Abstract
Abstract 1827
Poster Board I-853
It is now well established that cytogenetic abnormalities can affect the responses to therapies in multiple myeloma (MM) patients. Bortezomib, used alone or in combination with other agents, has been shown to overcome the adverse impact of several common unfavorable cytogenetic features. More recently, responses with lenalidomide and dexamethasone have been reported in patients with some types of unfavorable cytogenetics. Carfilzomib (CFZ) is a novel proteasome inhibitor that has demonstrated single agent activity in relapsed and/or refractory MM patients. The objective of this analysis was to provide the first preliminary information on the influence of cytogenetics in patients (pts) with relapsed and/or refractory MM treated with CFZ.
We evaluated 79 pts treated on two single agent CFZ studies (PX-171-003 and PX-171-004) in relapsed and/or refractory myeloma in which metaphase cytogenetics and/or FISH analysis for del 13q, t(4:14), and t(14;16) chromosomal abnormalities were available. Metaphase cytogenetics was conducted for all pts in the analysis; fluorescence in situ hybridization (FISH) results were available for 28 of the 79 pts. Twenty-one pts with relapsed and refratory MM (PX-171-003) and 58 pts with relapsed or refractory MM (PX-171-004) received CFZ at 20 mg/m2 IV on days 1, 2, 8, 9, 15, and 16 in a 28-day cycle for up to 12 cycles. For this analysis, responders were defined as pts who achieved at least a Minor Response (MR) [MR + Partial Response (PR) + Very Good Partial Response (VGPR) + Complete Response (CR)] by IMWG and EBMT criteria.
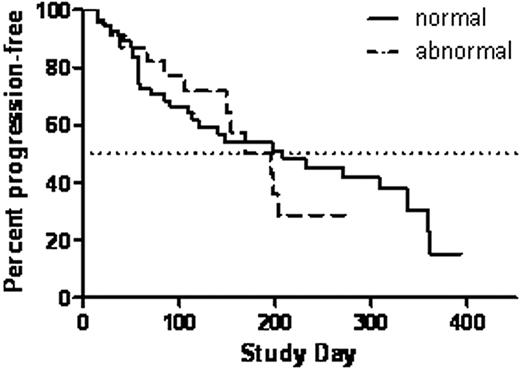
The median age of analysed pts was 63 yrs and 100% of pts were relapsed, with 70% refractory to their last therapy. Analysis of their histories demonstrated prior thalidomide treatment in 75% of pts, prior lenalidomide treatment in 57%, prior bortezomib treatment in 55%, and prior stem cell transplantation in 84%. The response rate (≥MR) for the entire group of patients was 40.5%. Twenty three of 79 pts had at least one of the abnormalities. The presence of del 13q, t(4;14), or t(14;16) did not significantly change the response rates, with 43.5% of pts with one or more abnormalities responding compared to 39.3% with none. The median time to progression (TTP) for all patients in this analysis was 203 days. The TTP for pts with one or more of the abnormalities was 195 days and was not significantly different from the TTP of 208 days for pts with none of the abnormalities (Figure; P > 0.05).
| Time to Progression . | |
|---|---|
| Normal | 208 Days |
| Abnormal | 195 Days |
| Time to Progression . | |
|---|---|
| Normal | 208 Days |
| Abnormal | 195 Days |
In this preliminary analysis, CFZ showed comparable activity in relapsed and relapsed/refractory MM with del 13q and/or t(4:14), and/or t(14;16) versus none of these abnormalities, with ≥MR in 43.5% vs. 39.3% of patients, and a TTP of 195 vs. 208 days, respectively. Updated efficacy data and TTP data will be presented at the meeting.
Jakubowiak:Millennium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Centocor Ortho Biotech: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Exelixis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers-Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Wang:Proteolix, Inc.: Research Funding. Jagannath:Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Siegel:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Stewart:Takeda-Millenium, Celgene, Novartis, Amgen: Consultancy; Takeda, Millenium: Research Funding; Genzyme, Celgene, Millenium, Proteolix: Honoraria. Kukreti:Celgene: Honoraria. Lonial:Celgene: Consultancy; Millennium: Consultancy, Research Funding; BMS: Consultancy; Novartis: Consultancy; Gloucester: Research Funding. McDonagh:Proteolix: Research Funding. Vallone:Proteolix, Inc.: Employment. Kauffman:Proteolix, Inc.: Employment. Vij:Proteolix: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal