Abstract
Abstract 1489
Poster Board I-512
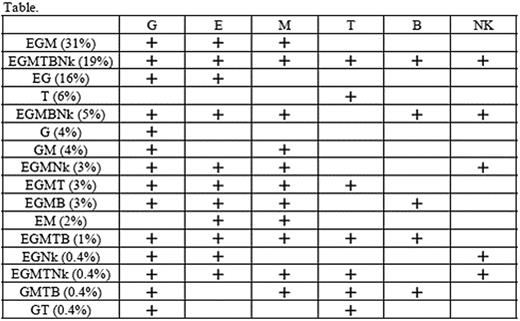
The hematopoietic stem cell (HSC) differentiation pathway in humans remains largely unknown due to the lack of an appropriate in vivo assay allowing the growth of HSCs as well as of clonal markers that enable the tracing of their progenies. Small populations of blood cells deficient in glycosylphosphatidylinositol-anchored proteins (GPI-APs) such as CD55 and CD59 are detectable in approximately 50% of patients with aplastic anemia (AA) and 15% of patients with refractory anemia (RA) of myelodysplastic syndrome defined by the FAB classification. Such blood cells with the paroxysmal nocturnal hemoglobinuria (PNH) phenotype (PNH-type cells) are derived from single PIGA mutant HSCs and their fate depends on the proliferation and self-maintenance properties of the individual HSCs that undergo PIG-A mutation by chance (Blood 2008;112:2160, Br J Haematol 2009 in press) Analyses of the PNH-type cells from a large number of patients on the diversity of lineage combination may help clarify the HSC differentiation pathway in humans because PIG-A mutant HSCs in patients with bone marrow failure appear to reflect the kinetics of healthy HSCs. Therefore, different lineages of peripheral blood cells were examined including glycophorin A+ erythrocytes (E), CD11b+ granulocytes (G), CD33+ monocytes (M), CD3+ T cells (T), CD19+ B cells (B), and NKp46+ NK cells (Nk) from 527 patients with AA or RA for the presence of CD55−CD59− cells in E and G, and CD55−CD59−CD48− cells in M,T, B, Nk with high sensitivity flow cytometry. Two hundred and twenty-eight patients (43%) displayed 0.003% to 99.1% PNH-type cells in at least one lineage of cells. The lineage combination patterns of PNH-type cells in these patients included EGM in 71 patients (31%), EGMTBNk in 43 (19%), EG in 37 (16%), T alone 14 (6%), EGMBNk in 11 (5%), G alone in 10 (4%), GM in 10 (4%), EGMNk in 7 (3%), EGMT in 7 (3%), EGMB in 6 (3%), EM in 5 (2%), EGMTB in 3 (1%), EGNk in 1 (0.4%), EGMTNk in 1 (0.4%), GMTB in 1 (0.4%), and GT in 1 (0.4%) (Table). All patterns included G or M, except for 14 patients displaying PNH-type T cells alone. No patients showed TB or TBNk patterns suggestive of the presence of common lymphoid progenitor cells. Peripheral blood specimens from 123 patients of the 228 patients possessing PNH-type cells were examined again after 3 to 10 months and all patients showed the same combination patterns as those revealed by the first examination. PIG-A gene analyses using sorted PNH-type cells from 3 patients revealed the same mutation in G and Nk for 1 patient and in G and T for 2 patients. These findings indicate that human HSCs may take a similar differentiation pathway to that of murine HSCs, the ‘myeloid-based model’ that was recently proposed by Kawamoto et al. (Nature 2008; 10:452), though the cases with PNH-type T cells alone remain to be elucidated.
Table. Lineages of cells containing PNH-type cells in patients with AA or RA. The number in the parenthesis denotes the proportion of patients showing each combination pattern in the total patients possessing PNH-type cells. (+ ; presence of PNH-type cells)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal