Abstract
In this issue of Blood, Zou and colleagues unravel a long-standing mystery—how megakaryocyte precursors make the transition from the collagen-rich osteoblastic bone marrow niche to the collagen-poor vascular niche while expressing 2 receptors that interact with collagen—GpVI and the α2β1 integrin.
We know that both GpVI and the α2β1 integrin are expressed on circulating platelets and that when platelets come into contact with collagen, there is a low-affinity interaction with GpVI. This generates an intraplatelet signal, which increases the affinity of the second receptor, α2β1, for collagen. α2β1 binding to collagen provides stable adhesion and allows the initiation of platelet plug formation.
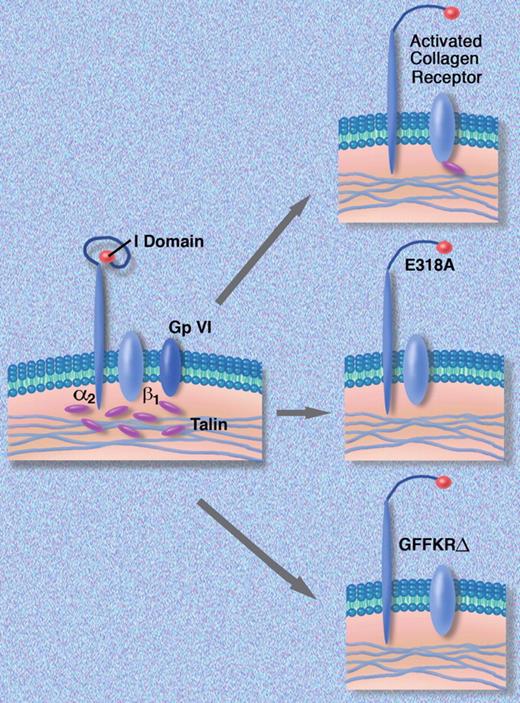
The effect of mutations introduced into the α2 polypeptide on activation of the α2β1 complex. Professional illustration by Marie Dauenheimer.
The effect of mutations introduced into the α2 polypeptide on activation of the α2β1 complex. Professional illustration by Marie Dauenheimer.
This study by Zou et al answers some important questions about the function of these receptors during megakaryocyte maturation.1 The authors employ an elegant synthesis of genetic engineering, cell culture, and mouse models to solve the puzzle. We know that GPVI and α2β1 are both expressed on mature megakaryocytes and, of course, on circulating platelets but not on megakaryocyte progenitors, which could explain how megakaryocytes escape from the collagen-rich osteoblastic niche. However, the authors show that expressing both proteins simultaneously (by transducing megakaryocyte progenitors with retroviruses containing GpVI and α2β1 polypeptide cDNAs) did not impair their development into mature megakaryocytes. Most of the article is devoted to testing a second hypothesis that megakaryocyte progenitors “down-regulate” or prevent the expression of α2β1 to allow them to escape from their collagen-rich niche.
The article builds on a rich literature on the regulation of integrin function. Integrins are transmembrane adhesive glycoproteins, which mediate a large number of cell-to-cell and cell-matrix interactions.2 Each integrin is made up of a single α and β subunit. Humans have 18 α and 8 β subunit polypeptides, which by a “mix and match” process, form 24 distinct receptors.3 To understand how integrins mediate cell-to-cell and cell-matrix interactions, one has to understand the difference between “outside-in” and “inside-out” signaling and how signaling leads to “affinity modulation” and “integrin switching.”
In addition to binding collagen, the α2β1 collagen receptor transduces signals in the cell that can cause shape change or a change in motility—outside-in signaling. However, α2β1 as well as other integrins like the platelet fibrinogen receptor α2β3 (also called GpIIb/IIIa) cannot bind ligand(s) until they are “activated.” This requires a signal from within the cell—inside-out signaling—which increases the affinity of the receptor for its ligand. The process is dynamic and receptor affinity can be reduced by changes in the inside-out signals or by internalization and removal of the activated receptor.
The molecular details of the affinity modulation of α2β1 are well understood and involve the linkage of the β1 subunit to the cytoskeleton via talin, which facilitates an interaction between the α and β subunit polypeptides. This then leads to the unfolding of the α2 polypeptide and exposure of the α2I domain, the specific region of the complex that interacts with collagen (figure). Based on this information, the authors engineered 2 mutant forms of the α2 polypeptide. The first, E318A, breaks an internal salt bridge, which exposes the I domain of the α2 polypeptide and creates a receptor that is locked into a high-affinity state and no longer responds to inside-out signals. The second mutation deletes 5 amino acids GFFKR, which are situated where the α2 polypeptide passes through the cell membrane. The GFFKR sequence interacts with complementary sequences on the β1 polypeptide to keep the protein complex in a quiescent inactive state. When the mutant α2 polypeptide was expressed in a rat basophil cell line (RBL-2H3), which makes only β1 polypeptides, GFFKRΔ α2β1 receptors were formed that enhanced cellular adhesion to collagen. They then inserted either wild-type α2 or 1 of the 2 mutant polypeptides into fetal liver cells that no longer made the α2 polypeptide and transplanted the transduced fetal liver cells into lethally irradiated mice. Platelets derived from animals receiving transplants with cells expressing in wild-type α2 had normal amounts of α2β1 on their platelets, while mice who received the mutant α2 receptors locked into a high-affinity state had no α2β1 protein on the surface of their platelets. α2β1 protein was present in platelet lysates, suggesting that during hematopoietic development, the cells internalized the high-affinity receptors.
Although one might conclude from these studies that the expression of high-affinity receptors in megakaryocyte progenitors would block their escape from the niche, the authors found that this was not so. Progenitors expressing high-affinity receptors were normally distributed in mouse marrow and were able to move through collagen-coated Boyden chamber filters. They did, however, down-regulate their high-affinity receptors in the process. This suggests that the down-regulation process is dynamic and perhaps temporally and spatially regulated (ie, occurring only “at the right time and in the right place”).
This study partially answers the question of how megakaryocyte progenitors escape from the osteoblastic niche by down-regulating their collagen receptors. This is an important first step and is “permissive” (ie, the progenitors would presumably remain locked in the osteoblastic niche if they could not shed their high-affinity collagen receptors). However, there has to be more to the story. How do the cells actually move or crawl from the osteoblastic to the vascular niche? Is there transient expression of high-affinity α2β1 receptors at the leading edge of the cell with loss of α2β1 receptors along the trailing edge of cells? Could this wave of receptor down-regulation provide a way for cells to crawl out of the niche? Is there an “integrin switch” leading to the expression of another integrin receptor that will bind to collagen with lower affinity or binds to other matrix constituents to facilitate cell movement? Are there inherited or acquired abnormalities of megakaryocyte production that could be due to progenitor trapping in the osteoblastic niche?
There are elegant techniques available for in vivo microscopy of the marrow space in living animals that could be used to study these questions4 and I am confident that these experiments will be or are being done. I look forward to more details regarding the movement of megakaryocyte and other progenitor cells from their respective niches so they can get on with the important business of producing blood cells.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal