Abstract
Allogeneic hematopoietic stem cell transplantation (alloHSCT) is the only potentially curative treatment available for patients with B-cell chronic lymphocytic leukemia (B-CLL). Here, we show that post-alloHSCT antibody repertoires can be mined for the discovery of fully human monoclonal antibodies to B-CLL cell-surface antigens. Sera collected from B-CLL patients at defined times after alloHSCT showed selective binding to primary B-CLL cells. Pre-alloHSCT sera, donor sera, and control sera were negative. To identify post-alloHSCT serum antibodies and subsequently B-CLL cell-surface antigens they recognize, we generated a human antibody-binding fragment (Fab) library from post-alloHSCT peripheral blood mononuclear cells and selected it on primary B-CLL cells by phage display. A panel of Fab with B-CLL cell-surface reactivity was strongly enriched. Selection was dominated by highly homologous Fab predicted to bind the same antigen. One Fab was converted to immunoglobulin G1 and analyzed for reactivity with peripheral blood mononuclear cells from B-CLL patients and healthy volunteers. Cell-surface antigen expression was restricted to primary B cells and up-regulated in primary B-CLL cells. Mining post-alloHSCT antibody repertoires offers a novel route to discover fully human monoclonal antibodies and identify antigens of potential therapeutic relevance to B-CLL and possibly other cancers. Trials described herein were registered at www.clinicaltrials.gov as nos. NCT00055744 and NCT00003838.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is a biologically and clinically heterogeneous hematologic malignancy characterized by a gradual accumulation of proliferating, resting, and dying CD5+CD19+CD23+ monoclonal B cells.1 Monoclonal antibodies (mAbs), alone or in combination with chemotherapy, hold substantial promise for first-line and second-line treatment of B-CLL. However, most preclinically and clinically investigated mAbs for the therapy of B-CLL target cell-surface antigens that are also expressed by healthy B cells and other blood cells of lymphoid and myeloid lineages.2-4 By contrast, mAbs to cell-surface antigens that are unique to or at least overexpressed on B-CLL cells may be less toxic and more active by allowing selective intervention with powerful antibody-drug conjugates, immunotoxins, and radioimmunoconjugates. A few differentially expressed B-CLL cell-surface antigens that may be suitable for selective mAb therapy have been discovered through gene expression profiling.5-8 A more direct antigen discovery strategy, termed SEREX, uses serum antibodies from patients with cancer for the screening of cDNA expression libraries.9,10 On the one hand, antigens that were identified by SEREX in a variety of cancers, including B-CLL,11 are predominantly intracellular proteins that do not allow mAb targeting. On the other hand, SEREX has become a valuable tool for the discovery of T-cell antigens because serum antibodies to intracellular proteins can induce CD8+ T-cell responses to peptide epitopes within the antigen by cross-presentation mediated through Fcγ receptors on dendritic cells.10
SEREX has also been applied to the discovery of antigens that mediate graft-versus-leukemia (GVL) activity after allogeneic hematopoietic stem cell transplantation (alloHSCT). Currently, alloHSCT is the only potentially curative treatment available for patients with B-CLL.12,13 Strong GVL activity is evident in B-CLL after alloHSCT from human leukocyte antigen (HLA)–matched related and unrelated donors.14 GVL and its counterpart graft-versus-host disease (GVHD) are believed to be mediated primarily by alloreactive donor T cells that recognize minor histocompatibility antigens, that is, HLA-displayed peptides derived from polymorphic proteins that are different in recipient and donor.15,16 In addition, GVL activity may be mediated by HLA-displayed peptides derived from antigens that are selectively expressed or overexpressed in leukemia cells.
Shifting the focus to another component of the adaptive immune system, there is growing interest in investigating whether alloHSCT-induced antibodies derived from donor B cells may also have a role in GVL activity, either indirectly through cross-presentation of antigens for induction of CD8+ T-cell responses or directly through tumor cell-surface targeting.17 With the use of SEREX, serum antibodies from patients who received an alloHSC transplant followed by donor lymphocyte infusion (DLI) led to the identification of potential GVL antigens in chronic myelogenous leukemia18-21 and multiple myeloma.22,23 Even for patients who received an alloHSC transplant not followed by DLI, SEREX identified candidate GVL antigens in mantle cell lymphoma24 and adult T-cell leukemia.25 Alloreactive antibodies directed against H-Y antigens encoded on the Y chromosome, including minor histocompatibility antigen DBY, were discovered in male recipients with female donors.26,27 Although most candidate GVL antigens discovered by SEREX were intracellular proteins, several cell-surface proteins that may mediate direct cytotoxicity of post-alloHSCT serum antibodies have also been identified.23,25,28 Collectively, these studies suggest that candidate GVL antigens in B-CLL may be discovered through post-alloHSCT serum antibodies, including cell-surface antigens suitable for selective mAb therapy.
Here, we investigate the hypothesis that alloHSCT induces a serum antibody response to B-CLL cell-surface antigens that can be harnessed for human mAb drug and target discovery through the generation and selection of post-alloHSCT antibody libraries. In clear contrast to SEREX, our approach was designed to (1) confine target discovery to cell-surface antigens and (2) concomitantly yield fully human mAbs of potential therapeutic utility. B-CLL in the context of alloHSCT may be particularly suited for this approach because of the typically slow disappearance of B-CLL cells.12,29 The continued presence of B-CLL antigens in the first several months after alloHSCT may support the formation of secondary antibody repertoires characterized by somatic hypermutation, class switch recombination, and receptor editing of immunoglobulin (Ig) genes in the reconstituting B-cell compartment.30,31
Methods
Clinical samples
Untreated patients with B-CLL (n = 13) and patients with B-CLL treated with allo-HSCT (n = 2) along with their HLA-matched sibling donors were enrolled in studies with protocols approved by the institutional review board at the Clinical Center, National Institutes of Health (NIH), and all patients gave informed consent in accordance with the Declaration of Helsinki. Plasma from patients with B-CLL and donors was prepared from blood and stored at −80°C. Peripheral blood mononuclear cells (PBMCs) were prepared from blood by using Lymphocyte Separation Medium (MP Biomedicals) and cryopreserved until use. PBMCs from healthy volunteers (n = 11) were prepared from freshly drawn blood obtained from the Department of Transfusion Medicine, Clinical Center, NIH. CD19+ and CD19− subpopulations were purified from human PBMCs by magnetic-activated cell sorting (MACS) with the use of CD19 MicroBeads (Miltenyi Biotec).
Cell lines
Epstein-Barr virus (EBV)–transformed B-lymphoblastoid cell lines (EBV-LCLs) were generated from PBMCs of a healthy volunteer (0745) and patients with B-CLL (18-7-3 and 18-1-12) as described.32 By using polymerase chain reaction (PCR) amplification of Ig heavy chain VDJ gene fragments from genomic DNA,33 EBV-LCL 0745 was found to be polyclonal as indicated by multiple bands. The same analysis showed that EBV-LCL 18-7-3 and 18-1-12 were monoclonal albeit different in HCDR3 lengths than the corresponding B-CLL cells, suggesting EBV transformation of normal B cells present in these patients with B-CLL. EBV-LCL 583 and 1363 were generated from PBMCs of patients with melanoma and kindly provided by Dr Suzanne L. Topalian (Surgery Branch, National Cancer Institute, NIH). B-CLL cell line EHEB was obtained from the German Collection of Microorganisms and Cell Cultures.34 B-CLL cell line 232-B4 was kindly provided by Dr Anders Rosén (Linköping University).35 Human Burkitt lymphoma B-cell lines Daudi, Raji, and Ramos and human mantle cell lymphoma B-cell line JeKo-1 were obtained from ATCC.
Control mAbs
Rituximab (Rituxan; Genentech and Biogen Idec) and alemtuzumab (Campath; Genzyme) were obtained from the pharmacy of the Clinical Center, NIH. The generation, expression, and purification of human anti–tetanus toxoid mAb TT11 IgG1 was described previously.36
Flow cytometry
Serum antibody detection after alloHSCT.
Multiparameter flow cytometry was performed in an LSR II instrument (Immunocytometry Systems; BD Biosciences). All incubation steps were on ice for 1 hour. Approximately 5 × 105 PBMCs prepared from a patient with untreated B-CLL (pilot experiment) or from the 2 patients with B-CLL treated with alloHSCT before induction chemotherapy (Table 1) were first blocked with 4% (vol/vol) normal goat serum in phosphate-buffered saline (PBS; used for all subsequent dilutions and washes) followed by 100 μg/mL unconjugated goat antibody-binding fragment (Fab) anti–human IgG polyclonal antibodies (pAbs; Jackson ImmunoResearch Laboratories Inc). After 2 washes, the cells were incubated with 1 μg/mL rituximab or alemtuzumab (pilot experiment) or with a 1:2 dilution of plasma from patients with B-CLL treated with allo-HSCT and donors or, as negative control, pooled human AB serum (Invitrogen). After 2 washes, the cells were incubated with 20nM goat F(ab′)2 anti–human IgG pAbs conjugated to Qdot 655 (Quantum Dot Corporation). The cells were costained with CD3-FITC/CD19–phycoerythrin (PE) Simultest reagent (BD Biosciences) for gating T cells and B cells and with propidium iodide for excluding dead cells from the analysis. After 2 more washes, a total of 20 000 gated events were collected for each sample in a list mode file, and data analysis was performed with FACS Convert and CellQuest software (BD Biosciences).
Characteristics of B-CLL patients treated with nonmyeloablative peripheral blood alloHSCT
| Patient . | A . | B . |
|---|---|---|
| Indication | Relapse | Relapse |
| Sex | Male | Female |
| Age* | 52 | 48 |
| Enrollment | 2003 | 2002 |
| Clinical trial† | NCT00055744 | NCT00003838 |
| Induction chemotherapy | EPOCH + FR | FC |
| Donor‡ | Brother | Brother |
| GVHD prophylaxis | CSP + MTX | CSP + MTX |
| Acute GVHD | No | No |
| Chronic GVHD | No | No |
| Response | PR; CR after DLI | CR |
| Current status§ | Molecular remission | Molecular remission |
| Patient . | A . | B . |
|---|---|---|
| Indication | Relapse | Relapse |
| Sex | Male | Female |
| Age* | 52 | 48 |
| Enrollment | 2003 | 2002 |
| Clinical trial† | NCT00055744 | NCT00003838 |
| Induction chemotherapy | EPOCH + FR | FC |
| Donor‡ | Brother | Brother |
| GVHD prophylaxis | CSP + MTX | CSP + MTX |
| Acute GVHD | No | No |
| Chronic GVHD | No | No |
| Response | PR; CR after DLI | CR |
| Current status§ | Molecular remission | Molecular remission |
EPOCH + FR indicates etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin plus fludarabine, rituximab; FC indicates fludarabine, cyclophosphamide; CSP + MTX, cyclosporine plus methotrexate; PR, partial response; CR, complete response; and DLI, donor lymphocyte infusion
At enrollment.
ClinicalTrials.gov identifier.
HLA-matched (6/6; A, B, and DR).
May 2009.
JML-1 IgG1 binding.
Approximately 5 × 105 cells (from a cell line, from PBMCs of patients with B-CLL, or from MACS-purified CD19+ PBMC subpopulations of healthy volunteers) were first incubated with pooled human AB serum for 20 minutes to block Fcγ receptors. All subsequent incubations were on ice for 1 hour. The cells were incubated with different concentrations (0.1-10 μg/mL) biotinylated JML-1 or TT11 IgG1 in 2% (vol/vol) fetal bovine serum (Hyclone) in PBS (used for all subsequent dilutions and washes), washed twice, and incubated with 2 μg/mL PE-coupled streptavidin (BD Biosciences). After 2 more washes, propidium iodide was added to exclude dead cells from the analysis, and flow cytometry was performed by using a FACSCalibur instrument (BD Biosciences). The data were analyzed with CellQuest software (BD Biosciences). In case of B-CLL PBMCs, the cells were costained with CD19–allophycocyanin (APC; BD Biosciences) for gating CD19+ and CD19− subpopulations.
Human Fab library generation
A human Fab library was generated from cryopreserved post-alloHSCT PBMCs from one patient (patient A) collected 6 months after transplantation. Total RNA was extracted from 2.5 × 107 PBMCs by using TRI Reagent (Molecular Research Center) and further purified by using the RNeasy Mini Kit (QIAGEN). Approximately 100 μg of total RNA was isolated and validated by agarose gel electrophoresis. First-strand cDNA synthesis from total RNA by using an oligo(dT) primer and SuperScript III reverse transcriptase (Invitrogen) were carried out according to the manufacturer's protocol. Vκ, Vλ, and VH encoding sequences were separately amplified from first-strand cDNA by a 35-cycle PCR by using the FastStart High-Fidelity PCR System (Roche) and combinations of 12 sense/1 antisense primers for Vκ, 20 sense/3 antisense primers for Vλ, and 19 sense/6 antisense primers for VH, for a total of 186 different combinations, encompassing all human germlines. (Note: The antisense primers for Vλ and VH align to Jλ and JH germlines, respectively, whereas the antisense primer for Vκ aligns to the Cκ encoding sequence). Human Cκ-pelB and Cλ-pelB encoding sequences required for the Vκ-Cκ-VH and Vλ-Cλ-VH cassette assembly, respectively, were amplified from pCκ37 and pCλ36 . Vκ-Cκ-VH and Vλ-Cλ-VH cassettes were assembled in one fusion step based on 3-fragment overlap extension PCR, digested with SfiI, and cloned into pC3C as described.37 Transformation of Escherichia coli strain XL1-Blue (Stratagene) by electroporation yielded 9.8 × 107 and 1.6 × 108 independent transformants for the κ and λ phagemid libraries, respectively. Randomly picked independent transformants from each library were analyzed for Fab expression by enzyme-linked immunoabsorbent assay (ELISA) and for sequence diversity by DNA fingerprinting by using AluI as described.38 With the use of VCSM13 helper phage (Stratagene), the pooled phagemid library was converted to a phage library as described39 and stored at 4°C after adding sodium azide to a final concentration of 0.02% (wt/vol).
Human Fab library selection
The human Fab library was selected on cryopreserved PBMCs (consisting of > 85% B-CLL cells) from an untreated patient with B-CLL (patient α) that had been maintained in 6-well tissue-culture plates for 1 to 2 days in RPMI 1640 (Invitrogen) supplemented with 5% (vol/vol) autologous serum. Five rounds of panning were carried out by using established phage display protocols.39 All incubations were at room temperature, unless noted otherwise. In the first round, the freshly reamplified phage library was preselected for functional Fab display through panning on rat anti-hemagglutinin (HA) mAb (Roche) immobilized to 3 wells of a 96-well ELISA plate (Costar 3690; Corning) at 500 ng/well. In the second round, 0.5 mL of fresh phage was first mixed with 0.5 mL of 5% (vol/vol) autologous serum in PBS and 0.5 mL of 1% (wt/vol) BSA in PBS. After adding sodium azide to a final concentration of 0.12% (wt/vol), the phage were incubated for 30 minutes. Primary B-CLL cells from one 6-well tissue-culture plate were harvested, collected through Lymphocyte Separation Medium, resuspended in 1.5 mL of 5% (vol/vol) autologous serum in PBS, counted (4.2 × 107), added to the 1.5-mL phage preparation in a 15-mL polypropylene tube, and incubated for 30 minutes with gentle agitation every 5 minutes. After washing 3 times with 15 mL of PBS, the cells were resuspended in 0.6 mL of PBS containing 10 mg/mL trypsin, shook at 37°C and 250 rpm for 30 minutes, and added to two 2-mL XL1-Blue cultures, resuming the phage display protocol. The third round was identical to the second round, except that 1.2 × 107 primary B-CLL cells were used. In the fourth round, selection for functional Fab display was repeated by using 2 wells with immobilized rat anti-HA mAb at 200 ng/well. The fifth round was identical to the second and third rounds, except that 5 × 107 primary B-CLL cells were used and 4 washes with 15 mL of PBS were carried out. Phage output-to-input ratios after each round were determined through output and input titering as described.39 One hundred randomly picked clones from the final output were analyzed for Fab expression by ELISA and for sequence diversity by DNA fingerprinting by using AluI as described.38
Whole-cell ELISA
Phage.
On the basis of input titering, polyclonal phage from the second, third, and fifth rounds were diluted to approximately 1011 phage in 75 μL of PBS and stored on ice. All subsequent steps were carried out in a V-bottom 96-well tissue-culture plate (Costar 3894; Corning) at room temperature with PBS for washing and dilution. Approximately 5 × 105 primary B-CLL cells from patient α were washed twice, resuspended in the 75-μL phage preparations or in PBS as negative control, and incubated for 1 hour on a rocker. Subsequently, the cells were washed twice and incubated with 100 μL of a 1:1000 dilution of mouse antiphage mAb conjugated to horseradish peroxidase (HRP; GE Healthcare) for 1 hour. Finally, the cells were washed twice, resuspended in 50 μL of HRP substrate solution,39 and incubated for 20 minutes. Absorbance at 405 nm was determined with an ELISA plate reader.
Crude Fab.
After induction of Fab expression with isopropyl β-D-1-thiogalactopyranoside as described,39 bacterial supernatants from 4 to 5 selected clones with identical fingerprints were pooled and concentrated 10-fold by using a 15-mL Amicon Ultra Centrifugal Filter Device with 10-kDa molecular weight cutoff (Millipore Corporation). Whole-cell ELISA was carried out by using 75 μL of concentrated supernatant and HRP-conjugated rat anti-HA mAb as described in the preceding paragraph.
Generation, expression, purification, and biotinylation of JML-1 IgG1
For the conversion of JML-1 Fab to JML-1 IgG1, the VH and light chain encoding sequences were PCR amplified by using appropriately designed primers and cloned into mammalian expression vector PIGG as described.37 With the use of 293fectin, 300 μg of PIGG–JML-1 plasmid was transiently transfected into 3 × 108 HEK 293F cells and kept in 300 mL of FreeStyle serum-free medium in a 500-mL spinner flask on a stirring platform at 75 rpm (CELLSPIN System; Integra) in a humidified atmosphere containing 8% CO2 at 37°C. After 4 days, the medium was collected after centrifugation, replaced for an additional 3 to 4 days, and collected again. Pooled supernatants were then processed, and IgG1 was purified by using a 1-mL recombinant Protein A HiTrap column (GE Healthcare) as described.37 The quality and quantity of purified IgG1 were determined by SDS–polyacrylamide gel electrophoresis and A280 absorbance. Purified JML-1 IgG1 and TT11 IgG1 were biotinylated by using the BiotinTag Micro-Biotinylation Kit (Sigma-Aldrich). The number of conjugated biotin molecules per IgG1 molecule was equivalent for JML-1 and TT11 IgG1.
Results
Detection of post-alloHSCT serum antibodies with B-CLL cell-surface reactivity
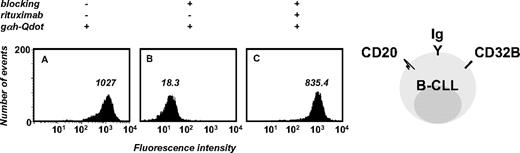
To detect presumably low concentrations of human serum antibodies against B-CLL cells in patients after alloHSCT, we developed a sensitive flow cytometry assay that used goat F(ab′)2 anti–human IgG pAbs conjugated to Qdot 655 nanocrystals (Figure 1). The detection of human serum antibodies specifically binding to B-CLL cell-surface antigens is complicated because B-CLL cells typically express transmembrane IgM, IgD, and Fcγ receptors such as CD32B, albeit at lower cell-surface densities than do normal B cells.40 To avoid detection of human Ig that is directly (as IgM or IgD) or indirectly (through binding to CD32B) displayed on the B-CLL cell surface, the assay required dual blocking with normal goat serum and unconjugated goat Fab anti–human IgG pAbs. Normal goat serum blocks Fcγ receptors and unconjugated goat Fab anti–human IgG pAbs were intended to block the binding of conjugated goat F(ab′)2 anti–human IgG pAbs to surface IgM and IgD epitopes that are shared with IgG (such as epitopes in the light chain and variable domain of the heavy chain). We used monovalent Fab for blocking to avoid artificial recruitment of serum antibodies and bivalent F(ab′)2 for detection to gain avidity. The assay was established by using rituximab (a chimeric mouse/human anti–human CD20 mAb with human IgG1κ constant domains) or alemtuzumab (a humanized anti–human CD52 mAb with human IgG1κ constant domains) and PBMCs from an untreated patient with B-CLL (Figure 1). These pilot experiments suggested the suitability and sensitivity of the assay for detecting the binding of post-alloHSCT serum antibodies to B-CLL cell-surface antigens.
Development of a sensitive flow cytometry assay to detect the binding of human antibodies to primary B-CLL cells. PBMCs (5 × 105, consisting of > 85% B-CLL cells) from a patient with untreated B-CLL were first incubated with normal goat serum to block Fcγ receptors (CD32B) followed by unconjugated goat Fab anti–human IgG pAbs to block cell-surface Ig (blocking). In the pilot experiment shown here, chimeric mouse/human anti–human CD20 mAb rituximab was the primary antibody that served as positive control for serum antibodies with human constant domains and cell-surface reactivity. Goat F(ab′)2 anti–human IgG pAbs conjugated to Qdot 655 nanocrystals (gαh-Qdot) were used as secondary antibodies. Substantial reactivity noted for the secondary antibody alone (A) was eliminated through cell-surface blocking (B), permitting detection of the primary antibody reactivity (C). The numbers inside each panel depict median fluorescence intensity (MFI).
Development of a sensitive flow cytometry assay to detect the binding of human antibodies to primary B-CLL cells. PBMCs (5 × 105, consisting of > 85% B-CLL cells) from a patient with untreated B-CLL were first incubated with normal goat serum to block Fcγ receptors (CD32B) followed by unconjugated goat Fab anti–human IgG pAbs to block cell-surface Ig (blocking). In the pilot experiment shown here, chimeric mouse/human anti–human CD20 mAb rituximab was the primary antibody that served as positive control for serum antibodies with human constant domains and cell-surface reactivity. Goat F(ab′)2 anti–human IgG pAbs conjugated to Qdot 655 nanocrystals (gαh-Qdot) were used as secondary antibodies. Substantial reactivity noted for the secondary antibody alone (A) was eliminated through cell-surface blocking (B), permitting detection of the primary antibody reactivity (C). The numbers inside each panel depict median fluorescence intensity (MFI).
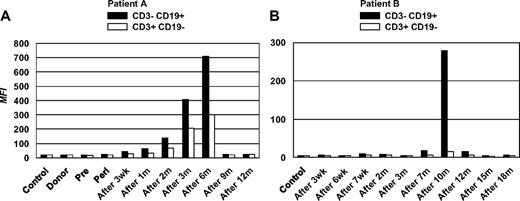
A complete set of frozen plasma samples from time points before, at, and after alloHSCT over a period of at least 1 year was available for 2 patients with refractory B-CLL who had received a nonmyeloablative alloHSC transplant from an HLA-matched sibling donor on 2 different protocols at the Clinical Center, NIH, and who remain in molecular remission 6 and 7 years later, respectively (Table 1). For each of the 2 patients, PBMCs from a time point before induction chemotherapy served as the source of primary B-CLL cells (CD3− CD19+) and T cells (CD3+ CD19−). By using the established assay, post-alloHSCT serum antibodies against B-CLL surface antigens were detected in both patients. Post-alloHSCT plasma from patient A showed a substantial but transient occurrence of serum antibodies with B-CLL cell-surface reactivity, peaking at 6 months after transplantation (Figure 2A). A parallel but weaker T-cell surface reactivity was also observed. By contrast, pre- and peri-alloHSCT plasma, plasma from the alloHSCT donor of patient A, and pooled control plasma from healthy volunteers were all negative. A similar pattern of serum antibody reactivity was seen for patient B (Figure 2B) from a different protocol. However, the transient B-CLL cell-surface reactivity was confined to later time points, peaked at 10 months after transplantation, and was not accompanied by T-cell surface reactivity. These patterns of serum antibody reactivity seen for patients A and B were reproducible in independent experiments.
Detection of post-alloHSCT human serum antibodies binding to primary B-CLL cells. PBMCs (5 × 105, consisting of > 85% B-CLL cells) harvested before induction chemotherapy from alloHSC transplant recipients patients A and B were incubated with plasma samples collected from each patient at the indicated time points. Plasma from alloHSCT donor (donor) or healthy volunteers (control) was included. The assay procedure described for Figure 1 was followed. By using a CD3-FITC/CD19-PE 2-color reagent, PBMCs were gated into B cells (dominated by B-CLL cells) and T cells. B-cell (■) and T cell–surface reactivities (□) are depicted as MFI for patient A (left) and patient B (right). Wk indicates, weeks; m, months.
Detection of post-alloHSCT human serum antibodies binding to primary B-CLL cells. PBMCs (5 × 105, consisting of > 85% B-CLL cells) harvested before induction chemotherapy from alloHSC transplant recipients patients A and B were incubated with plasma samples collected from each patient at the indicated time points. Plasma from alloHSCT donor (donor) or healthy volunteers (control) was included. The assay procedure described for Figure 1 was followed. By using a CD3-FITC/CD19-PE 2-color reagent, PBMCs were gated into B cells (dominated by B-CLL cells) and T cells. B-cell (■) and T cell–surface reactivities (□) are depicted as MFI for patient A (left) and patient B (right). Wk indicates, weeks; m, months.
The limited availability of post-alloHSCT plasma did not permit an extensive analysis of the reactivity of post-alloHSCT serum antibodies at these defined time points. However, as summarized in Table 2, some cell-surface reactivity with allogeneic B cells and third-party B cells was noted. Additional analyses with secondary antibodies specific for human Ig isotypes suggested that both IgG and IgM contributed to the transient B-CLL cell-surface reactivity (data not shown). Compared with clinical data, the peak in transient B-CLL cell-surface reactivity approximately paralleled the time points at which full donor chimerism was achieved, and the disappearance of B-CLL cells by flow cytometry and PCR was noted (data not shown). This suggested that the observed serum antibody response against B-CLL cells is an antigen-dependent phenomenon, probably involving both autologous and allogeneic epitopes of cell-surface antigens.
Cell-surface reactivity of post-alloHSCT serum antibodies
| . | Autologous B-CLL cells . | Autologous T cells . | Allogeneic B cells . | Third-party B cells . |
|---|---|---|---|---|
| Patient A after 6 m plasma | ++* | + | −† | Weak |
| Patient B after 10 m plasma | ++ | Weak | ++‡ | + |
| Rituximab | ++ (Patient A) | − (Patient A) | ++ | ++ |
| + (Patient B) | − (Patient B) | ++ | ++ | |
| Alemtuzumab | +++ (Patient A) | − (Patient A) | +++ | +++ |
| +++ (Patient B) | − (Patient B) | +++ | +++ |
| . | Autologous B-CLL cells . | Autologous T cells . | Allogeneic B cells . | Third-party B cells . |
|---|---|---|---|---|
| Patient A after 6 m plasma | ++* | + | −† | Weak |
| Patient B after 10 m plasma | ++ | Weak | ++‡ | + |
| Rituximab | ++ (Patient A) | − (Patient A) | ++ | ++ |
| + (Patient B) | − (Patient B) | ++ | ++ | |
| Alemtuzumab | +++ (Patient A) | − (Patient A) | +++ | +++ |
| +++ (Patient B) | − (Patient B) | +++ | +++ |
− indicates < 2; weak, ≥ 2 and < 5; +, ≥ 5 and < 20; ++, ≥ 20 and < 100; and +++, ≥ 100
Based on median fluorescence intensity (MFI) ratios calculated for sample over background.
Allogeneic B cells derived from PBMCs of patient A 15 months after transplantation.
Allogeneic B cells derived from PBMCs of the donor of patient B.
Generation and selection of a post-alloHSCT human Fab library by phage display
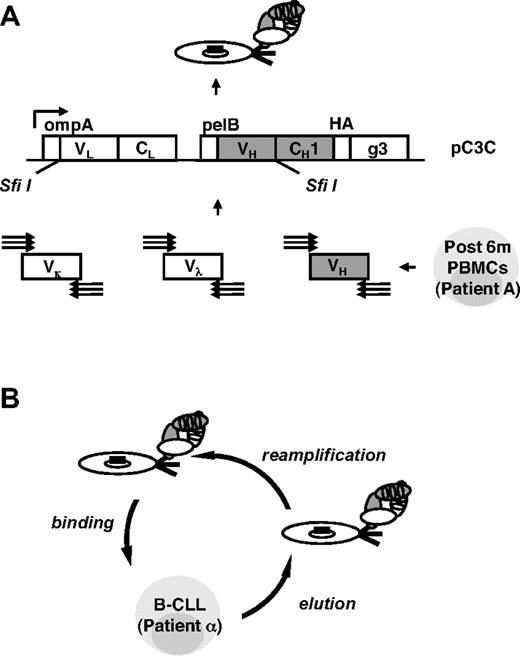
To identify post-alloHSCT serum antibodies and subsequently the cell-surface antigens they recognize, we used phage display vector pC3C36,37 for the generation of a human Fab library from post-alloHSCT PBMCs (Figure 3A). Total RNA was prepared from post-alloHSCT PBMCs collected from patient A at the peak of serum antibody response (6 months; Figure 2A), and human Vκ, Vλ, and VH encoding sequences were amplified by reverse transcription PCR. To include all human germlines, we used a total of 61 primers in 186 different and separate combinations as described previously for the generation of a naive human Fab library.36 Because of a depleted and not yet fully recovered B-cell repertoire in post-alloHSCT PBMCs, we expected a smaller number of successful primer combinations compared with normal PBMCs from a healthy volunteer. Although this was indeed the case, the difference was smaller than expected with a success rate of 71% for post-alloHSCT PBMCs compared with 89% for normal PBMCs (Table 3). This finding along with a library size of 2.6 × 108 independent Fab clones implied a high complexity of our post-alloHSCT human Fab library. Its integrity and diversity was confirmed by ELISA and DNA fingerprinting of unselected Fab clones.
Generation and selection of a post-alloHSCT human Fab library by phage display. (A) By using a total of 186 different and separate combinations, human Vκ, Vλ, and VH encoding sequences were amplified by reverse transcription PCR from alloHSCT (after 6m) PBMCs derived from patient A, assembled into Vκ-Cκ-VH and Vλ-Cλ-VH cassettes by 3-fragment overlap extension PCR, and cloned into phagemid pC3C by asymmetric SfiI ligation. The resulting post-alloHSCT human Fab library was subsequently converted from phagemid to phage by transformation of E coli and the addition of helper phage. (B) The post-alloHSCT human Fab library was selected by 3 rounds of panning on PBMCs derived from patient α with untreated B-CLL (round 2, round 3, and round 5). Two additional rounds of panning on immobilized rat anti-HA mAb were carried out (round 1 and round 4). The steps of 1 cell panning round are shown.
Generation and selection of a post-alloHSCT human Fab library by phage display. (A) By using a total of 186 different and separate combinations, human Vκ, Vλ, and VH encoding sequences were amplified by reverse transcription PCR from alloHSCT (after 6m) PBMCs derived from patient A, assembled into Vκ-Cκ-VH and Vλ-Cλ-VH cassettes by 3-fragment overlap extension PCR, and cloned into phagemid pC3C by asymmetric SfiI ligation. The resulting post-alloHSCT human Fab library was subsequently converted from phagemid to phage by transformation of E coli and the addition of helper phage. (B) The post-alloHSCT human Fab library was selected by 3 rounds of panning on PBMCs derived from patient α with untreated B-CLL (round 2, round 3, and round 5). Two additional rounds of panning on immobilized rat anti-HA mAb were carried out (round 1 and round 4). The steps of 1 cell panning round are shown.
Human Fab library after alloHSCT
| . | Post-alloHSCT PBMCs . | Normal PBMCs . |
|---|---|---|
| Successful primer combinations Vκ | 10/12 (83%) | 12/12 (100%) |
| Successful primer combinations Vλ | 41/60 (68%) | 59/60 (98%) |
| Successful primer combinations VH | 81/114 (71%) | 95/114 (83%) |
| Total of successful primer combinations | 132/186 (71%) | 166/186 (89%) |
| Phagemid | pC3C | Not applicable |
| Library size | 2.6 × 108 | Not applicable |
| E coli strain | XL1-Blue | Not applicable |
| Helper phage | VCSM13 | Not applicable |
| . | Post-alloHSCT PBMCs . | Normal PBMCs . |
|---|---|---|
| Successful primer combinations Vκ | 10/12 (83%) | 12/12 (100%) |
| Successful primer combinations Vλ | 41/60 (68%) | 59/60 (98%) |
| Successful primer combinations VH | 81/114 (71%) | 95/114 (83%) |
| Total of successful primer combinations | 132/186 (71%) | 166/186 (89%) |
| Phagemid | pC3C | Not applicable |
| Library size | 2.6 × 108 | Not applicable |
| E coli strain | XL1-Blue | Not applicable |
| Helper phage | VCSM13 | Not applicable |
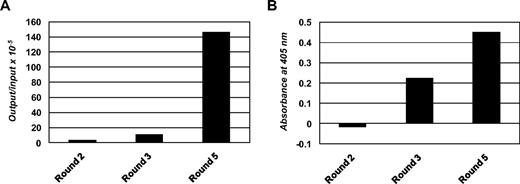
The post-alloHSCT human Fab library was selected by 3 rounds of panning on PBMCs from patient α with untreated B-CLL (Figure 3B). Two additional rounds of panning on immobilized rat anti-HA mAb were carried out before the first and after the second cell panning round to eliminate phage that did not display functional Fab with HA tag. The selection was monitored by phage output-to-input ratios (Figure 4A) and by whole-cell phage ELISA (Figure 4B), both of which showed the enrichment of phage displaying Fab with B-CLL cell-surface reactivity. Of 100 selected Fab clones that were analyzed by ELISA, whole-cell ELISA on PBMCs from patient α with untreated B-CLL, and DNA fingerprinting, 85 showed Fab expression, B-CLL cell-surface reactivity, and readable DNA fingerprints. Among these Fab clones, 73 belonged to 1 of 7 repeated DNA fingerprints and 63 belonged to 1 of 4 dominating patterns with 8 or more apparently identical Fab clones. Representative Fab clones, designated JML-1, -3, -7, and -13, each from 1 of the 4 dominating patterns, were further analyzed by DNA sequencing. The deduced amino acid sequences of the variable domains were compared with respect to germline origin, LCDR3 and HCDR3 sequences, and overall homology (Table 4). Strikingly, all 4 Fab clones were highly homologous with identical Vκ, Jκ, DH, and JH germline origins and identical LCDR3 and HCDR3 sequences. However, all Fab clones differed by at least 4 amino acids, and JML-1 had a different VH germline origin than did JML-3, -7, and -13 despite their identical HCDR3 sequences. A BLAST search of protein databases (http://blast.ncbi.nlm.nih.gov) showed that this HCDR3 sequence, GGQTIDI, is unique among rearranged heavy chains. Clearly, the high homology of the 4 dominating Fab clones, in particular their identical LCDR3 and HCDR3 sequences, imply recognition of the same antigen. JML-3, -7, and -13 showed approximately 2% overall deviation from their Vκ and VH germlines. Strong evidence for somatic hypermutation was found for the heavy chain of JML-1 with a deviation of approximately 7% from its VH germline (Table 4). On the basis of this observation and because crude Fab preparations from pooled clones with the JML-1 fingerprint consistently showed the strongest B-CLL cell-surface reactivity by whole-cell ELISA on PBMCs from patient α with untreated B-CLL (data not shown), we subsequently focused on JML-1.
Enrichment of phage displaying human Fab. (A) Phage output-to-input ratios increased approximately 50-fold over the 3 cell panning rounds, indicating the enrichment of phage displaying human Fab with cell-surface reactivity. (B) Polyclonal phage from the 3 cell panning rounds were analyzed for cell-surface reactivity in a whole-cell ELISA by using PBMCs derived from patient α with untreated B-CLL and mouse anti–phage mAb conjugated to HRP as detecting antibody. Shown are signals after subtraction of the signal obtained for the detecting antibody alone.
Enrichment of phage displaying human Fab. (A) Phage output-to-input ratios increased approximately 50-fold over the 3 cell panning rounds, indicating the enrichment of phage displaying human Fab with cell-surface reactivity. (B) Polyclonal phage from the 3 cell panning rounds were analyzed for cell-surface reactivity in a whole-cell ELISA by using PBMCs derived from patient α with untreated B-CLL and mouse anti–phage mAb conjugated to HRP as detecting antibody. Shown are signals after subtraction of the signal obtained for the detecting antibody alone.
Analysis of the amino acid sequences of selected human Fab
| . | JML-1 . | JML-3 . | JML-7 . | JML-13 . |
|---|---|---|---|---|
| Light chain | ||||
| Human germlines* | Vκ 1-39 | Vκ 1-39 | Vκ 1-39 | Vκ 1-39 |
| Jκ 3 | Jκ 3 | Jκ 3 | Jκ 3 | |
| Deviation from Vκ germline† | 0/95 | 2/95 | 1/95 | 3/95 |
| LCDR3 sequence | QQSYSTPFT | QQSYSTPFT | QQSYSTPFT | QQSYSTPFT |
| Sequence identity‡ to JML-1 | 100% | 98% | 99% | 97% |
| Sequence identity to JML-3 | 98% | 100% | 97% | 95% |
| Sequence identity to JML-7 | 99% | 97% | 100% | 96% |
| Sequence identity to JML-13 | 97% | 95% | 96% | 100% |
| Heavy chain | ||||
| Human germlines* | VH 3-9 | VH 3-30 | VH 3-30 | VH 3-30 |
| DH 3-10 | DH 3-10 | DH 3-10 | DH 3-10 | |
| JH 3 | JH 3 | JH 3 | JH 3 | |
| Deviation from VH germline† | 7/98 | 2/98 | 2/98 | 1/98 |
| HCDR3 sequence | GGQTIDI | GGQTIDI | GGQTIDI | GGQTIDI |
| Sequence identity to JML-1 | 100% | 89% | 90% | 88% |
| Sequence identity to JML-3 | 89% | 100% | 99% | 99% |
| Sequence identity to JML-7 | 90% | 99% | 100% | 98% |
| Sequence identity to JML-13 | 88% | 99% | 98% | 100% |
| . | JML-1 . | JML-3 . | JML-7 . | JML-13 . |
|---|---|---|---|---|
| Light chain | ||||
| Human germlines* | Vκ 1-39 | Vκ 1-39 | Vκ 1-39 | Vκ 1-39 |
| Jκ 3 | Jκ 3 | Jκ 3 | Jκ 3 | |
| Deviation from Vκ germline† | 0/95 | 2/95 | 1/95 | 3/95 |
| LCDR3 sequence | QQSYSTPFT | QQSYSTPFT | QQSYSTPFT | QQSYSTPFT |
| Sequence identity‡ to JML-1 | 100% | 98% | 99% | 97% |
| Sequence identity to JML-3 | 98% | 100% | 97% | 95% |
| Sequence identity to JML-7 | 99% | 97% | 100% | 96% |
| Sequence identity to JML-13 | 97% | 95% | 96% | 100% |
| Heavy chain | ||||
| Human germlines* | VH 3-9 | VH 3-30 | VH 3-30 | VH 3-30 |
| DH 3-10 | DH 3-10 | DH 3-10 | DH 3-10 | |
| JH 3 | JH 3 | JH 3 | JH 3 | |
| Deviation from VH germline† | 7/98 | 2/98 | 2/98 | 1/98 |
| HCDR3 sequence | GGQTIDI | GGQTIDI | GGQTIDI | GGQTIDI |
| Sequence identity to JML-1 | 100% | 89% | 90% | 88% |
| Sequence identity to JML-3 | 89% | 100% | 99% | 99% |
| Sequence identity to JML-7 | 90% | 99% | 100% | 98% |
| Sequence identity to JML-13 | 88% | 99% | 98% | 100% |
Based on DNA alignments using IMGT/V-QUEST (http://imgt.cines.fr)
Based on amino acid sequence alignments using IgBLAST (http://www.ncbi.nlm.nih.gov/igblast).
Based on amino acid sequences of the variable domains.
Generation and characterization of JML-1 IgG1
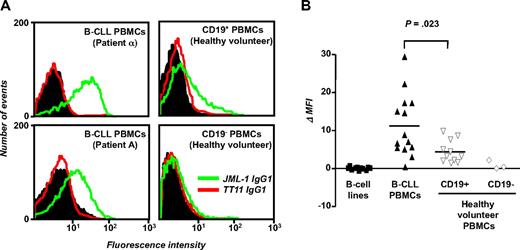
With the use of mammalian cell expression vector PIGG,41 JML-1 was converted from Fab to IgG1, expressed in HEK 293F cells, purified, and biotinylated. The previously described human anti–tetanus toxoid mAb TT11 IgG1,36 which was expressed, purified, and biotinylated in the same way as JML-1 IgG1, served as negative control. Flow cytometry confirmed the B-CLL cell-surface reactivity; JML-1 IgG1, but not TT11 IgG1, strongly bound to B-CLL cells from patient α that had been used for library selection. JML-1 IgG1 also recognized B-CLL cells from patient A, the alloHSC transplant recipient from whom the library had been generated (Figure 5A). Binding of JML-1 IgG1 to the CD19+ subpopulation of B-CLL PBMCs was evident with as little as 0.1 μg/mL, whereas no binding to the CD19− subpopulation of B-CLL PBMCs was seen with up to 10 μg/mL (data not shown). We next tested whether JML-1 IgG1 can bind to third-party B-CLL cells from untreated patients who were not involved in library selection and generation. Eleven of 12 patients with B-CLL tested showed JML-1 IgG1 but not TT11 IgG1 reactivity (Figure 5B), suggesting that the antigen recognized by JML-1 is broadly expressed in B-CLL. The level of expression in a given patient with B-CLL was found to be highly reproducible in independent experiments but was variable among different patients with B-CLL. No correlation with favorable or unfavorable prognostic markers of B-CLL1 was noted (data not shown). When PBMCs from healthy volunteers were separated by MACS into CD19+ and CD19− subpopulations, JML-1 IgG1 reactivity that also varied among different persons was noted only for the CD19+ subpopulation (Figure 5A-B). However, compared with primary B cells, the mean JML-1 IgG1 reactivity measured for primary B-CLL cells was significantly higher (P = .023, Mann-Whitney test; Figure 5B). Notably, none of 11 human B-cell lines we analyzed showed any JML-1 IgG1 reactivity. These included 2 EBV-transformed B-cell lines from patients with B-CLL; 3 EBV-transformed B-cell lines from healthy volunteers; and the previously described B-CLL cell lines EHEB and 232-B4, Burkitt lymphoma B-cell lines Daudi, Raji, and Ramos, and mantle cell lymphoma B-cell line JeKo-1 (Figure 5B). Collectively, these findings suggested that the antigen recognized by JML-1 is restricted to primary B cells and overexpressed in primary B-CLL cells.
Selective binding of JML-1 IgG1 to primary B-CLL cells and B cells. (A) B-CLL PBMCs (5 × 105) were incubated with 1 μg/mL biotinylated JML-1 IgG1 or 1 μg/mL biotinylated TT11 IgG1 and subsequently stained with CD19-APC to allow gating of B cells. In a parallel experiment, MACS-separated CD19+ and CD19− subpopulations of PBMCs from a healthy volunteer were used. Shown are flow cytometry profiles of B-CLL PBMCs from patient α (used for library selection; top left), from patient A (the alloHSC transplant recipient from whom the library originated; bottom left), and CD19+ and CD19− subpopulations of PBMCs from a representative healthy volunteer (top right and bottom right, respectively). The histograms show the binding of biotinylated JML-1 IgG1 (green) and biotinylated isotype control TT11 IgG1 (red) detected by PE-coupled streptavidin. The background signal obtained for the detection reagent alone is shown in black. (B) PBMCs from 14 patients with B-CLL (including patient α and patient A), PBMCs subpopulations from 11 (CD19+) and 3 (CD19−) healthy volunteers, and 11 B-cell lines (including B-CLL cell lines EHEB and 232-B4) were analyzed for JML-1 IgG1 and TT11 IgG1 binding as described above. Each data point depicts the MFI of an individual sample minus the MFI obtained for the detection reagent alone. Horizontal lines indicate arithmetic mean values; P, probability based on the Mann-Whitney test.
Selective binding of JML-1 IgG1 to primary B-CLL cells and B cells. (A) B-CLL PBMCs (5 × 105) were incubated with 1 μg/mL biotinylated JML-1 IgG1 or 1 μg/mL biotinylated TT11 IgG1 and subsequently stained with CD19-APC to allow gating of B cells. In a parallel experiment, MACS-separated CD19+ and CD19− subpopulations of PBMCs from a healthy volunteer were used. Shown are flow cytometry profiles of B-CLL PBMCs from patient α (used for library selection; top left), from patient A (the alloHSC transplant recipient from whom the library originated; bottom left), and CD19+ and CD19− subpopulations of PBMCs from a representative healthy volunteer (top right and bottom right, respectively). The histograms show the binding of biotinylated JML-1 IgG1 (green) and biotinylated isotype control TT11 IgG1 (red) detected by PE-coupled streptavidin. The background signal obtained for the detection reagent alone is shown in black. (B) PBMCs from 14 patients with B-CLL (including patient α and patient A), PBMCs subpopulations from 11 (CD19+) and 3 (CD19−) healthy volunteers, and 11 B-cell lines (including B-CLL cell lines EHEB and 232-B4) were analyzed for JML-1 IgG1 and TT11 IgG1 binding as described above. Each data point depicts the MFI of an individual sample minus the MFI obtained for the detection reagent alone. Horizontal lines indicate arithmetic mean values; P, probability based on the Mann-Whitney test.
Discussion
We report here the generation and selection of what is to our knowledge the first human post-alloHSCT antibody library. Adding to human immune, naive, and synthetic antibody repertoires,42,43 we have shown that the human post-alloHSCT antibody repertoire can be successfully mined by phage display to yield fully human mAbs of potential therapeutic relevance. Specifically, we present a strategy for exploiting post-alloHSCT antibody repertoires for concerted drug and target discovery in B-CLL therapy.
Finding a suitable time point for the generation of antibody libraries from post-alloHSCT PBMCs is complicated because B-cell reconstitution is variable and depends on a number of treatment-related and patient-specific parameters. We postulated that suitable time points could be narrowed by monitoring the appearance of post-alloHSCT serum antibodies with cell-surface reactivity which indicates the emerging ability of the reconstituted B-cell compartment to mount a potent serum antibody response. Although a substantial portion of this cell-surface reactivity may be mediated by antibodies that are polyspecific, autoreactive, or alloreactive, and therefore of limited use for broader mAb drug and target discovery, recent studies suggest the emergence of post-alloHSCT serum antibodies that selectively bind to tumor cell-surface antigens.23,25,28 As we show here, phage display provides a powerful tool for isolating and defining minor specificities of potentially broader utility from the post-alloHSCT antibody repertoire.
The cell-surface reactivity we detected in 2 patients with B-CLL after alloHSCT was highly transient with peaks at 6 months and 10 months, respectively. This finding is in agreement with other studies that observed peaks and waves for particular serum antibodies that emerged from post-alloHSCT antibody repertoires.28,44 By contrast, post-DLI antibody repertoires that have been mined for tumor specificity by using SEREX appear to sustain a constant level of serum antibodies over longer periods of time.17 These differences may be inherent to the divergence of reconstituting (post-alloHSCT) and reconstituted (post-DLI) B-cell compartments and may be shaped by innate and adaptive immune responses.
While the reconstituting antibody repertoire is probably dominated by natural antibodies45 that are part of the innate immune system and provide a first line of defense characterized by polyspecificity and low affinity, JML-1 and its homologues appear to be products of an adaptive immune response driven by the presence of antigen. In fact, the phage display approach we used for the selection of the JML-1 clonotype is based on monovalent Fab and thereby geared for the selection of high affinity found in secondary antibody repertoires.42 Affinity maturation by somatic hypermutation46 and receptor editing47 probably accounts for the amino acid sequence variation of JML-1, which showed the strongest B-CLL cell-surface reactivity, compared with JML-3, -7, and -13. Although we cannot exclude the possibility that PCR errors and PCR crossover artifacts contributed to this variation, a naive human antibody library that we generated with the same reagents and protocols for selection on various antigens did not yield a comparable panel of highly homologues antibodies with identical LCDR3 and HCDR3 sequences.36 Although our phage display approach involves random combination of heavy chain fragment and light chain, all selected Fab shared highly homologous chains with identical HCDR3 and LCDR3 sequences, suggesting (1) that both chains are necessary for antigen binding and (2) that the selected pair preexisted in the original post-alloHSCT antibody repertoire. It is thus tempting to speculate that JML-1 and its homologues contributed directly to GVL activity through tumor cell-surface targeting as has been suggested for post-alloHSCT and post-DLI serum antibodies.17 In follow-up studies, we are investigating the antitumor activity of JML-1 IgG1, including induction of apoptosis in B-CLL cells and mediation of complement-dependent cytotoxicity and antibody-dependent cellular cytotoxicity.
What do we know about the nature of the JML-1 antigen? The progressive elimination of B-CLL cells after alloHSCT could facilitate cross-presentation of several allogeneic antigens to the reconstituting immune system, and it is possible that at least some of the serum antibody reactivity we detected is directed against unmatched HLA (other than A, B, and DR; Table 1) of the alloHSC transplant recipient. Anti-HLA antibodies are frequently detected after organ transplantation and are thought to be involved in graft failure.48 Unlike an anti-HLA antibody, however, the dominant JML-1 clonotype showed B-cell lineage restricted reactivity against tumor and normal cells from different patients with B-CLL and healthy volunteers. In addition to its presence on the CD19+ subpopulation and its absence on the CD19− subpopulation of PBMCs, its absence on all B-cell lines tested excludes several cell-surface proteins that are currently investigated as targets for mAb therapy of B-CLL and other B-cell malignancies, including CD5, CD19, CD20, CD22, CD23, CD25, CD32B, CD37, CD38, CD40, CD45, CD52, CD74, CD79A, CD79B, CD80, CD200, HLA-DR, surface Ig, and ROR1.2-4 Its distinct expression profile also excludes B-CLL cell-surface proteins to which post-alloHSCT or post-DLI serum antibodies were previously discovered, such as OFA/iLR and BCMA.23,28 Notably, unlike SEREX, our method does not exclude non–peptidic epitopes, such as carbohydrates, lipids, and nucleic acids, which may or may not be associated with cell-surface proteins. Preliminary experiments suggested that the JML-1 antigen is constitutively expressed by B-CLL cells, and activation of B-CLL cells by either soluble recombinant human CD40L protein plus recombinant human IL-4 or a mitogen (lipopolysaccharide) did not result in significant changes in the cell-surface expression of JML-1 antigen on B-CLL cells or normal B cells from healthy volunteers (data not shown).
The identification of the JML-1 antigen is important for at least 2 reasons. First, it will allow the study of whether the JML-1 antigen is a common target of post-alloHSCT and post-DLI serum antibodies as well as alloreactive donor T cells. Second, knowing the JML-1 antigen will help to define its expression profile in normal adult tissues and its possible expression in other cancers which are important criteria for the therapeutic potential of JML-1 IgG1. Our method provides recombinant Fab or IgG in infinite supply for a variety of antigen discovery strategies, including protein microarray screening, cDNA expression cloning, and protein sequencing after immunoprecipitation.
A key advantage of our strategy is the fact that it yields fully human mAbs. Because of their potentially lower immunogenicity, fully human mAbs are considered superior to nonhuman, chimeric, and humanized mAbs in therapeutic applications that require repeated dosing.49 Post-alloHSCT antibody repertoires provide a novel route to the discovery of fully human mAbs. Expanding our strategy beyond B-CLL to other hematologic and solid malignancies that are treated with alloHSCT50 will determine its broader utility as an antibody drug and target discovery platform for cancer therapy.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs Robert M. Dean, Daniel H. Fowler, Ronald E. Gress, Frances T. Hakim, Gerald E. Marti, William G. Telford, Adrian Wiestner, and Wyndham H. Wilson for assistance and advice.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH (C.R.) and through a stipend from the National Foundation for Science, Higher Education and Technological Development of the Republic of Croatia (I.S.).
National Institutes of Health
Authorship
Contribution: S.B. and C.R. designed and supervised the experiments, interpreted data, and edited the manuscript; S.B., J.M.S., and I.S. performed experiments and analyzed data; R.S., R.W.C., S.Z.P., and M.R.B. participated in the design of the study and interpretation of data and edited the manuscript; and C.R. conceptualized the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christoph Rader, Experimental Transplantation and Immunology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, 9000 Rockville Pike, Bldg 10 CRC, Rm 3-3150, Bethesda, MD 20892-1203; e-mail: raderc@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal