Abstract
Mechanisms underlying apoptosis induced by concomitant interruption of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 (MEK/ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/Akt pathways were investigated in human leukemia cells. Inhibition of these pathways using the MEK inhibitor PD184352 or U0126 and the PI3K/Akt inhibitor perifosine strikingly induced apoptosis in multiple malignant human hematopoietic cells, and substantially reduced the colony-forming capacity of primary acute myeloblastic leukemia, but not normal CD34+ cells. These events were associated with pronounced Bim up-regulation, Mcl-1 down-regulation, marked Bak/Bax conformational change accompanied by Bax membrane translocation, and a pronounced increase in Bax/Bak association. Molecular studies using tet-inducible Akt, constitutively active MEK1, dominant-negative Akt, and MEK1 small interfering RNA revealed that inhibition of both MEK/ERK1/2 and Akt pathways plays a critical functional role in perifosine/PD184352-mediated lethality. Ectopic Mcl-1 expression potently inhibited perifosine/PD184352-induced apoptosis, as did Bak or Bax knockdown. Notably, knockdown of Bim, but not Bad, blocked Bak and Bax conformational change, inhibited Bax membrane translocation, diminished Bax/Bak binding, and sharply attenuated perifosine/PD184352-induced apoptosis. Finally, enforced expression of Bim significantly enhanced apoptosis induced by PI3K/Akt inhibitors, analogous to the effects of MEK1/2 inhibitors. Collectively, these findings suggest that Bim, and Mcl-1, but not Bad, integrate death signaling triggered by concomitant disruption of the PI3K/Akt and MEK1/2/ERK1/2 pathways in human leukemia cells.
Introduction
Evidence exists that dysregulated signaling pathways cooperate to promote the survival of transformed cells, and that interruption of more than one pathway may be required to maximize neoplastic cell death.1 The Ras/Raf/mitogen-activated protein kinase kinase 1/2 (MEK1/2)/extracellular signal-regulated kinase 1/2 (ERK1/2) and phosphatidylinositol 3-kinase (PI3K)/Akt cascades are frequently mutated in cancer cells, including those of hematopoietic origin,2,3 providing a rationale for their coordinated disruption as an anticancer strategy. Notably, it has recently been shown that concomitant interruption of Akt and ERK1/2 represents a potent stimulus for triggering transformed cell death.4,5
Extracellular signal-regulated kinase 1/2 (ERK1/2), a mitogen-activated protein kinase (MAPK) that lies downstream of Raf-1 and its target MEK1/2, represents a member of a MAPK family that includes p38 and c-Jun N-terminal kinase (JNK). These kinases are involved in cell proliferation, differentiation, and survival, among others. Although exceptions exist, ERK1/2 generally exerts antiapoptotic actions, whereas p38 MAPK and JNK promote apoptosis.6 The relative outputs of the JNK and ERK1/2 MAPK represent critical determinants of cell survival after environmental insults (eg, serum deprivation).7 Recently, MEK1/2 inhibitors have been developed, including PD184352 (CI-1040), its successor PD325901, and AZD6244, an agent that inhibits ERK1/2 activation in vitro and in vivo.8
Akt is a serine/threonine kinase whose activity is tightly regulated by PI3K. Akt is involved in cell survival, cell growth, and nutrient deprivation responses, among others.2 It signals downstream to glycogen synthase kinase-3α/β (GSK3α/β), m-TOR, FOXO, and other targets.2 Akt activation generally promotes cell survival due to inactivation of proapoptotic effectors such as Bad, and transcriptional repression of Bim.4,9 Recently, specific PI3K or Akt inhibitors have been developed. Among these, perifosine (NSC 63996) is an alkyl-lysophospholipid that has shown preclinical activity against both hematologic and nonhematologic malignancies10,11 and is under evaluation in leukemia and other malignancies.12 Perifosine-induced disruption of membrane localization and activation of Akt has been proposed as a mechanism of lethality.11,13 In human leukemia cells, the capacity of constitutively active Akt to rescue cells from perifosine14 implicates Akt disruption in perifosine-induced cell death.
In hematopoietic cells, the Akt and MEK1/2/ERK1/2 pathways cooperate to promote cell survival,15 raising the possibility that simultaneous interruption of these pathways may represent an effective antileukemic strategy. To test this hypothesis, we examined antileukemic interactions between perifosine and MEK1/2 inhibitors in human leukemia cells. Our results indicate that Akt and ERK1/2 pathway interruption synergistically induces apoptosis in human leukemia cells in association with Mcl-1 down-regulation, Bim accumulation, and Bax and Bak activation. They also suggest that in such cells, Bim rather than Bad plays a critical role in integrating death signals after concomitant interruption of the Akt and ERK1/2 cascades.
Methods
Cells and constructs
Human leukemia U937, HL60, MV4-11, and myeloma MM.1S cells were cultured, as previously reported.16 U937 cells overexpressing constitutively active forms of Akt (Myc-tagged myristoylated Akt),14 Jurkat cells expressing tetracycline-inducible constitutively active MEK1,16 and wild-type Bim17 constructs were previously described. Cells expressing tet-on inducible dominant-negative Akt were generated as follows: a triple mutant Akt construct in which lysine 179 was substituted with methionine and threonine 308 and serine 473 were mutated to alanine (Addgene) was subcloned into pcDNA4/TO (Invitrogen), and transfected into U937 cells stably expressing tet repressor protein using Amaxa nucleofector. Stable single-cell clones were selected with 100 μg/mL zeocin and tested for induced expression of dominant-negative Akt after 24-hour treatment with 2 μg/mL doxycycline.
Isolation of patient-derived leukemic blasts
Leukemic blasts were obtained from bone marrows of patients with acute myeloblastic leukemia (AML), French-American-British (FAB) subtype M2. These studies have been approved by the Investigational Review Board of Virginia Commonwealth University/Medical College of Virginia, and all patients provided informed consent in accordance with the Declaration of Helsinki. In each case, the percentage of blasts in the peripheral blood was more than 70%. Bone marrow was collected, and mononuclear cells were isolated, as previously described.18
Isolation of CD34+ cells
Normal bone marrow CD34+ cells were obtained with informed consent from patients undergoing routine diagnostic procedures for nonmyeloid hematopoietic disorders. CD34+ cells were isolated from mononuclear cell preparations, as previously described.19 In some experiments, normal CD34+ cells were obtained from Lonza.
Methylcellulose colony formation assays
A total of 104 normal human bone marrow CD34+ cells or 2 × 105 mononuclear cells isolated from the bone marrow of patients with AML was plated in Methocult GFH4434 (StemCell Technologies) in the presence or absence of the drugs. For CD34+ cells, after 10 days of culture, myeloid granulocyte-macrophage–colony-forming units (CFU) and CFU–erythroid and burst-forming units–erythroid were scored by morphologic assessment under an inverted microscope, as per the manufacturer's instructions. For leukemic colony formation, colonies were scored after 10 to 14 days of incubation, as previously described.20
Mutation analysis
Genomic DNA was isolated from primary AML blasts, according to the manufacturer's instructions (Qiamp; QIAGEN). Fms-like tyrosine kinase 3 (FLT3) mutation detection was performed, according to the manufactuer's instructions (Invivoscribe). Polymerase chain reaction and mutation detection by direct cycle sequencing for K-ras, phosphatidylinositol 3-kinase catalytic alpha (PIK3CA), Akt1, and phosphatase and tensin homolog (PTEN) were performed, as described previously.21-24
Reagents
Perifosine was provided by the Cancer Treatment and Evaluation Program of the National Cancer Institute. PD184352 was obtained as previously reported.25 The caspase inhibitor, zVAD-FMK, was purchased from Enzyme Systems Products. All reagents were formulated as recommended by their suppliers.
Knockdown studies involving stable transfection with short hairpin RNA and microRNA-adapted short hairpin RNA
U937 cells stably expressing short hairpin RNA (shRNA) directed against Bim, Bax, or Bak were generated, as previously described.26 The sequences used were as follows: Bax, 5′-ggtgccggaactgatcagaac-3′; Bak, 5′-ggattcagctattctggaa-3′. The constructs were verified by DNA sequencing and stably transfected into U937 cells using Amaxa nucleofector. Bim shRNA construct was previously described.26 U937 cells transfected with shRNA construct against green fluorescent protein (shGFP)26 were used as a control for various shRNA-expressing cells.
To knock down Bad, a microRNA-adapted shRNA (shRNAmir) designed against human Bad (5′-acgtgctcactaccaaatgtta-3′) was purchased from Open Biosystems and transfected into U937 cells, as above. U937 cells transfected with enhanced GFP (EGFP)–shRNAmir construct were used as controls for Bad shRNAmir cells. Knockdown MEK1 and Mcl-1 experiments were carried out by transient transfection using smart pool MEK1 or Mcl-1 small interfering RNA (siRNA) and nonspecific siRNA as a negative control (Dharmacon).
Assessment of apoptosis
Apoptotic cells were routinely identified by annexin V–fluorescein isothiocyanate staining, as previously described.27
Immunoprecipitation and immunoblotting
Cells were lysed in 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) buffer,14 after which 500 μg of protein lysates was subjected to immunoprecipitation using designated antibodies. Immunoblotting was performed using the immunoprecipitates or the whole-cell lysates, as previously described in detail.27 Primary antibodies were as follows: polyclonal Bax, Bcl-2, and Mcl-1 (BD Pharmingen); caspase-8 (Alexis); poly(adenosine 5′-diphosphate-ribose) polymerase (BIOMOL Research Laboratories); phospho-ERK1/2 (Thr202/Tyr204), phospho-Akt (Ser473), phospho-p70S6K (Thr389), phospho-p90RSK, cleaved caspase-9, and cleaved caspase-3 (Cell Signaling Technology); Akt, p70S6K, apoptosis-inducing factor (AIF), second mitochondria-derived activator of caspase/direct IAD binding protein with low pT (Smac/DIABLO), and cytochrome c (Santa Cruz Biotechnology); and Bim, Bak, and α-tubulin (Calbiochem).
Bax and Bak conformational change
Cells were lysed in CHAPS buffer, and protein lysates were subjected to immunoprecipitation using anti-Bax 6A7 (Sigma-Aldrich) or anti-Bak Ab-1 antibodies (Calbiochem) that recognize only Bax or Bak protein that has undergone conformational change. Immunoprecipitates were then subjected to immunoblotting analysis using anti-Bax or anti-Bak polyclonal antibodies.
Subcellular fractionation
Cytosolic and membrane fractions were separated as previously described.18
Statistical analysis
The significance of differences between experimental conditions was determined using Student t test for unpaired observations. Synergistic interactions were evaluated using median dose effect analysis using the Calcusyn software program (Biosoft).28
Results
MEK1/2 inhibitors synergistically potentiate perifosine lethality in malignant hematologic cells
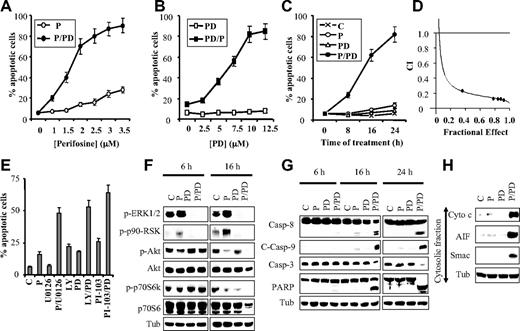
Recently, several groups, including our own, have reported that perifosine increases MEK1/2/ERK1/2 activation in various cells,11,14,29 prompting the hypothesis that MEK1/2/ERK1/2 activation might attenuate perifosine lethality. To test this hypothesis, U937 cells were exposed to various perifosine concentrations with or without 10μM PD184352 for 24 hours, after which apoptosis was monitored by annexin V/propidium iodide (PI). Whereas PD184352 was essentially nontoxic, it substantially increased the lethality of 1.5μM perifosine, and induced very extensive apoptosis at perifosine concentrations equal to or higher than 2.0μM (Figure 1A). Perifosine alone (1.0-3.5μM) exerted only minimal toxicity. PD184352 (5μM) significantly increased apoptosis by 2.5μM perifosine, and PD184352 concentrations of 10.0μM resulted in near-maximal lethality (Figure 1B). Time course analysis of cells exposed simultaneously to 2.5μM perifosine and 10 μM PD184352 revealed a very modest increase in cell death at 8 hours, but more extensive lethality after 16 to 24 hours (Figure 1C). Median dose effect analysis of cells exposed to perifosine and PD184352 for 24 hours at a fixed ratio yielded combination index values considerably less than 1.0, indicating a highly synergistic interaction (Figure 1D). Parallel studies revealed that coexposure of U937 cells to perifosine and PD184352 for 24 hours resulted in a marked diminution in total viable cell numbers, whereas individual treatment had only modest effects (supplemental Figure 1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). In addition, [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] assays, which reflect both cell proliferation and viability, produced similar results (supplemental Figure 1B). Combined exposure to the MEK1/2 inhibitor U0126 and perifosine, or to PD184352 and PI3K inhibitor PI-103 or LY294002, also markedly increased apoptosis in U937 cells (Figure 1E). Lastly, parallel studies revealed that simultaneous administration of perifosine and PD184352 also resulted in a pronounced increase in cell death in MV4-11 leukemia cells (FLT3 mutated), HL-60 promyelocytic leukemia cells, and MM.1S multiple myeloma cells (supplemental Figure 2).
Inhibition of MEK/ERK activity strikingly enhances perifosine-mediated apoptosis in human leukemia cells. (A) U937 cells were exposed for 24 hours to the designated concentration of perifosine (P) alone (○) or in conjunction with 10μM PD184352 (PD, ●), after which the percentage of apoptotic cells was determined by annexin V analysis, as described in “Assessment of apoptosis.” (B) U937 cells were exposed to the designated concentration of PD184352 alone (□) or in combination with 2.5μM perifosine (■), after which apoptosis was determined, as above. (C) Cells were exposed to perifosine (2.5μM) and PD184352 (10μM) alone or in combination for the indicated intervals, after which the percentage of apoptotic cells was determined as above. (D) Median dose effect analysis of apoptosis induction by perifosine and PD184352. U937 cells were exposed to various concentrations of PD184352 and perifosine at a fixed ratio (4:1), after which apoptosis was monitored at 24 hours by annexin V/PI analysis. Combination index values were determined in relation to the fractional effect using a commercially available software program, as described in “Statistical analysis.” Combination index values less than 1.0 correspond to a synergistic interaction. (E) U937 cells were exposed to perifosine (2.5μM) and U0126 (10μM), PD184352 (10μM), LY294002 (LY; 20μM), and PI-103 (3μM) either individually or in the designated combinations for 24 hours, after which the percentage of apoptotic cells was determined by annexin V analysis. (F-G) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for the indicated intervals, after which protein lysates were prepared and subjected to Western blot analysis using designated antibodies. For this and all subsequent Western blot analysis, blots were subsequently reprobed with anti-tubulin (Tub) antibodies to document equivalent loading and transfer. The results of a representative study are shown; 2 additional experiments yielded equivalent results. (H) Cells were treated with PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 16 hours, after which mitochondria-free cytosolic fractions were obtained, as described in “Subcellular fractionation,” and subjected to Western blot analysis to monitor release of cytochrome c, AIF, and Smac/DIABLO into the cytosol.
Inhibition of MEK/ERK activity strikingly enhances perifosine-mediated apoptosis in human leukemia cells. (A) U937 cells were exposed for 24 hours to the designated concentration of perifosine (P) alone (○) or in conjunction with 10μM PD184352 (PD, ●), after which the percentage of apoptotic cells was determined by annexin V analysis, as described in “Assessment of apoptosis.” (B) U937 cells were exposed to the designated concentration of PD184352 alone (□) or in combination with 2.5μM perifosine (■), after which apoptosis was determined, as above. (C) Cells were exposed to perifosine (2.5μM) and PD184352 (10μM) alone or in combination for the indicated intervals, after which the percentage of apoptotic cells was determined as above. (D) Median dose effect analysis of apoptosis induction by perifosine and PD184352. U937 cells were exposed to various concentrations of PD184352 and perifosine at a fixed ratio (4:1), after which apoptosis was monitored at 24 hours by annexin V/PI analysis. Combination index values were determined in relation to the fractional effect using a commercially available software program, as described in “Statistical analysis.” Combination index values less than 1.0 correspond to a synergistic interaction. (E) U937 cells were exposed to perifosine (2.5μM) and U0126 (10μM), PD184352 (10μM), LY294002 (LY; 20μM), and PI-103 (3μM) either individually or in the designated combinations for 24 hours, after which the percentage of apoptotic cells was determined by annexin V analysis. (F-G) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for the indicated intervals, after which protein lysates were prepared and subjected to Western blot analysis using designated antibodies. For this and all subsequent Western blot analysis, blots were subsequently reprobed with anti-tubulin (Tub) antibodies to document equivalent loading and transfer. The results of a representative study are shown; 2 additional experiments yielded equivalent results. (H) Cells were treated with PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 16 hours, after which mitochondria-free cytosolic fractions were obtained, as described in “Subcellular fractionation,” and subjected to Western blot analysis to monitor release of cytochrome c, AIF, and Smac/DIABLO into the cytosol.
Combined exposure of human leukemia cells to perifosine and PD184352 results in Akt and MEK/ERK1/2 inactivation in association with marked caspase activation and mitochondrial injury
As previously observed by several groups, including our own,11,14,29 exposure of U937 cells to perifosine alone for 6 or 16 hours resulted in ERK1/2 activation (Figure 1F), manifested by increased phosphorylation of ERK1/2 and its downstream target p90RSK. Coexposure to PD184352 essentially abrogated basal and perifosine-stimulated ERK1/2 activation. In contrast, perifosine markedly diminished Akt phosphorylation, an event also observed in cells coexposed to PD184352. Perifosine/PD184352-mediated inhibition of MEK/ERK1/2 and PI3K/Akt was associated with diminished phosphorylation of the mTOR substrate p70S6K (Figure 1F); cleavage/activation of procaspases-8, -9, and -3; and poly(adenosine 5′-diphosphate-ribose) polymerase degradation, particularly after 16 and 24 hours (Figure 1G). Consistent with these findings, individual exposure (16 hours) of cells to PD184352 or perifosine minimally induced cytosolic release of cytochrome c, AIF, and Smac/DIABLO, whereas effects were pronounced with combined treatment (Figure 1H).
Akt and MEK inactivation contribute functionally to perifosine/PD184352 lethality
To assess the functional significance of Akt inactivation in PD184352/perifosine lethality, U937 cells ectopically expressing a myristoylated, constitutively active form of Akt (Akt-CA4 and Akt-CA7) were used.14 Akt-CA4 and Akt-CA7 cells displayed persistent expression of phosphorylated Akt and its downstream target GSK3α/β after PD184352/perifosine exposure, whereas empty-vector cells (pUSEamp) did not (Figure 2A). Notably, both Akt-CA4 and Akt-CA7 cells were significantly less sensitive to PD184352/perifosine-mediated lethality than pUSEamp controls (Figure 2B; P < .01). Conversely, induction of a doxycycline-responsive dominant-negative Akt construct with doxycyline (Figure 2C, inset) markedly enhanced PD184352-mediated apoptosis (P < .02; Figure 2C). Furthermore, knockdown of MEK1 with siRNA significantly enhanced perifosine-mediated cell death (P < .05; Fig 2D). Collectively, these findings argue that Akt and MEK inactivation play critical functional roles in cell death induced by perifosine and MEK inhibitors in human leukemia cells.
MEK and Akt inactivation plays a key role in perifosine/PD-mediated lethality, and this treatment induces apoptosis in and diminishes the colony-forming ability of primary AML cells. (A) Two clones (Akt-CA4 and Akt-CA7) of U937 cells expressing constitutively active Akt (myristoylated Akt) and the empty-vector pUSEamp were left untreated or treated with perifosine and PD184352 for 16 hours, after which protein lysates were prepared and subjected to Western blot analysis to monitor the levels of total Akt and the degree of its phosphorylation. Endo-Akt indicates endogenous Akt. Alternatively, extent of apoptosis was determined using annexin V analysis (B). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for empty-vector pUSEamp cells (P < .01). (C) U937 cells inducibly expressing dominant-negative Akt were left untreated or treated for 48 hours with 2 μg/mL doxycycline, after which the cells were either lysed and Western blot performed (C inset) or exposed to PD184352 (10 or 15μM) for an additional 24 hours and then subjected to annexin V/PI analysis. Values represent the means for 3 separate experiments ± SD. *Significantly higher than values obtained in the absence of doxycycline (P < .02). (D) U937 cells were transiently transfected with siRNA against MEK1 (siMEK1) or negative control siRNA (siNC) for 48 hours and then treated with 3μM perifosine or 10μM PD184352. The extent of apoptosis was monitored at 48 hours after treatment using annexin V staining assay. Values represent the means for 3 separate experiments ± SD. *Significantly higher than values obtained for siNC-transfected cells (P < .05). (E) Leukemic blasts were isolated, as described in “Isolation of patient-derived leukemic blasts,” from the bone marrow of 4 patients with AML (FAB classification M2), exposed to perifosine (7.5μM) and PD (10μM) for 24 hours, after which the extent of apoptosis was determined using annexin V analysis. (F) Alternatively, protein lysates were prepared and subjected to Western blot analysis. (G) Normal CD34+ cells were isolated, as described in “Isolation of CD34+ cells,” from the bone marrow of normal subjects (nonleukemic) and exposed to perifosine (7.5μM) and PD184352 (10μM) alone or in combination for 24 hours, after which cell viability was determined by flow cytometry using the annexin V staining assay. (H) Primary AML (patient nos. 2 and 4) and normal CD34+ cells (Lonza) were plated in methylcellulose in the presence of perifosine (7.5μM) and PD184352 (10μM) alone or in combination for 10 to 14 days, after which the CFUs were enumerated and expressed as a percentage of untreated cells, as described in “Methylcellulose colony formation assays.” (E-G) Each sample was analyzed in triplicate; values represent the means ± SD.
MEK and Akt inactivation plays a key role in perifosine/PD-mediated lethality, and this treatment induces apoptosis in and diminishes the colony-forming ability of primary AML cells. (A) Two clones (Akt-CA4 and Akt-CA7) of U937 cells expressing constitutively active Akt (myristoylated Akt) and the empty-vector pUSEamp were left untreated or treated with perifosine and PD184352 for 16 hours, after which protein lysates were prepared and subjected to Western blot analysis to monitor the levels of total Akt and the degree of its phosphorylation. Endo-Akt indicates endogenous Akt. Alternatively, extent of apoptosis was determined using annexin V analysis (B). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for empty-vector pUSEamp cells (P < .01). (C) U937 cells inducibly expressing dominant-negative Akt were left untreated or treated for 48 hours with 2 μg/mL doxycycline, after which the cells were either lysed and Western blot performed (C inset) or exposed to PD184352 (10 or 15μM) for an additional 24 hours and then subjected to annexin V/PI analysis. Values represent the means for 3 separate experiments ± SD. *Significantly higher than values obtained in the absence of doxycycline (P < .02). (D) U937 cells were transiently transfected with siRNA against MEK1 (siMEK1) or negative control siRNA (siNC) for 48 hours and then treated with 3μM perifosine or 10μM PD184352. The extent of apoptosis was monitored at 48 hours after treatment using annexin V staining assay. Values represent the means for 3 separate experiments ± SD. *Significantly higher than values obtained for siNC-transfected cells (P < .05). (E) Leukemic blasts were isolated, as described in “Isolation of patient-derived leukemic blasts,” from the bone marrow of 4 patients with AML (FAB classification M2), exposed to perifosine (7.5μM) and PD (10μM) for 24 hours, after which the extent of apoptosis was determined using annexin V analysis. (F) Alternatively, protein lysates were prepared and subjected to Western blot analysis. (G) Normal CD34+ cells were isolated, as described in “Isolation of CD34+ cells,” from the bone marrow of normal subjects (nonleukemic) and exposed to perifosine (7.5μM) and PD184352 (10μM) alone or in combination for 24 hours, after which cell viability was determined by flow cytometry using the annexin V staining assay. (H) Primary AML (patient nos. 2 and 4) and normal CD34+ cells (Lonza) were plated in methylcellulose in the presence of perifosine (7.5μM) and PD184352 (10μM) alone or in combination for 10 to 14 days, after which the CFUs were enumerated and expressed as a percentage of untreated cells, as described in “Methylcellulose colony formation assays.” (E-G) Each sample was analyzed in triplicate; values represent the means ± SD.
Cotreatment with perifosine and PD184352 results in apoptosis induction and a marked reduction in colony formation of primary AML blasts while largely sparing normal CD34+ cells
Parallel studies revealed that simultaneous administration of perifosine and PD184352 produced at least additive increases in cell death in primary leukemic blasts isolated from 6 of 8 patients with AML (FAB classification M2). Four representative samples, including 3 responding (nos. 1, 2, and 4) specimens and one nonresponding specimen (no. 3), are shown in Figure 2E. Western blot analysis performed on extracts obtained from these specimens revealed that all responding samples displayed discernible basal Akt and ERK1/2 phosphorylation (supplemental Figure 3A). Analysis of specimens nos. 2 and 4 revealed further activation of ERK1/2 after perifosine treatment, and inactivation of both Akt and ERK1/2 with perifosine/PD184352 exposure, analogous to results obtained in cell lines (Figure 2F). In contrast, nonresponding specimen no. 3 displayed minimal basal Akt and ERK1/2 phosphorylation, and no ERK1/2 activation with perifosine (supplemental Figure 3A-B). Mutation analysis revealed that 3 responding specimens harbored FLT3 mutations, including internal tandem duplication (FLT3/ITD) in 2 patients (nos. 2 and 6) and a point mutation (FLT3/D835) in one patient (no. 5). Of these, 2 of 2 with a sufficient number of assayable cells displayed basal Akt and ERK1/2 activation (supplemental Figure 3A and data not shown). In contrast, no mutations were detected in K-ras, PIK3CA, Akt1, or PTEN in any sample (supplemental Table 1).
Interestingly, identical perifosine and PD184352 exposures, alone or in combination, exhibited minimal toxicity toward normal CD34+ cells (Figure 2G). Furthermore, normal CD34+ cell colony formation was not substantially affected by perifosine and PD184352, although PD184352 slightly diminished myeloid lineage colonies (Figure 2H). In contrast, a marked decrease in AML colony formation was observed after treatment with perifosine or PD184352 alone, and this was more pronounced with the combination (Figure 2H). Together these observations suggest that dual disruption of MEK/ERK1/2 and PI3K/Akt may effectively induce cell death in leukemia cells exhibiting activation of both pathways.
Perifosine/PD184352-mediated lethality involves Bax and Bak
In general, apoptotic signals converge toward the multidomain proapoptotic proteins Bax and Bak. Whereas Bak is localized in the organelle membranes, particularly in the mitochondria, where it undergoes conformational change upon apoptotic stimuli, Bax is primarily distributed in the cytosol in nonapoptotic cells. Upon diverse apoptotic stimuli, Bax undergoes conformational change, accompanied by translocation into the mitochondrial membrane, promoting the release of proapoptotic mitochondrial proteins (eg, cytochrome c) into the cytosol and inducing apoptosis.30,31 Therefore, the effects of combined ERK1/2 and PI3K/Akt inhibition were examined in relation to Bak and Bax conformational change and intracellular Bax localization. Whereas treatment (16 hours) with perifosine or PD184352 minimally induced Bak or Bax conformational change, effects of combined treatment were pronounced (Figure 3A). Similarly, individual agents only modestly induced Bax translocation, whereas combined treatment dramatically increased mitochondrial translocation (Figure 3A). In contrast, perifosine and PD184352 treatment had minimal effects on Bax or Bak expression (Figure 3B). Furthermore, combined treatment with perifosine and PD184352 resulted in a pronounced increase in Bak/Bax association, whereas no effects were observed when perifosine or PD184532 was administered individually (Figure 3C). These findings indicate that perifosine and PD184352 cooperate to promote Bax and Bak conformational change, Bax mitochondrial translocation, and Bak/Bax binding. To investigate the functional roles of Bax and Bak in perifosine/PD184352-mediated apoptosis, Bax or Bak was knocked down in U937 cells by stably transfecting them with shRNA (Figure 3D). Notably, Bax or Bak knockdown cells displayed significant resistance to perifosine/PD184352-mediated apoptosis, comparable with resistance to VP16 (Figure 3E). These findings indicate that Bax and Bak play significant functional roles in apoptosis induced by simultaneous MEK1/2/ERK1/2 and PI3K/Akt inhibition.
Exposure to perifosine/PD184352 results in Bak and Bax conformational change, whereas knockdown of these molecules markedly attenuates apoptosis. (A) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 16 hours, after which cells were lysed in buffer containing 1% CHAPS; conformationally changed Bak and Bax protein were immunoprecipitated using anti-Bak Ab1 and anti-Bax 6A7 antibodies, respectively, and subjected to Western blot analysis using polyclonal Bak and Bax antibodies (top panel). Alternatively, cytosolic and membrane fractions were separated, as indicated in “Subcellular fractionation,” and subjected to Western blot analysis (bottom panels). (B) U937 cells were exposed to PD184352 and perifosine alone or in combination for 6 or 16 hours, after which protein lysates were prepared and subjected to Western blot analysis. (C) U937 cells were treated and lysed as in panel A and subjected to immunoprecipitation using anti-Bak antibodies. The immunoprecipitates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with polyclonal Bax antibodies. Input lysates were subjected to Western blot and probed with Bak antibodies. (D) U937 cells stably transfected with shGFP, Bax (shBax), or Bak (shBak) were treated with perifosine and PD184352, or with VP16 (2.5μM) for 24 hours, after which protein lysates were prepared and subjected to Western blot analysis. Alternatively, the extent of apoptosis was determined using annexin V staining (E). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for shGFP cells (P < .01).
Exposure to perifosine/PD184352 results in Bak and Bax conformational change, whereas knockdown of these molecules markedly attenuates apoptosis. (A) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 16 hours, after which cells were lysed in buffer containing 1% CHAPS; conformationally changed Bak and Bax protein were immunoprecipitated using anti-Bak Ab1 and anti-Bax 6A7 antibodies, respectively, and subjected to Western blot analysis using polyclonal Bak and Bax antibodies (top panel). Alternatively, cytosolic and membrane fractions were separated, as indicated in “Subcellular fractionation,” and subjected to Western blot analysis (bottom panels). (B) U937 cells were exposed to PD184352 and perifosine alone or in combination for 6 or 16 hours, after which protein lysates were prepared and subjected to Western blot analysis. (C) U937 cells were treated and lysed as in panel A and subjected to immunoprecipitation using anti-Bak antibodies. The immunoprecipitates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with polyclonal Bax antibodies. Input lysates were subjected to Western blot and probed with Bak antibodies. (D) U937 cells stably transfected with shGFP, Bax (shBax), or Bak (shBak) were treated with perifosine and PD184352, or with VP16 (2.5μM) for 24 hours, after which protein lysates were prepared and subjected to Western blot analysis. Alternatively, the extent of apoptosis was determined using annexin V staining (E). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for shGFP cells (P < .01).
Mcl-1 down-regulation is required for perifosine/PD184352-mediated apoptosis
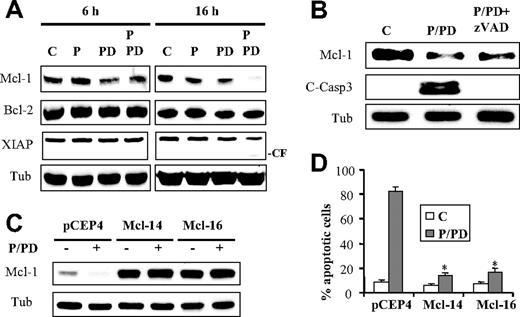
In view of the critical role of Mcl-1 in malignant hematopoietic cell survival,32,33 expression and function of this antiapoptotic protein were examined in relation to treatment with perifosine and PD184352. As shown in Figure 4A, expression of Mcl-1 was slightly reduced after exposure to PD184352 with or without perifosine for 6 hours, but was substantially diminished in cells exposed to both agents for 16 hours. Dose-response studies showed marked Mcl-1 down-regulation by 10 μM PD184352, which abrogated ERK1/2 phosphorylation, but diminished effects with PD184352 concentrations up to 5 μM (data not shown). No changes were noted in the levels of Bcl-2 protein, or other antiapoptotic family members Bcl-xL (data not shown) or X-linked inhibitor of apoptosis (XIAP), although a cleaved fragment of the latter was noted after 16 hours of combined treatment (Figure 4A). Because Mcl-1 represents a caspase target,34 the caspase dependence of PD184352/perifosine-mediated Mcl-1 down-regulation was examined. Addition of the pan-caspase inhibitor zVAD-fmk, which blocked caspase-3 cleavage, had little effect on reduced Mcl-1 expression in cells exposed to PD184352/perifosine (Figure 4B), arguing that Mcl-1 down-regulation is unlikely to reflect caspase-mediated cleavage.
Perifosine/PD184352-mediated lethality involves Mcl-1 down-regulation. (A) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 6 or 16 hours, after which protein lysates were prepared and subjected to Western blot analysis using designated antibodies (CF = cleavage fragment). (B) U937 cells were pretreated with zVAD-fmk (25μM) for 1 hour before treatment with perifosine and PD184352 for 16 hours. Then Mcl-1 protein levels and caspase-3 cleavage were monitored by Western blot analysis. (C) Two clones (Mcl1-14 and Mcl1-16) of U937 cells overexpressing Mcl-1 and empty-vector cells (pCEP4) were treated with perifosine and PD184352 for 16 hours, after which Mcl-1 protein levels were monitored by Western blot analysis. Alternatively, the extent of apoptosis was monitored at 24 hours after treatment using annexin V staining assay (D). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for empty-vector pCEP4 cells (P < .005).
Perifosine/PD184352-mediated lethality involves Mcl-1 down-regulation. (A) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 6 or 16 hours, after which protein lysates were prepared and subjected to Western blot analysis using designated antibodies (CF = cleavage fragment). (B) U937 cells were pretreated with zVAD-fmk (25μM) for 1 hour before treatment with perifosine and PD184352 for 16 hours. Then Mcl-1 protein levels and caspase-3 cleavage were monitored by Western blot analysis. (C) Two clones (Mcl1-14 and Mcl1-16) of U937 cells overexpressing Mcl-1 and empty-vector cells (pCEP4) were treated with perifosine and PD184352 for 16 hours, after which Mcl-1 protein levels were monitored by Western blot analysis. Alternatively, the extent of apoptosis was monitored at 24 hours after treatment using annexin V staining assay (D). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for empty-vector pCEP4 cells (P < .005).
To assess the functional significance of Mcl-1 down-regulation in perifosine/PD184352-induced lethality, U937 cells ectopically expressing Mcl-1 (Mcl-14 and Mcl-16) were used. Notably, these cells were considerably more resistant to Mcl-1 down-regulation after perifosine/PD184352 exposure than their empty-vector counterparts (Figure 4C; pCEP4). Furthermore, ectopic expression of Mcl-1 (eg, in Mcl-14 and Mcl-16 cells) dramatically reduced the lethality of the perifosine/PD184352 regimen compared with empty-vector controls (Figure 4D; P < .005). Conversely, Mcl-1 knockdown with siRNA enhanced perifosine/PD184352-mediated lethality (supplemental Figure 4). These findings suggest that Mcl-1 down-regulation in cells exposed to PD184352/perifosine plays a significant functional role in the lethality of this regimen.
Bim, but not Bad, activation is required for perifosine/PD184352-mediated Bak and Bax conformational change, membrane translocation of Bax, and apoptosis
In view of the central roles that the BH3-only proteins Bim (Bcl-2–interacting mediator of cell death), Bad (Bcl-2 antagonist of cell death), and Bid play in apoptosis induced by various stimuli, including disruption of the MEK/ERK1/2 or Akt pathways,4,17 expression and phosphorylation of these proteins were examined. Perifosine increased Bad phosphorylation at 6 hours, a phenomenon that presumably stems from MEK1/2/ERK1/2 activation in view of its abrogation by PD184352 (Figure 5A), events previously associated with apoptosis induction.4 Expression of Bid, which is cleaved by caspases during apoptosis, displayed no change after 6-hour exposure to both agents, but was clearly reduced after 16 hours (Figure 5A). Notably, PD184352 diminished Bim phosphorylation and induced a pronounced accumulation of Bim at 6 hours as well as 16 hours. Bim accumulation and Mcl-1 down-regulation were also observed in MV4-11 cells (supplemental Figure 5) and in AML cells obtained from 2 patients (specimens nos. 2 and 4; Figure 5B), which displayed high basal MEK/ERK1/2 and Akt activation, as well as increased cell death after perifosine/PD184352 exposure (Figure 2F and supplemental Figure 3A). However, no major changes were observed in AML specimen no. 3 (Figure 5B), which exhibited minimal basal Akt or ERK1/2 phosphorylation (supplemental Figure 3A-B), and which did not show increased lethality with combined exposure (Figure 2F). In addition, inducible activation of a constitutively active MEK1 construct with doxycyline resulted in a pronounced Bim down-regulation (Figure 5C left panel) and markedly diminished apoptosis by the PI3K/Akt inhibitor perifosine or LY294002 (Figure 5C right panel). These findings support the notion that Bim expression is negatively regulated by ERK1/2 activation.17
Knockdown of Bim significantly diminishes perifosine/PD184352-mediated cell death. (A) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 6 or 16 hours, after which protein lysates were prepared and subjected to Western blot analysis using designated antibodies. (B) Primary AML mononuclear cells isolated from 3 patients were treated with perifosine (7.5μM) and PD184352 (10μM) alone or in combination for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor Bim and Mcl-1 protein levels. (C left panel) Jurkat cells (MT6) inducibly expressing constitutively active MEK1 were left untreated or treated for 48 hours with 2 μg/mL doxycycline, after which cells were analyzed for phospho-ERK1/2 and Bim expression by Western blot. Alternatively, cells were treated with perifosine (8μM) or LY294002 (50μM) for 48 hours, after which cell death was assessed by annexin V analysis (C right panel). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained in the absence of doxycycline (P < .01). (D) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5 μM) alone or in combination for 16 hours, after which cells were lysed in CHAPS buffer and subjected to immunoprecipitation assay using Bim antibodies. The immunoprecipitates were then subjected to immunoblot analysis with either Mcl-1 or Bim antibodies. Input lysates were also subjected to Western blot analysis to monitor Bim levels. (E) Two clones (shBim-8 and shBim-13) of U937 cells in which Bim was knocked down using shRNA and their control counterpart shGFP-transfected cells were treated with perifosine and PD184352 alone or in combination for 24 hours, after which the extent of apoptosis was determined using the annexin V staining assay. Alternatively, protein lysates were prepared and Western blot was performed (F). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for shGFP-transfected cells; P < .01. (C inset) Western blot performed on lysates prepared from these cells before treatment.
Knockdown of Bim significantly diminishes perifosine/PD184352-mediated cell death. (A) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5μM) alone or in combination for 6 or 16 hours, after which protein lysates were prepared and subjected to Western blot analysis using designated antibodies. (B) Primary AML mononuclear cells isolated from 3 patients were treated with perifosine (7.5μM) and PD184352 (10μM) alone or in combination for 24 hours, after which cells were lysed and subjected to Western blot analysis to monitor Bim and Mcl-1 protein levels. (C left panel) Jurkat cells (MT6) inducibly expressing constitutively active MEK1 were left untreated or treated for 48 hours with 2 μg/mL doxycycline, after which cells were analyzed for phospho-ERK1/2 and Bim expression by Western blot. Alternatively, cells were treated with perifosine (8μM) or LY294002 (50μM) for 48 hours, after which cell death was assessed by annexin V analysis (C right panel). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained in the absence of doxycycline (P < .01). (D) U937 cells were exposed to PD184352 (10μM) and perifosine (2.5 μM) alone or in combination for 16 hours, after which cells were lysed in CHAPS buffer and subjected to immunoprecipitation assay using Bim antibodies. The immunoprecipitates were then subjected to immunoblot analysis with either Mcl-1 or Bim antibodies. Input lysates were also subjected to Western blot analysis to monitor Bim levels. (E) Two clones (shBim-8 and shBim-13) of U937 cells in which Bim was knocked down using shRNA and their control counterpart shGFP-transfected cells were treated with perifosine and PD184352 alone or in combination for 24 hours, after which the extent of apoptosis was determined using the annexin V staining assay. Alternatively, protein lysates were prepared and Western blot was performed (F). Values represent the means for 3 separate experiments ± SD. *Significantly lower than values obtained for shGFP-transfected cells; P < .01. (C inset) Western blot performed on lysates prepared from these cells before treatment.
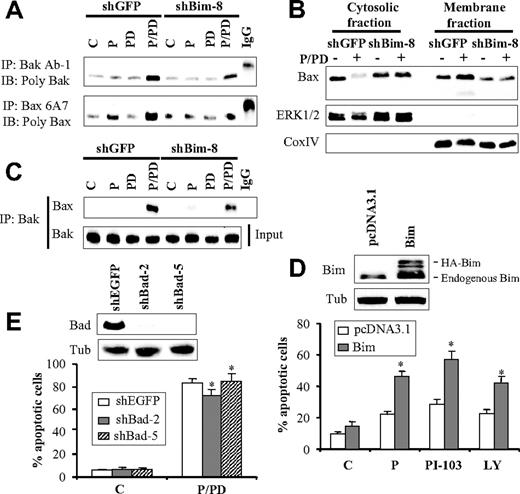
Because Mcl-1 may exert its antiapoptotic activity by neutralizing Bim, the effects of these agents on binding of Bim to Mcl-1 were assessed by coimmunoprecipitation assays. Treatment of cells with PD184352 markedly increased the amount of Bim sequestered by Mcl-1 (Figure 5D). Notably, this effect was substantially attenuated by cotreatment with perifosine. Given the findings that cotreatment with perifosine and PD184352 increased Bim protein levels and diminished Bim sequestration by Mcl-1, the functional role of Bim in perifosine/PD184352-mediated lethality was investigated. To this end, U937 cells were stably transfected with a Bim-specific shRNA construct. As shown in Figure 5E (inset), 2 clones exhibiting significant Bim knockdown were significantly more resistant to perifosine/PD184352-mediated lethality than their control counterparts (Figure 5E). Caspase-9 and caspase-3 activation was significantly attenuated in Bim knockdown cells, reflected by a marked diminution in their respective cleavage products compared with control cells (Figure 5F). Furthermore, Bak and Bax conformation analysis revealed that Bim knockdown markedly attenuated perifosine/PD184352-mediated Bak and Bax conformational change (Figure 6A). Consistent with these findings, perifosine/PD184352-mediated Bax translocation to the mitochondria was essentially abrogated (Figure 6B), and Bak/Bax association was largely inhibited in Bim knockdown cells (Figure 6C). Together, these findings suggest that Bim plays an important functional role in perifosine/PD184352-mediated lethality in U937 cells by promoting Bak and Bax conformational change, mitochondrial translocation of Bax, and Bak/Bax binding. To evaluate further the functional role of Bim in perifosine/PD184252-mediated apoptosis, Bim expression was enforced in U937 cells by stable transfection with a Bim construct (Figure 6D top panel). Notably, these cells were significantly more sensitive to the lethal effects of the PI3K/Akt inhibitor perifosine PI-103 or LY294002 compared with control cells transfected with a pcDNA3.1 construct (P < .05 in each case; Figure 6D bottom panel).
Role of Bim in Bak and Bax activation and apoptosis mediated by perifosine and PD184352 cotreatment. (A) U937 cells in which Bim was knocked down with shRNA and their control counterpart shGFP-transfected cells were exposed to perifosine (2.5μM) and PD184352 (10μM) for 16 hours, after which cells were lysed in CHAPS buffer and subjected to Bak and Bax conformation analysis, as indicated in “Bax and Bak conformational change.” Alternatively, protein lysates were prepared from cytosolic and membrane fractions, as described in “Subcellular fractionation,” and subjected to Western blot analysis (B). (C) Bim knockdown cells and their control counterparts were treated and lysed as in (A) and analyzed for Bak and Bax binding, as described in Figure 3C. (D) U937 cells were stably transfected with a wild-type Bim construct, and clones exhibiting increases in Bim expression were pooled and used for subsequent experiments (D top panel). Bim-overexpressing cells were exposed to perifosine (2.5μM), PI-103 (3μM), or LY294002 (20μM) for 24 hours, after which the extent of apoptosis was monitored using annexin V analysis assay (D bottom panel). Values represent the means for 3 separate experiments ± SD. *Significantly higher than values for pcDNA3.1-transfected cells (P < .05 in each case). (E) Protein lysates were prepared from 2 clones (shBad-2 and shBad-5) of U937 cells in which Bad was knocked down using shRNAmir, or their control counterparts transfected with EGFP-shRNAmir (shEGFP), after which they were subjected to Western blot analysis (E top panel). These cells were treated with perifosine and PD184352 for 24 hours, after which the extent of cell death was monitored by the annexin V analysis assay (D bottom panel). Values represent the means for 3 separate experiments ± SD. *Not significantly different from values for shEGFP-transfected cells (P > .05 in each case).
Role of Bim in Bak and Bax activation and apoptosis mediated by perifosine and PD184352 cotreatment. (A) U937 cells in which Bim was knocked down with shRNA and their control counterpart shGFP-transfected cells were exposed to perifosine (2.5μM) and PD184352 (10μM) for 16 hours, after which cells were lysed in CHAPS buffer and subjected to Bak and Bax conformation analysis, as indicated in “Bax and Bak conformational change.” Alternatively, protein lysates were prepared from cytosolic and membrane fractions, as described in “Subcellular fractionation,” and subjected to Western blot analysis (B). (C) Bim knockdown cells and their control counterparts were treated and lysed as in (A) and analyzed for Bak and Bax binding, as described in Figure 3C. (D) U937 cells were stably transfected with a wild-type Bim construct, and clones exhibiting increases in Bim expression were pooled and used for subsequent experiments (D top panel). Bim-overexpressing cells were exposed to perifosine (2.5μM), PI-103 (3μM), or LY294002 (20μM) for 24 hours, after which the extent of apoptosis was monitored using annexin V analysis assay (D bottom panel). Values represent the means for 3 separate experiments ± SD. *Significantly higher than values for pcDNA3.1-transfected cells (P < .05 in each case). (E) Protein lysates were prepared from 2 clones (shBad-2 and shBad-5) of U937 cells in which Bad was knocked down using shRNAmir, or their control counterparts transfected with EGFP-shRNAmir (shEGFP), after which they were subjected to Western blot analysis (E top panel). These cells were treated with perifosine and PD184352 for 24 hours, after which the extent of cell death was monitored by the annexin V analysis assay (D bottom panel). Values represent the means for 3 separate experiments ± SD. *Not significantly different from values for shEGFP-transfected cells (P > .05 in each case).
Finally, previous studies in human breast cancer cells indicated that the proapoptotic protein Bad played a central role in lethality due to dual inhibition of PI3K/Akt and MEK/ERK1/2 pathways.4 However, Bad knockdown studies with shRNAmir revealed that U937 cells in which Bad was essentially eliminated (Figure 6E top panel) remained fully sensitive to perifosine/PD184352-mediated apoptosis (Figure 6E bottom panel). These findings suggest that Bad does not play a major role in perifosine/PD184352-mediated apoptosis, at least in these human leukemia cells.
Discussion
The generally cytoprotective role played by the MEK1/2/ERK1/2 and PI3K/Akt signaling cascades has focused attention on the use of MEK1/2 and PI3K/Akt inhibitors to enhance the activity of conventional cytotoxic agents. However, it has been shown that simultaneous interruption of 2 complementary signaling pathways potently induces apoptosis in transformed cells.1 In this context, concomitant interruption of the MEK1/2/ERK1/2 (ie, by PD184352) and PI3K/Akt (ie, by perifosine) pathways represents a particularly attractive strategy. In support of this notion, synergistic induction of apoptosis by inhibitors of these pathways was observed in various human hematopoietic malignant cell lines, as well as in primary AML blasts. Significantly, this treatment substantially reduced the colony-forming capacity of 2 AML patient specimens, but minimally reduced normal CD34+ cells colony formation. These findings suggest that PI3K/Akt and ERK1/2 pathways cooperate to promote leukemia cell survival.4,15 Notably, induction of a tet-responsive dominant-negative Akt construct markedly enhanced MEK1/2 inhibitor-mediated lethality, whereas a constitutively active Akt construct sharply reduced perifosine/PD184352 lethality. Conversely, knockdown of MEK1 with siRNA markedly enhanced perifosine-mediated cell death, whereas inducible expression of constitutively active MEK1 strikingly reduced cell death mediated by PI3K/Akt inhibitors (ie, perifosine or LY294002). Consistent with previous studies using the mTOR inhibitor rapamycin or RAD001, perifosine also induced clear ERK1/2 activation, raising the possibility that perifosine may activate MEK/ERK1/2 through its inhibitory action on Akt/mTOR/p70S6K, leading to the activation of the PI3K/Ras pathway.35 Collectively, these findings reinforce the notion that disruption of a single pathway, that is, PI3K/Akt or MEK/ERK1/2, may not be optimal for efficient leukemic cell killing; instead, both pathways may need to be disabled. It is important to note that both ERK1/2 and Akt phosphorylate and regulate the expression of various Bcl-2 family members involved in cell death/survival decisions, including the short-lived antiapoptotic multidomain Mcl-1 and the BH3-only domain Bad and Bim, among others.4,9,36 It is, therefore, plausible that death signaling triggered by simultaneous PI3K and MEK1/2/ERK1/2 pathway interruption may be integrated at the level of one or more of such Bcl-2 family members.
In the present study, Mcl-1 was the primary antiapoptotic Bcl-2 family member down-regulated in response to ERK1/2 and PI3K/Akt inactivation. Although Mcl-1 is degraded by caspases, the failure of the broad caspase inhibitor zVAD-fmk to block Mcl-1 down-regulation argues against this possibility. Mcl-1 expression is regulated by ERK1/2 at the transcriptional level and at the posttranslational level through direct phosphorylation on threonine 163, which attenuates Mcl-1 degradation.37 Conversely, GSK3, a kinase inactivated by Akt, phosphorylates Mcl-1 on serine 159, an event that promotes Mcl-1 degradation.38 Consequently, both transcriptional and posttranscriptional mechanisms may be involved in Mcl-1 down-regulation after perifosine/PD184352 exposure. The finding that ectopic Mcl-1 expression substantially attenuated PD184352/perifosine-induced apoptosis implicates Mcl-1 down-regulation in the lethality. It is noteworthy that Mcl-1 plays a critical role in malignant hematopoietic cell survival, particularly leukemia and myeloma cells.32,33 In such cells, Mcl-1 down-regulation, for example, by antisense oligonucleotides, may be sufficient to induce apoptosis.33 However, other studies suggest that Mcl-1 down-regulation per se is insufficient to trigger cell death.39 A possible explanation for these discrepancies may reflect differential expression levels of proapoptotic proteins (ie, Bad, Bim, Noxa) that are sequestered by Mcl-1.40 Thus, whereas Mcl-1 down-regulation is unlikely to be solely responsible for perifosine/PD184352 lethality, diminished Mcl-1 expression in all probability cooperates with other events (eg, up-regulation of Bim) to promote apoptosis.
It is currently thought that the antiapoptotic activity of Mcl-1 primarily involves interactions with Bak and Bim.41-43 In this context, MEK/ERK1/2 controls Bim expression at both transcriptional and posttranslational levels. MEK/ERK1/2 activation induces Bim transcriptional repression through an uncertain mechanism,44,45 as well as through increased Bim phosphorylation and proteasomal degradation.17,46 Conversely, MEK/ERK1/2 inhibition leads to Bim accumulation due to enhanced Bim transcription and Bim dephosphorylation that sensitize cells to apoptosis.17 PI3K/Akt pathway antiapoptotic activity also involves Bim regulation at both the transcriptional and posttranslational levels. Specifically, Bim transcription is enhanced by FOXO, a transcription factor negatively regulated by Akt. Akt also directly phosphorylates Bim at serine 87, an event that protects cells from apoptosis.47 In the present studies, PD184352 treatment alone or in combination with perifosine induced pronounced Bim accumulation, presumably due to increased Bim transcription as well as diminished phosphorylation and degradation. In support of the latter notion, PD184352 failed to enhance Bim accumulation in cells expressing a phosphorylation-defective Bim construct in which serines 55, 65, and 100 were mutated to alanine (M.R. and S.G., unpublished data, November 2007). In addition, induction of a tet-responsive constitutively active MEK construct markedly down-regulated Bim and diminished cell death induced by PI3K/Akt inhibitors (ie, perifosine or LY294002). The findings that Bim knockdown by shRNA abrogated perifosine/PD184352-mediated Bak and Bax conformational changes and mitochondrial translocation of Bax, and significantly reduced apoptosis, and ectopic expression of Bim significantly sensitized U937 cells to perifosine and other PI3K/Akt inhibitors argue that Bim plays a critical role in leukemia cell death stemming from concomitant Akt and ERK1/2 pathway inactivation. Although the mechanism by which Bim promotes apoptosis remains uncertain, it has been shown that Bim, by binding to antiapoptotic Bcl-2 family members such as Mcl-1, Bcl-2, and Bcl-xL, releases the proapoptotic multidomain Bcl-2 family members Bak and Bax. Once released, Bax and Bak permeabilize the mitochondrial outer membrane, triggering release of apoptogenic factors such as cytochrome c, AIF, and Smac/DIABLO.48-50 Evidence for direct activation of Bax by Bim also exists.51,52 Whether in the present context Bim acts directly or indirectly, Bim up-regulation in all likelihood plays an important functional role in synergistic induction of leukemic cell death by simultaneous interruption of the MEK1/2/ERK1/2 and PI3K/Akt pathways.
It is important to note that combined ERK1/2 and PI3K/Akt inactivation induced Bim accumulation in conjunction with a marked increase in Bim unassociated with Mcl-1, presumably reflecting diminished Mcl-1 protein levels. Furthermore, whereas perifosine alone only slightly reduced Bim protein levels, most likely due to MEK1/2/ERK1/2 activation,17,46 it significantly reduced the Bim/Mcl-1 association. In addition to the slight decline in both Mcl-1 and Bim levels, this phenomenon may reflect the fact that Bim phosphorylation lowers its binding to Mcl-1, as previously described.46 It is conceivable that these events cooperate to trigger the following: (1) an increase in free Bim leading to direct activation of Bax, and/or (2) increased binding of Bim to Bcl-2 and Bcl-xL, actions that may displace Bak and Bax from antiapoptotic proteins. In this context, down-regulation of Mcl-1 has also been shown to contribute to untethering of Bak and promotion of Bak activation.53 In any event, the ultimate consequence of these actions is Bax/Bak activation and induction of apoptosis. This model is consistent with the observations that Bax or Bak knockdown with shRNA markedly diminished perifosine/PD184352-mediated apoptosis in leukemia cells.
Recent results involving malignant epithelial (breast) cancer cells suggested that Bad phosphorylation status represents the primary integrator of cell death signaling after interruption of the Akt and EGFR/MAPK pathways.4 Consistent with these findings, combined exposure to perifosine and PD184352 modestly reduced Bad phosphorylation. However, knockdown Bad with shRNA, in marked contrast to Bim, failed to protect leukemia cells from perifosine/PD184352-mediated lethality, suggesting that Bad is not a critical factor in lethality in this setting. Discrepancies between this and previous reports may therefore reflect cell type-specific roles of Bad phosphorylation in integrating death signals after concomitant interruption of the MEK1/2 and Akt pathways.
Notably, combined perifosine/PD184352 treatment resulted in significant increases in cell death in 6 of 8 primary AML blast specimens assayed, but had little effect on normal progenitor CD34+ cells, raising the possibility that at least some leukemic cells may be more dependent than their normal counterparts on intact Akt and ERK1/2 pathways for survival. However, documentation of the selectivity of this strategy toward leukemic cells will require additional studies, including in vivo evaluation. Interestingly, 2 primary AML specimens that displayed an increase in cell death after perifosine/PD184352 exposure displayed discernible basal Akt and ERK1/2 phosphorylation, and further ERK1/2 activation after perifosine treatment, whereas nonresponding cells did not. Moreover, 3 of 6 responding primary specimens harbored either FLT3/ITD or FLT3/D835 mutations, and 2 of these with a sufficient number of cells for assay displayed basal Akt/ERK1/2 activation. Such observations are consistent with previous findings demonstrating a connection between FLT3 dysregulation and Akt and ERK1/2 pathway activation.54,55 It is tempting to speculate that AML cells, particularly those exhibiting FLT3 dysregulation, may be addicted to these pathways, thus accounting for their susceptibility to this regimen. However, in view of the small number of primary AML specimens analyzed, the heterogeneous effects of Akt and ERK1/2 inactivation on Bim and Mcl-1 expression, and the absence of K-ras, PIK3CA, Akt1, or PTEN mutations in these specimens, it is clear that larger sample numbers will be necessary to define the relationship between susceptibility to the perifosine/PD184352 regimen and both the basal signaling status as well as the genetic background of individual blast specimens. Such studies are underway.
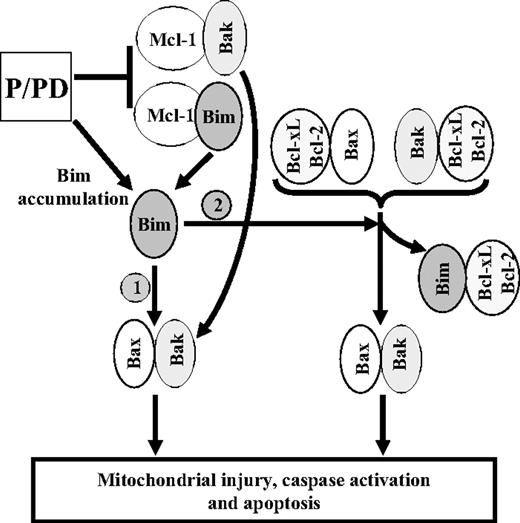
Collectively, these findings support a human leukemia model in which concomitant MEK/ERK1/2 and PI3K/Akt pathway disruption triggers Bim accumulation (Figure 7). This may occur through increased transcription and diminished proteasomal degradation secondary to dephosphorylation. Simultaneously, Mcl-1, which neutralizes Bim and Bak, undergoes marked down-regulation at both the transcriptional and posttranslational levels. Bim accumulation combined with Mcl-1 down-regulation cooperate to allow Bim to activate Bax directly and/or to displace Bak and Bax from Bcl-2/Bcl-xL, promoting Bax/Bak activation. These events trigger mitochondrial outer membrane permeabilization and release of cytochrome c, which induce apoptosis. This mechanism is recapitulated in at least some primary specimens, but given AML cell heterogeneity, additional factors are likely to be involved. Together, such findings provide insights into the mechanisms by which simultaneous interruption of the PI3K/Akt and MEK/ERK1/2 pathways cooperate to trigger leukemic cell death. They also raise the possibility that concurrent disruption of these pathways may represent an effective antileukemic strategy.
Proposed mechanistic model by which dual disruption of PI3K/Akt and MEK/ERK1/2 pathways induces apoptosis. Dual disruption of the MEK/ERK1/2 and PI3K/Akt pathways results in Bim accumulation, most likely through enhanced transcription and diminished degradation by the proteasome system as a consequence of Bim dephosphorylation. In addition, this treatment also diminishes Mcl-1 protein levels through a caspase-independent process, leading to Bim and Bak release from Mcl-1. These events may involve 2 possible mechanisms: (1) direct activation of Bax by accumulation of Bim; and (2) binding of Bim to Bcl-2 and Bcl-xL, thereby displacing Bax and Bak from these antiapoptotic proteins. Both mechanisms lead to Bax and Bak conformational change and activation, mitochondrial outer membrane permeabilization, release of cytochrome c, and other proapoptotic factors, culminating in apoptosis induction.
Proposed mechanistic model by which dual disruption of PI3K/Akt and MEK/ERK1/2 pathways induces apoptosis. Dual disruption of the MEK/ERK1/2 and PI3K/Akt pathways results in Bim accumulation, most likely through enhanced transcription and diminished degradation by the proteasome system as a consequence of Bim dephosphorylation. In addition, this treatment also diminishes Mcl-1 protein levels through a caspase-independent process, leading to Bim and Bak release from Mcl-1. These events may involve 2 possible mechanisms: (1) direct activation of Bax by accumulation of Bim; and (2) binding of Bim to Bcl-2 and Bcl-xL, thereby displacing Bax and Bak from these antiapoptotic proteins. Both mechanisms lead to Bax and Bak conformational change and activation, mitochondrial outer membrane permeabilization, release of cytochrome c, and other proapoptotic factors, culminating in apoptosis induction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA93738, CA100866, and P50CA130805 from the National Institutes of Health; award R6059-06 from the Leukemia & Lymphoma Society of America; Lymphoma Specialized Programs of Research Excellence (SPORE) from the NIH Award P50CA130805; an award from the V Foundation; and an award from the Department of Defense.
National Institutes of Health
Authorship
Contribution: M.R. designed and performed the research, analyzed data, and wrote the manuscript; A.A., J.R.H., T.R.C., and M.M. performed the research; A.F.-G. performed the research and assisted in data analysis; H.H. provided vital analytical tools; P.D. assisted in writing the manuscript; and S.G. designed research, identified patient samples, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Grant, Division of Hematology/Oncology, MCV Station Box 230, Virginia Commonwealth University, Richmond, VA 23298; e-mail: stgrant@vcu.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal