Abstract
STATs are constitutively activated in several malignancies. In primary mediastinal large B-cell lymphoma and Hodgkin lymphoma (HL), inactivating mutations in SOCS1, an inhibitor of JAK/STAT signaling, contribute to deregulated STAT activity. Based on indications that the SOCS1 mutations are caused by the B cell–specific somatic hypermutation (SHM) process, we analyzed B-cell non-HL and normal B cells for mutations in SOCS1. One-fourth of diffuse large B-cell lymphoma and follicular lymphomas carried SOCS1 mutations, which were preferentially targeted to SHM hotspot motifs and frequently obviously inactivating. Rare mutations were observed in Burkitt lymphoma, plasmacytoma, and mantle cell lymphoma but not in tumors of a non–B-cell origin. Mutations in single-sorted germinal center B cells were infrequent relative to other genes mutated as byproducts of normal SHM, indicating that SOCS1 inactivation in primary mediastinal large B-cell lymphoma, HL, diffuse large B-cell lymphoma, and follicular lymphoma is frequently the result of aberrant SHM.
Introduction
STAT transcription factors are constitutively activated in several malignancies, often resulting from genomic alterations that deregulate STAT-activating kinases or interfere with termination of STAT signaling.1,2 SOCS1, whose expression is induced by activated STATs, terminates JAK/STAT signaling by binding to and marking phosphorylated JAK for proteasomal degradation.1,3 Function-impairing mutations in SOCS1 have recently been observed in primary mediastinal B-cell lymphoma (PMBL) and classic and nodular lymphocyte–predominant Hodgkin lymphoma (HL) and were associated with accumulation of several phosphorylated STATs.4-9
Analysis of the mutation pattern of SOCS1 in nodular lymphocyte–predominant HL indicated a somatic hypermutation (SHM) origin.7 The SHM process is largely restricted to rearranged immunoglobulin V-genes in germinal center (GC) B cells.10 However, SHM occasionally also targets other genes. Rare BCL6 and FAS mutations present in GC but not naive B cells were ascribed as byproducts of “normal” SHM,11,12 whereas mutations in PIM1, c-MYC, RHOH, and PAX5, present in several B-cell lymphomas but not in normal GC B cells, were ascribed to “aberrant” SHM.12-18
Given the indications that SOCS1 is a target of SHM, SOCS1 inactivation by SHM could, in addition to HL and PMBL, also contribute to lymphomagenesis in other GC or post-GC B cell–derived lymphomas. We thus analyzed the SOCS1 mutation status of other B-cell lymphomas, and, to clarify whether SOCS1 mutations are the result of (aberrant) SHM, also in normal GC and naive B cells, T-cell lymphomas, and solid tumors with constitutive activated STATs.
Methods
Tissue samples
Tissue samples were from the files of the Senckenberg Institute of Pathology and originally submitted for diagnostic purposes. Approval for this study was obtained from the University of Frankfurt School of Medicine Institutional Review Board. Informed consent was obtained in accordance with the Declaration of Helsinki.
Isolation of single tonsillar GC and naive B cells
Single GC and naive tonsillar B cells were sorted by fluorescence-activated cell sorter as CD20+CD38intermediateIgD− and IgD+CD23+CD27−CD38− cells, respectively, in 10 μL of 1× polymerase chain reaction (PCR) buffer (supplemental data, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Sequence analysis of SOCS1 and V gene rearrangements
Genomic DNA was extracted from sections of frozen samples and various cell lines. DNA (10-100 ng) was used as template for amplification of the complete open reading frame of SOCS1 in a single-round PCR. PCR products were purified and directly sequenced. In cases with several mutations, genomic DNA was diluted and aliquots of various dilutions were amplified in a 2-round nested PCR. PCR products obtained from dilutions where 30% to 50% of PCRs yielded PCR products were directly sequenced. For amplification of SOCS1 from sorted single cells, cells were digested with proteinase K and a 2-round nested PCR was performed. PCR products were directly sequenced. Amplification of VH gene rearrangements to control the single-cell sort was performed as previously described19 (supplemental data).
Results and discussion
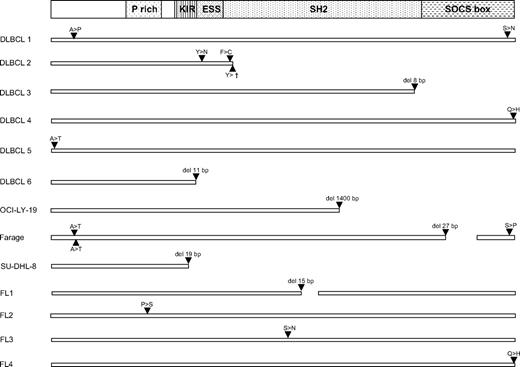
The complete open reading frame and 60-bp untranslated region of SOCS1, all encoded by one exon, were amplified in a single PCR from genomic DNA of frozen samples of 5 types of B-cell non-Hodgkin lymphomas and directly sequenced. Mutations were detected in 7 of 26 diffuse large B-cell lymphoma (DLBCL) biopsies and 5 of 21 DLBCL cell lines, 4 of 15 follicular lymphomas (FLs) and in rare cases of Burkitt lymphoma, plasmacytoma, and mantle cell lymphoma (Table 1; supplemental Table 1). Sixteen of 19 B-cell non-Hodgkin lymphoma cases with mutations carried single, whereas 3 samples carried several mutations (Figure 1). Further analysis of these 3 samples revealed that the mutations were always on one SOCS1 allele (Figure 1).
SOCS1 mutations in lymphomas, solid tumors, and single GC and naive B cells
| Tumor or cell type . | No. of tumor samples with mutations/no. of tumor samples analyzed (%) . | No. of tumor samples or sequences with replacement mutations . | No. of sequences from B cells with mutations/no. of sequences from B cells analyzed* . | Overall mutation frequency, percentage† . | |
|---|---|---|---|---|---|
| 5′ fragment . | 3′ fragment . | ||||
| DLBCL | 7/26 (27) | 6 | 0.036 | ||
| DLBCL cell lines | 5/21 (24) | 3 | 0.034 | ||
| Follicular lymphoma | 4/15 (27) | 4 | 0.019 | ||
| Burkitt lymphoma | 1/14 (7) | 1 | 0.005 | ||
| Plasmacytoma | 1/15 (6) | 1 | 0.005 | ||
| Mantle cell lymphoma | 1/15 (6) | 0 | 0.005 | ||
| Anaplastic large cell lymphoma | 0/16 | ||||
| Breast cancer | 0/16 | ||||
| Gastrointestinal stroma tumor | 0/11 | ||||
| Donor 1 GC B cell | 1 | 1/22 | 0/30 | 0.005 | |
| Donor 2 GC B cell | 2 | 3/25 | 0/25 | 0.015 | |
| Donor 3 naive B cell | 0/20 | 0/20 | |||
| Donor 3 GC B cell | 0/20 | 0/20 | |||
| All GC B cells | 3 | 4/67 | 0/75 | 0.007 | |
| Tumor or cell type . | No. of tumor samples with mutations/no. of tumor samples analyzed (%) . | No. of tumor samples or sequences with replacement mutations . | No. of sequences from B cells with mutations/no. of sequences from B cells analyzed* . | Overall mutation frequency, percentage† . | |
|---|---|---|---|---|---|
| 5′ fragment . | 3′ fragment . | ||||
| DLBCL | 7/26 (27) | 6 | 0.036 | ||
| DLBCL cell lines | 5/21 (24) | 3 | 0.034 | ||
| Follicular lymphoma | 4/15 (27) | 4 | 0.019 | ||
| Burkitt lymphoma | 1/14 (7) | 1 | 0.005 | ||
| Plasmacytoma | 1/15 (6) | 1 | 0.005 | ||
| Mantle cell lymphoma | 1/15 (6) | 0 | 0.005 | ||
| Anaplastic large cell lymphoma | 0/16 | ||||
| Breast cancer | 0/16 | ||||
| Gastrointestinal stroma tumor | 0/11 | ||||
| Donor 1 GC B cell | 1 | 1/22 | 0/30 | 0.005 | |
| Donor 2 GC B cell | 2 | 3/25 | 0/25 | 0.015 | |
| Donor 3 naive B cell | 0/20 | 0/20 | |||
| Donor 3 GC B cell | 0/20 | 0/20 | |||
| All GC B cells | 3 | 4/67 | 0/75 | 0.007 | |
In 4 cases of DLBCL (cases 1, 4, 5, and 6) with SOCS1 mutations, where normal tissue could be separated from the tumor by microdissection, the absence of SOCS1 mutations in the normal tissue revealed the somatic origin of the mutations in the lymphomas. All plasmacytomas were extramedullary. All anaplastic large cell lymphomas were ALK+ and the breast cancers HER2+ in immunohistochemistry. Successful sorting of naive and GC B cells by fluorescence-activated cell sorter was controlled by amplification of VH genes from 20 naive and 20 GC B cells. Whereas all GC B cells carried somatic mutations (average mutation frequency, 8.8%; range, 3.2%-22.2%), all naive B cells were unmutated. One DLBCL carried only a mutation in the 5′ untranslated region. Of the DLBCL cell lines, OCI-LY-1 carried a silent and OCI-LY-18 a mutation in the 3′ untranslated region. The mutations in the DLBCL cell lines and primary samples with replacement mutations are shown in Figure 1 and supplemental Table 1. The DLBCL cell lines OCI-LY-3, -4, -7, -8, -10, SU-DHL-4, -5, -6, -7, -10, DB, HAT, Karpas 422, Pfeiffer, Toledo, and WSU carried no SOCS1 mutations. With the exception of Farage, all mutations were detected as double sequences in sequence electropherograms.
Length of the 5′ fragment, 305 bp; 3′ fragment, 513 bp.
Single nucleotide missense mutations and deletions. For tumor samples, mutation frequencies were calculated assuming that 2 alleles were amplified. For sequences from B cells, mutation frequencies were calculated assuming that always 1 allele was amplified. However, in 3 of 4 cells with mutations, the mutations were detected as double peaks, indicating that, probably from a considerable fraction of cells, 2 alleles were amplified. Assuming that 2 alleles were amplified from 75% of the cells, the mutation frequency for all 3 donors together would be 0.005%.
SOCS1 mutations in DLBCL and FL. (Top) A scheme of the SOCS1 protein with functionally important regions.20 For DLBCL and FL primary cases and DLBCL cell lines, only replacement mutations are shown. For missense mutations, the amino acid exchanges are shown in the single letter code. In DLBCL2, a nonsense mutation, in DLBCL3 and 6, and SU-DHL-8, small out-of-frame deletions (resulting from reading frame shifts 84, 48, and 19 amino acids, respectively, unrelated to SOCS1 followed by stop codons were encoded) and in OCI-LY-19 a large deletion/insertion (encoding 14 amino acids unrelated to SOCS1 before a stop codon) caused obviously function-impairing truncations of SOCS1 protein, and the same applies to the 9- and 5-amino-acid deletions in functionally important regions in Farage and FL1. The mutations in the Farage cell line were detected as single peaks in sequence electropherograms, indicative of a lack of an unmutated allele. All other mutations in primary cases and cell lines appeared as double peaks. Thus, a second unmutated allele was always present in the 4 cell lines with double peaks. In biopsies the unmutated alleles could be the result of the presence of a second unmutated allele in the tumor cells but also originate from nontumor cells present in the lymphoma biopsies. For the 2 DLBCL samples with several mutations, dilution of the genomic DNA and subsequent 2-round PCRs to amplify single molecules revealed that in both cases all mutations were on one SOCS1 allele.
SOCS1 mutations in DLBCL and FL. (Top) A scheme of the SOCS1 protein with functionally important regions.20 For DLBCL and FL primary cases and DLBCL cell lines, only replacement mutations are shown. For missense mutations, the amino acid exchanges are shown in the single letter code. In DLBCL2, a nonsense mutation, in DLBCL3 and 6, and SU-DHL-8, small out-of-frame deletions (resulting from reading frame shifts 84, 48, and 19 amino acids, respectively, unrelated to SOCS1 followed by stop codons were encoded) and in OCI-LY-19 a large deletion/insertion (encoding 14 amino acids unrelated to SOCS1 before a stop codon) caused obviously function-impairing truncations of SOCS1 protein, and the same applies to the 9- and 5-amino-acid deletions in functionally important regions in Farage and FL1. The mutations in the Farage cell line were detected as single peaks in sequence electropherograms, indicative of a lack of an unmutated allele. All other mutations in primary cases and cell lines appeared as double peaks. Thus, a second unmutated allele was always present in the 4 cell lines with double peaks. In biopsies the unmutated alleles could be the result of the presence of a second unmutated allele in the tumor cells but also originate from nontumor cells present in the lymphoma biopsies. For the 2 DLBCL samples with several mutations, dilution of the genomic DNA and subsequent 2-round PCRs to amplify single molecules revealed that in both cases all mutations were on one SOCS1 allele.
SHM preferentially targets RGYW hotspot motifs and generates single nucleotide substitutions more frequently than insertions and deletions.21 Taking DLBCLs and FLs together, hotspots were 5.4 times more frequently mutated than expected (30% of mutations in hotspots, whereas 5.6% of the amplified SOCS1 region are hotspots), and single nucleotide substitutions were much more frequent than deletions (23 vs 6). This indicates that the mutations in SOCS1 in DLBCLs and FLs are likely the result of SHM.
In 13 of 16 mutated DLBCLs and FLs, the mutations caused alterations in the SOCS1 protein, which were in 7 cases most probably function-impairing (Figure 1). Three DLBCLs carried only silent mutations or mutations in untranslated regions. The ratio of replacement to silent mutations was 3.5, only marginallly above the ratio of 3.0 for unselected mutations. It is thus probable that a considerable fraction of the SOCS1 mutations was not selected during lymphomagenesis.
To clarify whether SOCS1 is accidentally targeted by normal SHM in GC B cells, we sorted single GC and naive B cells from tonsils and amplified SOCS1 in nested 2 round PCRs in 2 overlapping fragments, which were directly sequenced. All 40 PCR fragments from naive B cells were unmutated (Table 1). Taking all GC cells together, single mutations were detected in 4 of 67 (5.9%) 5′ SOCS1 fragments and 0 of 75 3′ SOCS1 fragments, with an overall mutation frequency of 0.007%. In comparable studies, mutation frequencies in BCL6 and CD95 were 0.1% and 0.03% and significantly higher compared with naive B cells, whereas PAX5 and RHOH showed frequencies of 0.03% and 0.005%, which were comparable with naive B cells.11,12 SOCS1 is thus also compared with other accidentally mutated genes, rarely mutated in normal GC cells.
STATs are also constitutively activated in several tumors not derived from B cells, such as anaplastic large cell lymphoma, gastrointestinal stromal tumors, and breast cancer.2,22,23 SOCS1 was amplified and sequenced from several samples of these entities and was always unmutated (Table 1). Mutations in SOCS1 are thus restricted to B-cell lymphomas, in line with an SHM origin.
Taking the restriction of SOCS1 mutations to the GC-derived lymphomas PMBL, HL, DLBCL, and FL together with the SOCS1 mutation pattern, it is most probable that the mutations originate from SHM. As SOCS1 mutations were infrequent in GC B cells but frequent in HL, PMBL, DLBCL, and FL, all entities in which aberrant SHM has been observed, and as there were no indications for stringent selection of rare SOCS1 mutations occurring as byproducts of normal SHM, the SOCS1 mutations are most probably the result of aberrant SHM. The so far known targets of aberrant SHM are protooncogenes. Because of the preferential targeting of normal and aberrant SHM to the 1500 bp downstream of promoters, most mutations were in 5′ untranslated regions and introns and therefore largely without obvious functional consequences, although the aberrant SHM process may promote the occurrence of translocations.12 In the case of SOCS1, encoded by 2 exons separated by a short intron, the complete coding region is within 1500 bp downstream of the SOCS1 promoter, and mutations in the coding region thus occur at high frequencies. As most mutations in coding regions are deleterious, a large fraction will inactivate the tumor suppressor gene SOCS1 and could promote lymphomagenesis when both alleles would be inactivated. However, in all samples but one cell line, in addition to a mutated an unmutated SOCS1 allele was always present. Besides inactivating mutations and larger genomic deletions, SOCS1 can be inactivated by hypermethylation24 ; and indeed, in 2 DLBCL cell lines with SOCS1 mutations, no SOCS1 RNA expression from the unmutated alleles could be detected (supplemental data), indicating that SOCS1 hypermethylation may also occur in DLBCLs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sabine Albrecht and Ekatherini Hadzoglou for excellent technical assistance and Laura Pasqualucci and Margaret Shipp for providing us with reagents.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (BR 1238/6-3) and the Deutsche Krebshilfe (107572).
Authorship
Contribution: A.M. and C.R. designed and performed research and analyzed and interpreted data; M.S. and E.O. performed research and analyzed data; W.B. analyzed and interpreted data; M.-L.H. provided samples and interpreted data; R.K. designed research and analyzed and interpreted data; and A.B. designed research, analyzed and interpreted data, and drafted the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Bräuninger, Gerhard-Domagk-Institute for Pathology, University of Münster, Gerhard-Domagk-Str 17, 48149 Münster, Germany; e-mail: andreas.braeuninger@ukmuenster.de.
References
Author notes
A.M. and C.R. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal