobilization in response to chemotactic agonists.11 To further assess the responsiveness of neonatal PMNs to these inflammatory agonists we examined synthesis of IL-8, an event that requires signaling from surface receptors to complex gene expression pathways.5 Term and preterm PMNs responded to stimulation with PAF or LPS with synthesis and release of this chemokine (Figure 2D and data not shown). Taken together, these experiments indicate that term and preterm PMNs have functional receptors that recognize LPS and PAF and that failure to form NETs in response to these inflammatory mediators (Figure 1) is not exclusively due to developmentally delayed signaling via intracellular pathways downstream from these receptors, although in some cases defects in signaling may contribute to the phenotype of neonatal neutrophil dysfunction.
PMNs from term and preterm neonates respond to LPS and PAF, excluding developmental deficiency in these signaling systems as a mechanism of defective NET formation. (A) Expression of mRNA for TLR4, the PAF receptor, and GAPDH in PMNs isolated from each subject group was determined by semiquantitative RT-PCR. (B) FITC-labeled LPS uptake was determined in each subject group by immunocytochemistry and confocal microscopy. (C) Intracellular Ca++ release was determined by fluorometry following preincubation with Fluo4, a Ca++-dependent fluorochrome, and stimulation with the indicated concentrations of PAF. The data bars indicate mean plus or minus SEM fluorescence in 3 separate experiments with PMNs from adult, term, and preterm subjects. (D) Secretion of IL-8 at baseline and at 240 minutes was determined by ELISA following stimulation of PMNs with PAF (10 nM) or LPS (100 ng/mL). The data indicate mean plus or minus SEM supernatant IL-8 concentrations in 3 separate experiments with PMNs from each subject group. A similar pattern was seen after a 120-minute incubation time (not shown).
PMNs from term and preterm neonates respond to LPS and PAF, excluding developmental deficiency in these signaling systems as a mechanism of defective NET formation. (A) Expression of mRNA for TLR4, the PAF receptor, and GAPDH in PMNs isolated from each subject group was determined by semiquantitative RT-PCR. (B) FITC-labeled LPS uptake was determined in each subject group by immunocytochemistry and confocal microscopy. (C) Intracellular Ca++ release was determined by fluorometry following preincubation with Fluo4, a Ca++-dependent fluorochrome, and stimulation with the indicated concentrations of PAF. The data bars indicate mean plus or minus SEM fluorescence in 3 separate experiments with PMNs from adult, term, and preterm subjects. (D) Secretion of IL-8 at baseline and at 240 minutes was determined by ELISA following stimulation of PMNs with PAF (10 nM) or LPS (100 ng/mL). The data indicate mean plus or minus SEM supernatant IL-8 concentrations in 3 separate experiments with PMNs from each subject group. A similar pattern was seen after a 120-minute incubation time (not shown).
In additional studies using live cell imaging we found that neonatal PMNs do not form NETs in response to PMA (Figure S1B), a pharmacologic agonist that acts distal to surface receptors and is the stimulus that has largely been used to characterize NET generation by adult PMNs.27,32 Finally, we also found that incubation of adult PMNs with live E coli or S aureus induces formation of NET lattices, consistent with previous observations that bacteria stimulate NET generation.32 In contrast, term neonatal PMNs did not form NETs in response to bacteria in parallel incubations (data not shown). Thus, these experiments in aggregate document a defect in NET formation when neonatal PMNs are activated with pharmacologic agonists and microorganisms, in addition to receptor-mediated pathways activated by LPS and PAF. To exclude the possibility that neonatal PMNs release a soluble inhibitor of NET formation, we incubated adult PMNs and neonatal PMNs in various ratios. Ratios as high as 3 term or preterm PMNs to 1 adult PMN did not inhibit NET formation, arguing against this as a mechanism for the defect (Figure S2).
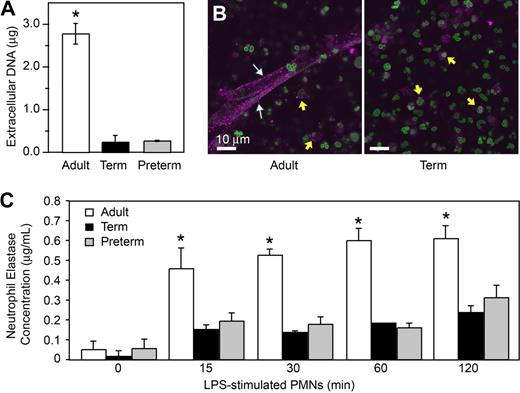
Using electron microscopy, we also examined NET formation by control and LPS-stimulated PMNs isolated from preterm and term infants and adults. LPS-stimulated PMNs isolated from adult donors generated extensive extracellular lattices (Figure 1C left panels). These structures are similar to those detected by live cell imaging (Figure 1A,B). In contrast, complex extracellular NETs were absent when control or LPS-stimulated neonatal PMNs were examined by electron microscopy (Figure 1C middle and right panels). We then used an assay in which extracellular DNA was quantitated by fluorometry using a cell-impermeable fluorescent dye (Figure 3A) as a correlate to detection of NET formation by light and electron microscopy (Figure 1). We found a significant increase in supernatant extracellular DNA content following stimulation of adult PMNs with LPS, but much lower levels in samples from LPS-stimulated PMNs isolated from either term or preterm infants (Figure 3A).
Stimulated PMNs from term and preterm infants extrude less DNA and release less neutrophil elastase, a surrogate marker of NET formation, than do PMNs from adults. (A) Supernatant DNA content was determined via fluorometry by quantitation of a non–cell-permeable DNA dye (Sytox Orange) in samples from PMNs stimulated with LPS (100 ng/mL) for 1 hour. The data are expressed as mean plus or minus SEM values for extracellular DNA. The asterisk indicates a significant difference (P < .001) between values from adult PMNs and those from term and preterm neutrophils. (B) NET-associated neutrophil elastase was examined by immunocytochemical analysis of PAF-stimulated and control PMNs from healthy adults and term infants. Stimulated PMNs from adults (left) extruded NETs that stained robustly for NE (magenta fluorescence; white arrows). Stimulated PMNs isolated from term infants (right) failed to form NET lattices containing extracellular NE. NE was detected in primary granules in PMNs from both term neonates and adults (yellow arrows). Extracellular and intracellular DNA counterstaining as in Figure 1 demonstrated both nuclear and, depending on the source of PMNs (adult vs neonate), extracellular NET-associated DNA. (C) NET-associated NE activity was determined in DNase-treated incubations of control PMNs and neutrophils stimulated with LPS (100 ng/mL) for 120 minutes. Mean plus or minus SEM NE concentrations are shown. The asterisk indicates a significant difference (P < .05) in NE concentration in DNase-treated samples from neonatal PMNs compared with the DNase-treated samples from adult PMNs. The images and data in Figure 3 are from a minimum of 3 separate experiments in each case.
Stimulated PMNs from term and preterm infants extrude less DNA and release less neutrophil elastase, a surrogate marker of NET formation, than do PMNs from adults. (A) Supernatant DNA content was determined via fluorometry by quantitation of a non–cell-permeable DNA dye (Sytox Orange) in samples from PMNs stimulated with LPS (100 ng/mL) for 1 hour. The data are expressed as mean plus or minus SEM values for extracellular DNA. The asterisk indicates a significant difference (P < .001) between values from adult PMNs and those from term and preterm neutrophils. (B) NET-associated neutrophil elastase was examined by immunocytochemical analysis of PAF-stimulated and control PMNs from healthy adults and term infants. Stimulated PMNs from adults (left) extruded NETs that stained robustly for NE (magenta fluorescence; white arrows). Stimulated PMNs isolated from term infants (right) failed to form NET lattices containing extracellular NE. NE was detected in primary granules in PMNs from both term neonates and adults (yellow arrows). Extracellular and intracellular DNA counterstaining as in Figure 1 demonstrated both nuclear and, depending on the source of PMNs (adult vs neonate), extracellular NET-associated DNA. (C) NET-associated NE activity was determined in DNase-treated incubations of control PMNs and neutrophils stimulated with LPS (100 ng/mL) for 120 minutes. Mean plus or minus SEM NE concentrations are shown. The asterisk indicates a significant difference (P < .05) in NE concentration in DNase-treated samples from neonatal PMNs compared with the DNase-treated samples from adult PMNs. The images and data in Figure 3 are from a minimum of 3 separate experiments in each case.
To further characterize and quantify differences in NET generation by neonatal PMNs, we examined neutrophil elastase (NE) by immunocytochemistry and activity assays using samples from control or LPS-stimulated PMNs. NE is associated with NETs, and NE activity in PMN supernatants was reported to be a quantifiable surrogate marker for NET formation.27,29,32 Anti-NE staining confirmed the association of this antibacterial enzyme with NETs formed by adult PMNs following stimulation with PAF (Figure 3B) or LPS (not shown). In contrast, NETs displaying NE were not detected by immunocytochemistry in incubations of stimulated neonatal PMNs, although NE was present in primary granules of PMNs from term neonates examined in parallel (Figure 3B and data not shown). We next assayed NET-associated NE activity as an index of NET formation32 at baseline and at multiple time points following stimulation of adult and neonatal PMNs with LPS. NET-associated NE activity in adult PMNs increased over the 120-minute incubation period. In parallel, NE activity associated with PMNs isolated from term and preterm infants was markedly lower (Figure 3C), a pattern consistent with measurements of extracellular DNA (Figure 3A) and NE in extracellular NETs evaluated by immunocytochemistry (Figure 3B and data not shown). Treatment of PMNs with the NE inhibitor at a high concentration (1 mg/mL), but not a lower concentration (100 μg/mL), reduced NET formation, suggesting the possibility that NE activity contributes to NET formation (data not shown).
The defect in NET formation by neonatal PMNs is associated with impaired extracellular bacterial killing
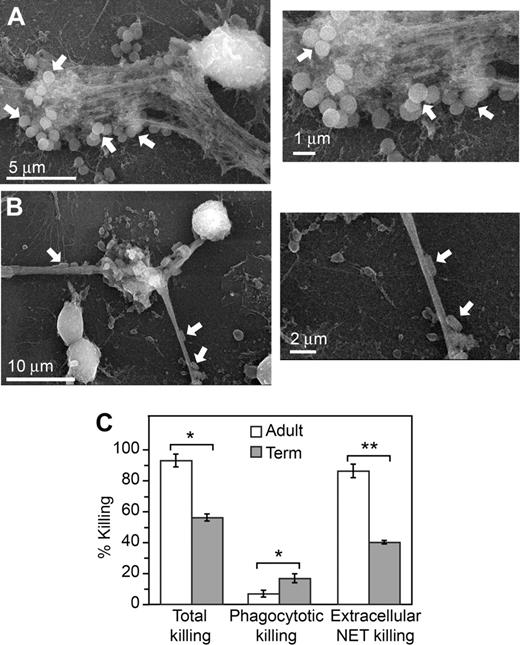
We then determined whether the defect in NET formation by neonatal PMNs is associated with bacterial killing deficits, as assessed by an in vitro killing assay. Extracellular lattices that formed when adult PMNs were incubated with E coli or S aureus trapped and localized the bacteria when examined by scanning electron microscopy (Figure 4A-B), consistent with previous reports that bacteria are immobilized by NETs.27,29 Using protocols adapted from the literature,29,30,41 we examined total, intracellular, and extracellular killing of E coli by PMNs from adults and term neonates. E coli are a common cause of severe infections and sepsis in infants13 (Table S1). By inhibiting phagocytosis and ingestion-dependent intracellular killing by pretreatment of PMNs with cytochalasins we determined the component of bacterial killing that occurs extracellularly; disruption of extracellular, NET-mediated killing by digestion of NETs with DNase allowed the intracellular component of bacterial killing to be distinguished. We found that total bacterial killing by PMNs isolated from term newborns was decreased compared with that of neutrophils isolated from adults. Total bacterial killing by PMNs from term neonates has previously been reported to be variable depending on the physiologic state of the infant.20,42,43 Whereas the component of bacterial killing dependent on phagocytosis was not decreased in neonatal PMNs, extracellular bacterial killing was markedly impaired (Figure 4). This result is consistent with deficient extracellular NET formation (Figures 1,3) and with previous observations that NETs contribute a major component of extracellular antibacterial activity.27,29,30 It also demonstrates that impaired NET formation by PMNs isolated from term infants contributes to decreased bacterial killing in vitro.
Extracellular bacterial killing associated with NET formation is deficient in neonatal PMNs. NET trapping of S aureus (A) and E coli (B) was examined by scanning electron microscopy following incubation of the bacteria with unstimulated PMNs from adult donors for 60 minutes. These images are representative of 3 different experiments. (C) Total bacterial killing by PMNs isolated from adults and term infants following stimulation with LPS (100 ng/mL) for 1 hour was determined (left bars). To determine NET-associated extracellular bacterial killing, phagocytosis and intracellular bacterial killing were blocked by pretreatment with cytochalasins B and D (right bars). DNase treatment was used to degrade NETs formed following LPS stimulation and block NET-associated bacterial killing to thus determine phagocytotic, intracellular bacterial killing (middle bars). See “Bacterial killing assay” for additional details. The bars indicate mean bacterial killing plus or minus SEM in 3 separate experiments. An asterisk indicates a significant difference (P < .05) in total bacterial killing and phagocytotic, intracellular bacterial killing by PMNs isolated from term infants compared with PMNs isolated from healthy adults. A double asterisk indicates a significant difference (P < .001) in extracellular, NET-associated bacterial killing by PMNs isolated from term infants compared with PMNs isolated from adults.
Extracellular bacterial killing associated with NET formation is deficient in neonatal PMNs. NET trapping of S aureus (A) and E coli (B) was examined by scanning electron microscopy following incubation of the bacteria with unstimulated PMNs from adult donors for 60 minutes. These images are representative of 3 different experiments. (C) Total bacterial killing by PMNs isolated from adults and term infants following stimulation with LPS (100 ng/mL) for 1 hour was determined (left bars). To determine NET-associated extracellular bacterial killing, phagocytosis and intracellular bacterial killing were blocked by pretreatment with cytochalasins B and D (right bars). DNase treatment was used to degrade NETs formed following LPS stimulation and block NET-associated bacterial killing to thus determine phagocytotic, intracellular bacterial killing (middle bars). See “Bacterial killing assay” for additional details. The bars indicate mean bacterial killing plus or minus SEM in 3 separate experiments. An asterisk indicates a significant difference (P < .05) in total bacterial killing and phagocytotic, intracellular bacterial killing by PMNs isolated from term infants compared with PMNs isolated from healthy adults. A double asterisk indicates a significant difference (P < .001) in extracellular, NET-associated bacterial killing by PMNs isolated from term infants compared with PMNs isolated from adults.
Oxygen radical signaling of NET formation is interrupted in neonatal PMNs
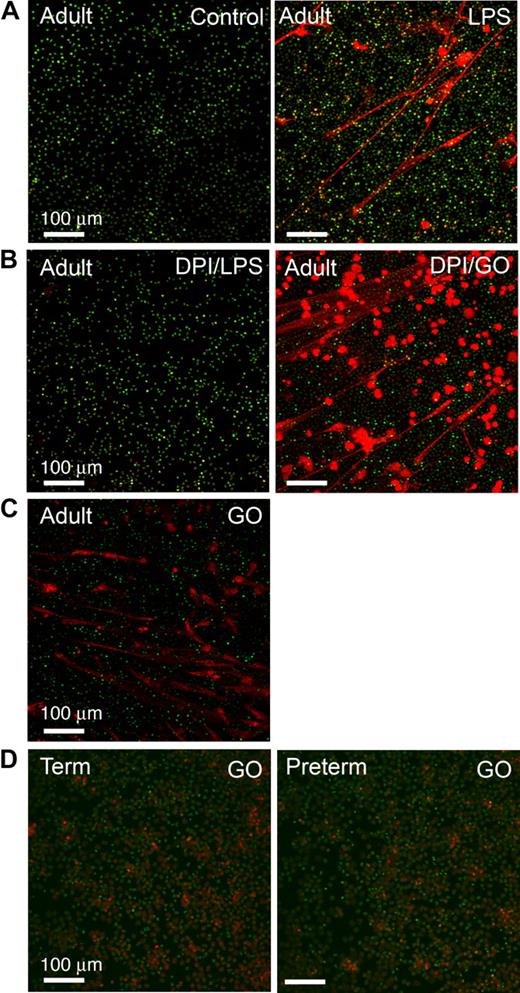
PMNs from subjects with chronic granulomatous disease (CGD), a genetic deficiency of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity, and adult PMNs with NADPH oxidase disabled by the pharmacologic agent diphenylene iodonium (DPI) are unable to form NETs.32 Exogenous cell-permeable ROSs restore the ability of CGD PMNs to form NETs, leading to the conclusion that ROSs function as signaling molecules that activate a downstream pathway to a unique “NET death” response and associated extracellular NET formation32 (also see “Introduction”). To examine this potential mechanism in neonatal PMNs, we first documented that inhibition of ROS generation by pretreatment with DPI dramatically reduced NET formation by LPS-stimulated adult PMNs (Figure 5B). Treatment with glucose/glucose oxidase (GO) to generate ROSs, including cell-permeant hydrogen peroxide,32 reversed the defect in pericellular extrusion of DNA and complex extracellular NET lattice formation by PMNs pretreated with DPI (Figure 5B). GO also induced NET formation by adult PMNs in the absence of stimulation with LPS or other agonists (Figure 5C). These results are similar to that previously reported for PMNs from healthy adults and CGD patients.32 We then examined ROS signaling in neonatal PMNs and found that GO does not complement the defect in NET formation in term or preterm neonatal PMNs (Figure 5D, and data not shown) under conditions in which it induced NET formation in control or DPI-treated adult PMNs studied in parallel (Figure 5B-C). Incubations using GO in a concentration range from 200 to 1000 mU/mL yielded the same result. We also found that PMNs from term and preterm neonates stimulated with PMA are able to generate endogenous ROSs as qualitatively assayed by nitroblue tetrazolium reduction (Figure S1A), consistent with previous observations that term neutrophils have NADPH oxidase activity21,44 ; nevertheless, term and preterm PMNs treated with PMA were unable to form organized extracellular NETs (Figure S1B). Together, these results demonstrate that the defect in NET formation and extracellular bacterial killing by neonatal PMNs (Figures 1,3,Figure 4–5) is not due to inability to generate endogenous ROSs because of developmental deficiency of NADPH oxidase activity.11,21 Thus, additional signal transduction signaling events governing NET formation are required.
ROSs induce NET formation by adult PMNs but do not complement defective NET formation by PMNs isolated from newborn infants. NET formation was detected by live cell imaging with confocal microscopy (20× objective) using cell-permeant (green fluorescence) and impermeant (red fluorescence) DNA dyes as in Figure 1. All images are representative of 4 different experiments using PMNs from each subject group. (A) PMNs isolated from adults were incubated with buffer alone (control) or stimulated with LPS (100 ng/mL) for 60 minutes. (B) Adult PMNs were pretreated with DPI (20 μM) and then incubated for 60 minutes with either LPS (100 ng/mL) or exogenous ROSs generated by treatment with glucose/GO (1 mM/200 mU/mL). DPI inhibited endogenous ROS generation. (C) Adult PMNs were treated with glucose/GO (1 mM/200 mU/mL) alone without LPS or other agonist stimulation. (D) PMNs isolated from term (left panel) or preterm (right panel) infants were stimulated with exogenous ROSs by treatment with glucose/GO (1 mM/1000 mU/mL). Additional incubations using GO in concentrations from 200 mU/mL (ie, as with adult PMNs in panels B and C) to 1000 mU/mL yielded similar results (not shown).
ROSs induce NET formation by adult PMNs but do not complement defective NET formation by PMNs isolated from newborn infants. NET formation was detected by live cell imaging with confocal microscopy (20× objective) using cell-permeant (green fluorescence) and impermeant (red fluorescence) DNA dyes as in Figure 1. All images are representative of 4 different experiments using PMNs from each subject group. (A) PMNs isolated from adults were incubated with buffer alone (control) or stimulated with LPS (100 ng/mL) for 60 minutes. (B) Adult PMNs were pretreated with DPI (20 μM) and then incubated for 60 minutes with either LPS (100 ng/mL) or exogenous ROSs generated by treatment with glucose/GO (1 mM/200 mU/mL). DPI inhibited endogenous ROS generation. (C) Adult PMNs were treated with glucose/GO (1 mM/200 mU/mL) alone without LPS or other agonist stimulation. (D) PMNs isolated from term (left panel) or preterm (right panel) infants were stimulated with exogenous ROSs by treatment with glucose/GO (1 mM/1000 mU/mL). Additional incubations using GO in concentrations from 200 mU/mL (ie, as with adult PMNs in panels B and C) to 1000 mU/mL yielded similar results (not shown).
Discussion
PMN defects and other facets of neonatal immune deficiency contribute to sepsis and additional infectious syndromes in newborns, and are major causes of morbidity, mortality, and health-related expense. NET formation by PMNs is a previously unrecognized antibacterial effector activity with unexpected mechanistic features.27,32,45 Impaired NET formation and extracellular bacterial killing by PMNs from human neonates, as we report here, are novel defects in the antimicrobial repertoire of this critical defensive cell that further demonstrate the complexity of the developmental immunodeficiency of newborn infants. Our studies also provide new insights into the mechanism of NET formation by human PMNs.
NET lattices formed by activated PMNs capture and contain both Gram-positive and Gram-negative bacteria.27,29,30,32 Physical capture and containment of pathogens by intracellular factors released into the extracellular space by innate immune effector cells is an evolutionarily conserved host defense mechanism.46 PMN NETs are also studded with elastase27,29 (Figure 3), an enzyme with activity against bacterial proteins and virulence factors, and with histones,27,32 which have antimicrobial properties.47 Thus NETs may be spatially restricted repositories that provide high local concentrations of antimicrobial factors.27,29
NET-associated killing of several bacterial and fungal species occurs in vitro27-30,41 and is reported to complement and act in temporal sequence with phagocytosis and intracellular killing.32 NET formation in vivo is reported in models of experimental shigellosis, necrotizing fasciitis, and pneumococcal pneumonia, as well as in human appendicitis.27,29,30 Furthermore, bacterial strains that express DNases or endonucleases that are competent to degrade NETs can escape capture and killing in vitro and in vivo, contributing to their pathogenicity and virulence in disease models.29,30 Additional factors may protect some bacteria, including Streptococcus pneumoniae, from killing by NETs.41 Thus, evolving evidence indicates that NET formation by PMNs is an important mechanism of extracellular containment and elimination of specific pathogens, and that evasion of NETs represents a biologic capacity that contributes to the ability of bacteria to successfully infect and injure the host.
Defective NET formation and extracellular bacterial killing by primary human neutrophils, as we report here, are novel deficiencies that have not previously been identified in PMNs from neonates of any gestational age or in leukocytes from older subjects. Defective elimination of pathogens due to relatively decreased phagocytic capacity, reduced generation of ROSs, and lower levels of granule elastase, bactericidal/permeability-increasing protein, and lactoferrin contribute to impaired bacterial killing in neonatal neutrophil dysfunction.11,21,48 Our findings indicate that deficient PMN NET formation and extracellular bacterial containment and killing by this mechanism may also be critical determinants of impaired microbial killing in severe neonatal infections such as sepsis and pneumonia. Contributions of individual antimicrobial defenses likely depend on the degree of maturity of the infant and the infecting organism(s). Although our studies of extracellular bacterial killing were done only with PMNs from term infants because of small numbers of leukocytes retrieved from limited volumes of blood from preterm babies, many of whom are neutropenic at birth,10,11,18 we documented defective NET formation by PMNs from both gestational age groups. Because the severity of neonatal PMN dysfunction is, in general, related to developmental maturity, this suggests that extracellular killing by neutrophils from premature infants is likely to also be defective and may be profound in very premature babies.
In addition to identifying a new component of the complex phenotype of neonatal neutrophil dysfunction, our studies provide unique evidence for an intricate pathway to NET formation by human neutrophils. Studies of adult PMNs treated with PMA indicate that NET formation involves an active cell death mechanism leading to disruption of the nuclear membrane, disintegration of the nucleus and cytoplasmic granules with mixing of chromatin and granular constituents, and local extrusion of DNA, histones, and enzymes.27,32 Whereas this process appears to be required, NETs form under conditions in which some PMNs are grossly intact (Figures 1,5). It is unknown whether active participation by viable PMNs is also required for formation of complex, 3-dimensional NET lattices (C.C.Y., unpublished studies, July 2007). PMA-stimulated mononuclear leukocytes were reported to not form NETs,32 indicating that cellular and nuclear specializations influence the response; it is thus interesting to consider that the unusual and characteristic multilobe morphology and organization of the PMN nucleus may in some way contribute to this process. Morphologic observations and TUNEL and annexinV analyses argue against classic mechanisms of necrosis and apoptosis in the “NET death” pathway in PMNs.32 In addition, agonists that delay apoptosis, including LPS and IL-8, induce NET formation27 (and this study). Neonatal PMNs have a constitutive delay in apoptosis compared with adult PMNs, in part secondary to decreased functional expression of caspase-3.10,44,49 Thus, they might be predicted to more robustly form NETs compared with adult PMNs. We found the opposite, further disassociating NET formation from apoptosis.
The mechanism of cell death and consequent NET formation by adult PMNs activated with PMA or S aureus requires generation of ROSs and is deficient in neutrophils from patients with CGD or PMNs with pharmacologically inactivated NADPH oxidase.32 This suggests that intracellular oxygen radicals signal to downstream components of a pathway that controls NET formation.32 The functional phenotype of neonatal PMNs, in which ROS generation is preserved (albeit at lower levels than in adults, depending on the degree of maturity) (Figure S1A) but NET formation is defective, provided a unique system in which to directly test this hypothesis. We found that exogenous, cell-permeant ROSs generated by glucose/GO induced NET formation by adult PMNs with DPI-inactivated NADPH oxidase. This result is consistent with the response of PMNs from subjects with CGD.32 Nevertheless, ROSs did not complement the defect in NET formation in term neonatal PMNs, which have NADPH oxidase activity equivalent to that of adult PMNs,21,44 or in preterm PMNs. This result demonstrates that the mechanism of NET lattice formation by human PMNs involves events distal and/or parallel to ROS generation. ROSs, including hydrogen peroxide, have activities that influence tyrosine phosphorylation and other regulatory checkpoints in signal transduction cascades, in addition to being effectors of oxidant injury.50 Molecular signaling cascades and intracellular kinases are differentially expressed and regulated in neonatal versus adult PMNs (C.C.Y. et al, manuscript in preparation), potentially providing a system for further dissection of pathways to NET formation.
The definition of primary immunodeficiencies is undergoing re-examination, and it has been proposed that most humans are immunodeficient if susceptibility to a severe infectious disease is a defining feature of an immunodeficient phenotype.51 Even if immunodeficiencies are less broadly framed, neonatal neutrophil deficiency may be the most common of these syndromes. Our studies of deficient NET formation and extracellular bacterial killing by preterm and term neonatal PMNs indicate that additional features of this complex developmental condition remain to be defined. It has also been observed that there is particular value added when studying the “human model” in the context of host-pathogen interactions and antimicrobial systems.51 If this is true, further characterization of effector mechanisms of PMNs and other immune cells from human neonates will yield insights broadly relevant to both host defense and injurious inflammation.6
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The nurses and staff of the Newborn Intensive Care and Labor and Delivery units at the University of Utah Medical Center provided essential help in identifying patients. Ann Croft of ARUP Laboratories provided bacterial cultures and helpful information regarding killing assays. We thank members of our group for invaluable discussions, advice, and technical assistance and particularly thank Jason Foulks, PhD, who provided important assistance with intracellular Ca++ release assays. We are grateful to Gopal Marathe, PhD, for information regarding PAF signaling. Linn Steele provided invaluable help in preparation of the paper, as did Diana Lim in preparation of the figures.
This work was supported by National Institutes of Health (NIH; Bethesda, MD) grants (K08 HD049699 [C.C.Y.], 5R01 HL066277-07 [A.S.W.], and 5R37 HL044525-20 [G.A.Z.]); The Children's Health Research Center of the University of Utah (Salt Lake City, UT) and the Primary Children's Medical Center Foundation (Salt Lake City, UT; awards to C.C.Y. and N.L.T.); and a Flight Attendant Medical Research Institute (FAMRI; Miami, FL) Young Clinical Scientist Award (M.L.M.).
National Institutes of Health
Authorship
Contribution: C.C.Y. performed, directed, and interpreted experiments, and wrote major portions of the paper; M.J.C., E.S.H., N.L.T., A.M.M., M.L.M., N.B.C., C.K.R., K.H.A., and C.A.P. performed experiments, provided key experimental approaches, and/or interpreted data; A.S.W. interpreted central findings and edited portions of the paper; and G.A.Z. provided overall direction for the project, reviewed and analyzed all experiments, wrote sections of the paper, and edited the entire paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian C. Yost or Guy A. Zimmerman, Program in Human Molecular Biology & Genetics, Bldg 533, Rm 4220, 15 North 2030 East, Salt Lake City, UT 84112; e-mail: christian.yost@hmbg.utah.edu or guy.zimmerman@hmbg.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal