Abstract
Abstract 2431
Poster Board II-408
Nicotinamide (NAM), is a form of VitB3 that recognized and inhibits SIRT1, the human ortholog of the yeast Sir2 class III NAD+-dependent histone deacetylase. We have previously demonstrated that NAM inhibits in vitro differentiation and enhances expansion, migration, homing and NOD/SCID engraftment efficacy of cord blood (CB)-derived CD34+ cells cultured with cytokines.
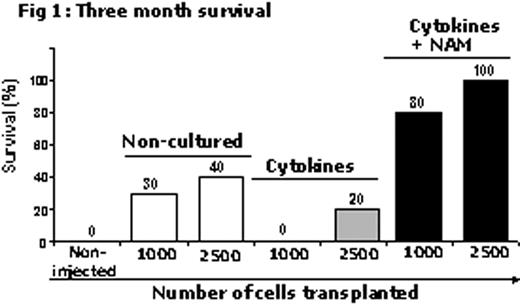
In the current study, the in vivo function of ex vivo cultured cells with NAM was tested in a congenic mice model (BALB/C, CD45.1/CD45.2) for BM transplantation. Purified CD117+ BM cells from BALB/C CD45.1 mice were cultured with a combination of 4 cytokines (FLT3, SCF, TPO, IL-6, 50 ng/ml each), with and without 0.5mM NAM for three weeks. Numbers of CFUc, CD117+ and CD117+Lin- cells were significantly (p < 0.05) higher in cultures treated with NAM as compared with cultured treated with cytokines alone. Non-cultured, freshly purified CD117+ cells (1000 and 2500 cells/mice) and their total progeny following expansion with or without NAM were transplanted into ablated (1000 Rad) CD45.2 mice (n = 10/cohort), 24h post irradiation (Fig 1). Three months post transplantation, all the mice in the control group (non-transplanted) died. The percent survival of mice transplanted with cells cultured with cytokines and NAM was remarkably higher over the survival of mice in the cohort transplanted with cells cultured with cytokines alone or non-cultured cells (Fig 1). FACS analysis (CD45.1-donor / CD45.2-host) of peripheral blood from mice transplanted with NAM cultured cells show 80% donor cell chimerism (CD45.1), 3 and 6 months post transplantation. Percent of donor derived Gr-1+ and CD3+ cells were similar in mice transplanted with non-cultured or NAM cultured cells. Percentages of donor cell chimerism (CD45.1) in secondary mice transplanted with total BM cells derived from primary recipients originally transplanted with non-cultured and NAM cultured cells were 47 and 73, respectively, 6 weeks after the secondary transplantation.
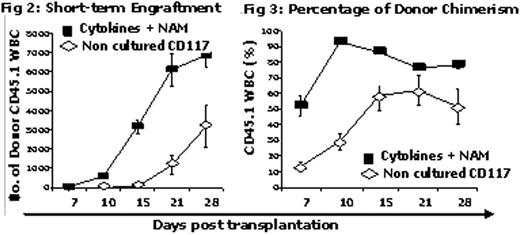
In a different experiment, to follow time to engraftment during the first month post transplantation, mice transplanted with non-cultured cells or cells cultured with cytokines and NAM (n = 10/cohort) were bled at weekly intervals and peripheral blood samples were counted for WBC and analyzed by the FACS to determine donor cell chimerism and lineage engraftment. The results show accelerated engraftment (Fig 2) and higher levels of donor cell chimerism (Fig 3) in the cohort transplanted with NAM cultured cells relative to the cohort transplanted with non-cultured cells. Number of granulocytes, T, NK and B cells during the first month post transplant were also significantly (p<0.05) higher in mice transplanted with cells cultured with cytokines and NAM relative to their levels in mice transplanted with non-cultured cells.
The results obtained in the congenic mice model for BMT suggest that NAM promotes expansion in ex vivo cultures of short and long-term repopulating cells, as demonstrated by accelerated donor derived engraftment during the first month post transplantation, higher survival of mice, sustained donor cell chimerism 6 month post transplantation and successful reconstitution of secondary recipients. NAM is thus a novel molecule that may be used to stimulate and expand hematopoietic repopulating cells, fasten post transplant engraftment and hopefully improve transplantation outcome. Current studies are designed to elucidate NAM mode of action.
Peled:Gamida-Cell: Employment, Equity Ownership. Shoham:Gamida Cell: Employment. Aschengrau:Gamida Cell: Employment. Yackoubov:Gamida Cell: Employment. Frei:Gamida Cell: Employment. Nagler:Gamida Cell: Arnon Nagler, Consultancy. Peled:Gamida Cell: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal