Abstract
Recent reports describe hematopoietic abnormalities in mice with targeted instability of the mitochondrial genome. However, these abnormalities have not been fully described. We demonstrate that mutant animals develop an age-dependent, macrocytic anemia with abnormal erythroid maturation and megaloblastic changes, as well as profound defects in lymphopoiesis. Mice die of severe fatal anemia at 15 months of age. Bone-marrow transplantation studies demonstrate that these abnormalities are intrinsic to the hematopoietic compartment and dependent upon the age of donor hematopoietic stem cells. These abnormalities are phenotypically similar to those found in patients with refractory anemia, suggesting that, in some cases, the myelodysplastic syndromes are caused by abnormalities of mitochondrial function.
Introduction
The myelodysplastic syndromes (MDSs) are a heterogeneous group of aging-associated disorders characterized by peripheral-blood cytopenias with hypercellular and dysplastic bone marrow.1 Several lines of evidence implicate disruptions of mitochondrial physiology as an important factor in the development of human MDS. First, mitochondria play a prominent role in the regulation of apoptosis,2 a generally accepted mechanism for the ineffective hematopoiesis of MDS.3 Furthermore, the ringed sideroblasts observed in many patients with MDS result from iron deposition within mitochondria of developing erythroblasts.4 Likewise, Pearson syndrome, which presents in infancy with a profound sideroblastic anemia, is caused by a large-scale deletion of mitochondrial DNA (mtDNA).5,6 Finally, certain drugs, including chloramphenicol7 and linezolid,8 cause dyserythropoiesis by disrupting mitochondrial protein synthesis. Hence, mitochondrial dysfunction may contribute to the development of human MDS.
Mitochondria serve critical functions in cellular metabolism, pyrimidine biosynthesis,9 and apoptosis.2 These functions are regulated in part by a distinct mitochondrial genome that replicates within the organelle, independent of nuclear chromosomal DNA. The unique features of mtDNA replication impact on the genetics of mitochondrial diseases. First, acquisition of mtDNA mutations can occur independent of cell division and therefore arise in quiescent somatic tissues.10 Second, both mutated and wild-type (WT) mtDNAs can exist in the same cell, a condition called heteroplasmy. In somatic cells, segregation of mitochondrial genomes occurs randomly during mitosis,11 with important implications for the extent of heteroplasmy and consequent expressivity of disorders caused by mtDNA mutations.
Whether somatic mtDNA mutations contribute to human MDS is controversial. Two groups12-14 identified pathogenetic mtDNA mutations in unfractionated bone-marrow samples from some patients with MDS. However, Shin et al15 demonstrated nearly equivalent levels of mtDNA mutations in both patients with MDS and volunteer subjects not known to have hematologic disease. Most recently, Wulfert et al16 demonstrated heteroplasmic point mutation rates of 38% in patients with refractory anemia and 68% in patients with refractory anemia with excess blasts in transformation compared with 48% in patients with chronic myeloid leukemia. Overall, the various methods used in these reports, as well as the difficulties inherent in separating individual variation from pathogenic changes, have made it difficult to reach firm conclusions regarding mtDNA mutations in patients with MDS.
In light of these conflicting results, we sought to determine whether mitochondrial dysfunction resulting from targeted mutagenesis of mtDNA could recapitulate features of human MDS. Experimental models to explore the role of mitochondrial dysfunction in aging have only recently become available. Replication of mammalian mtDNA is controlled by DNA polymerase gamma (Polg), a protein encoded by the nuclear genome. Polg has 2 enzymatic activities: a C-terminal polymerase and an N-terminal “proofreading” exonuclease. To generate mice with accelerated rates of mtDNA mutation, 2 independent groups selectively ablated Polg proofreading activity in mice by creating a germ-line missense mutation (Polg D257A) within the exonuclease domain.17,18 Homozygous mutant animals (PolgA/A) exhibit numerous features of accelerated aging, including progressive anemia. The purpose of the current study was to better define the hematopoietic abnormalities associated with the decreased mitochondrial function of PolgA/A mice. We show that these animals develop a progressive, ultimately fatal megaloblastic anemia that is associated with both erythrodysplasia and impaired lymphopoiesis, 2 findings that frequently are observed in human patients with MDS. Overall, these data demonstrate that mitochondrial dysfunction in the hematopoietic compartment can generate key features of human MDS and suggest that the Polg D257A model is a valid system with which to study the biology of these poorly understood disorders.
Methods
Mice
Polg D257A mice were generated and maintained as described in supplemental Methods (available on the Blood website; see the Supplemental Materials link at the top of the online article). B6.SJL mice were procured at 4 to 6 weeks of age (National Cancer Institute). All Polg mice were bred and housed at a pathogen-free facility in microisolator cages. β-thalassemia intermedia mice (β-globin+/th−3) and WT littermate controls (β-globin+/+) were provided by Mitchell Weiss (Children's Hospital of Philadelphia) and housed as described previously.19 Mice studies were performed following Institutional Animal Care and Use Committee approval from the University of Pennsylvania and Roswell Park Cancer Institute.
Peripheral-blood analysis
Peripheral blood was collected in ethylene diamine tetra-acetic acid–treated tubes (Microvette 100, Sarsted). Samples were diluted 6-fold with phosphate-buffered saline containing 1% bovine serum albumin and then used to prepare Wright-stained smears and for analysis on an Advia 2120 hematology analyzer, using the provided mouse archetype software (Siemens Health Diagnostics Inc).
Flow cytometric analysis
All antibodies were obtained from BioLegend or BD Biosciences. After the study mice were humanely killed, single-cell suspensions from bone marrow, spleen, and/or thymus were prepared and analyzed as described in supplemental Methods.
Morphologic evaluation
Sorted bone-marrow cell populations (2 × 105 cells per slide) were spun in a Cytospin 2 (Thermo Shandon Inc), air-dried, then stained with Wright-Giemsa by the use of a modified technique for murine cells (Scott Kogan, University of California, San Francisco, e-mail communication, May 2007; see supplemental Methods). Images were obtained with the use of a Nikon 80i with a 100×/0.5-1.3 Oil Iris lens (Nikon Inc) with an attached DFC320 camera that uses FireCam 1.4 software (Leica Microsystems). Image sizes were calibrated by the use of a 1-mm stage micrometer (Fisher Scientific), and all images were cropped and scaled in parallel with the resulting scale bar without further modification.
Transplantation
Three days after starting antibiotic prophylaxis (0.05% neomycin and 100 μ/mL polymyxin), B6.SJL mice received a single dose of lethal irradiation (950 cGy). The following day, single-cell suspensions of bone marrow were isolated from donor mice, and 5 × 106 viable nucleated cells were injected into the retroorbital sinus of recipient B6.SJL mice as previously described.20 Transplant recipient mice were maintained on freshly changed antibiotic water for 3 weeks after transplant.
Mitochondrial function
After the mice were humanely killed, mitochondria were isolated from livers and spleens as described in supplemental Methods. Protein concentration was quantified with the use of the BCA assay (Pierce) with bovine serum albumin as standard. Enzyme assays for the mtDNA-dependent enzyme cytochrome oxidase (complex IV) and the nuclear DNA-dependent enzyme citrate synthase were performed as described in supplemental Methods. Each sample was assayed in duplicate or triplicate by the use of different protein concentrations.
Statistics
All plotted data are reported as mean plus or minus SEM. All statistical tests, as described in detail in supplemental Methods, were 2-sided and tested at a nominal significance level of P less than .05.
Results
PolgA mice develop age-related macrocytic anemia and lymphopenia
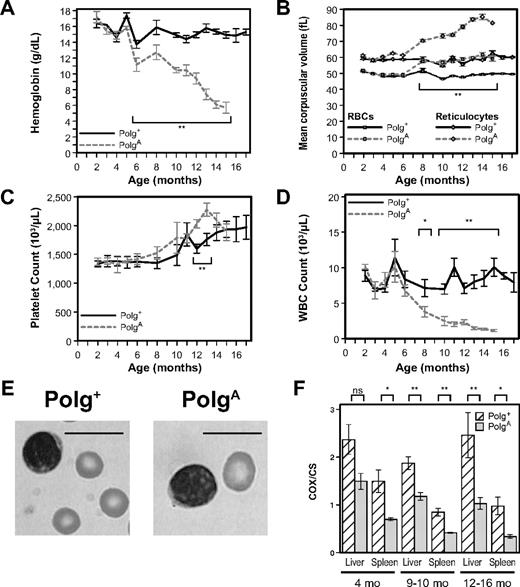
To define the hematopoietic phenotype of PolgA/A mice, we monitored blood counts serially in a cohort of 15 mice (5 Polg+/+, 5 Polg+/A, and 5 PolgA/A) from age 2 to 17 months. No difference in peripheral-blood counts was observed between Polg+/+ and Polg+/A mice (data not shown), and therefore data from Polg+/+ and Polg+/A mice have been combined and referred to as Polg+, whereas data from PolgA/A mice will be referred to as PolgA. From 1 to 4 months of age both hemoglobin (Hgb) levels (Figure 1A) and mean corpuscular volume (Figure 1B, squares) were indistinguishable between genotypes. From 6 to 10 months of age the Hgb levels of PolgA mice were moderately decreased in comparison with Polg+ mice, with significant anemia appearing as early as 6 months of age (P < .001) that worsened only modestly up to 10 months of age (P < .001). From the age of 11 months onwards, however, the Hgb levels of PolgA mice worsened rapidly, resulting in their demise at 15 months of age. The Hgb levels of littermate Polg+ mice remained stable throughout the experiment, which was terminated when the Polg+ mice reached 17 months of age (Figure 1A). Macrocytosis became evident in PolgA mice shortly after the development of their anemia (Figure 1B squares), with a statistically significant difference between the genotypes from 8 months of age until the termination of the experiment (P < .001). This phenotype also worsened significantly with aging and was readily appreciated on examination of peripheral blood (Figure 1E). As with the Hgb levels, the MCVs of littermate Polg+ mice remained stable throughout the experiment.
PolgA mice develop age-related macrocytic anemia and leukopenia associated with dysfunction of mitochondria isolated from both liver and spleen. (A-D) Peripheral-blood samples from both Polg+ and PolgA mice were analyzed from 2 to 17 months of age. Each point represents the mean ± SEM of Polg+ mice (n = 3-12, solid lines) and PolgA mice (n = 3-9, dotted lines). (A) Hemoglobin levels. (B) Mean corpuscular volume of all RBCs (□) and of reticulocytes only (◇). (C) Platelet count. (D) White blood cell (WBC) count. (E) Representative Wright-Giemsa–stained images of RBCs and lymphocytes from peripheral-blood smears of 15-month-old mice. Bars represent 10 microns. (F) The relative activity of the mtDNA-dependent mitochondrial enzyme cytochrome c oxidase (COX) was compared with that of the nuclear DNA-dependent mitochondrial enzyme citrate synthase (CS) in mitochondria isolated from the livers and spleens of both Polg+ and PolgA mice at the indicated ages. Each bar represents the mean ± SEM of mitochondria isolated from liver or spleen, respectively, of Polg+ mice (n = 3-6, cross-hatched) and PolgA mice (n = 3-5, gray) at the indicated age. *P < .05 (statistical significance) by Student t test; **P < .01
PolgA mice develop age-related macrocytic anemia and leukopenia associated with dysfunction of mitochondria isolated from both liver and spleen. (A-D) Peripheral-blood samples from both Polg+ and PolgA mice were analyzed from 2 to 17 months of age. Each point represents the mean ± SEM of Polg+ mice (n = 3-12, solid lines) and PolgA mice (n = 3-9, dotted lines). (A) Hemoglobin levels. (B) Mean corpuscular volume of all RBCs (□) and of reticulocytes only (◇). (C) Platelet count. (D) White blood cell (WBC) count. (E) Representative Wright-Giemsa–stained images of RBCs and lymphocytes from peripheral-blood smears of 15-month-old mice. Bars represent 10 microns. (F) The relative activity of the mtDNA-dependent mitochondrial enzyme cytochrome c oxidase (COX) was compared with that of the nuclear DNA-dependent mitochondrial enzyme citrate synthase (CS) in mitochondria isolated from the livers and spleens of both Polg+ and PolgA mice at the indicated ages. Each bar represents the mean ± SEM of mitochondria isolated from liver or spleen, respectively, of Polg+ mice (n = 3-6, cross-hatched) and PolgA mice (n = 3-5, gray) at the indicated age. *P < .05 (statistical significance) by Student t test; **P < .01
To determine whether the observed increase in red blood cell (RBC) size reflected alterations in RBC development, we next confined our analyses of MCV to reticulocytes. Although the MCV of reticulocytes from PolgA mice was essentially identical to that of Polg+ mice through 6 months of age, it increased sharply from 8 months of age until the termination of the experiment (Figure 1B, diamonds, P < .001). The platelet counts of both Polg+ and PolgA mice remained stable up to 6 months of age and then increased in parallel at 8 to 10 months of age (Figure 1C). Although the difference in platelet counts at both 12 and 13 months of age reached statistical significance (P < .005), the biologic significance of this difference is unclear, given the subsequent loss of statistical significance attributable to the combination of decreasing platelet levels of PolgA mice near the time of their ultimate demise and increasing platelet levels of aging Polg+ mice. The white blood cell count of PolgA mice was consistent with that of Polg+ mice up to 4 months of age, but by 8 months of age the PolgA mice became significantly lymphopenic (P = .046), remaining so for the duration of the experiment (Figure 1D, P < .002). The lymphopenia of these mice appeared to involve both major lymphoid lineages because the relative percentage of T cells and B cells was identical between genotypes (data not shown). Of note, PolgA mice never developed leukocytosis or other signs of leukemic conversion. Overall, these results demonstrate that aging PolgA mice develop a profound macrocytic anemia associated with marked B-cell and T-cell defects.
Development of anemia in PolgA is associated with mitochondrial dysfunction
To determine whether mitochondrial dysfunction develops with aging in PolgA mice, we isolated mitochondria from both liver and spleen from both Polg+ and PolgA mice at 4, 9 to 10, and 12 to 14 months of age and then determined the specific activities of cytochrome oxidase (COX) and citrate synthase (CS) in mitochondrial protein extracts. The activity of COX is dependent upon 3 mtDNA-encoded polypeptides, whereas the activity of CS is completely independent of mtDNA. Given the differing genomic origins of these 2 mitochondrial protein complexes, the ratio of their specific activities provides a direct assessment of mtDNA-dependent protein function. As early as 4 months of age, a decreased COX/CS ratio was observed in mitochondria from PolgA mice (Figure 1F, 4 months, gray bars) compared with Polg+ mice (cross-hatched bars), although the difference was statistically significant in the spleen (P < .005) but not the liver (P = .068). By 9 to 10 months of age the difference in the COX/CS ratio was significant in both liver (P < .005) and spleen (P < .01), and these differences remained significant in mice older than 12 months of age (Figure 1F). This progressive impairment of COX activity relative to CS activity in aging PolgA mice is consistent with the direct impairment of mtDNA-dependent mitochondrial function caused by the loss of Polg proofreading activity.
Analysis of erythroid development
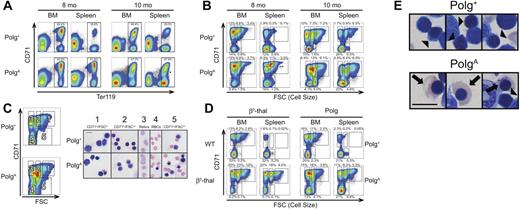
To characterize alterations in erythropoiesis associated with the macrocytosis and anemia of PolgA mice, the relative expression of the erythroid marker Ter119 and the transferrin receptor (CD71) in bone marrow and splenic erythroblasts were analyzed by flow cytometry in cohorts of Polg+ and PolgA mice at 6, 8, and 10 months of age. As demonstrated in the top row of Figure 2A, both marrow and spleen of Polg+ mice contained populations of Ter119+ erythroid precursors with a greater frequency of Ter119+ cells in the marrow than in the spleen. The relative frequency of various stages of erythroid development were explored in greater detail through the analysis of the Ter119+ region (boxes in Figure 2A) by both CD71 and forward light scatter (FSC), a surrogate for cell size, as described by Liu et al.21 As demonstrated in the top row of Figure 2B, the analysis of both bone marrow and spleen revealed 4 distinct populations, with polychromatophilic normoblasts (population 2 in Figure 2C) and reticulocytes (population 3) predominating in the Ter119+ population of normal bone marrow and mature RBCs (population 4) predominating in the Ter119+ population of normal spleen. To determine the sensitivity of this analytic method to alterations in erythropoiesis, we next analyzed both bone marrow and spleen from 6-month-old thalassemic mice, or their WT littermate controls, in parallel with 10-month-old Polg+ and PolgA mice. This mouse model of β-thalassemia intermedia has been previously described22 and features a prominent expansion of erythropoiesis that exceeds the reserve capacity of the bone marrow, resulting in an expansion of splenic erythropoiesis, a response termed “stress erythropoiesis.”23 As shown in the left-hand panels of Figure 2D, the significant anemia observed in the thalassemic mouse (Hgb 7.8 g/dL) was associated with a stress erythropoietic response characterized by an expansion of polychromatophilic normoblasts and reticulocytes in both marrow and spleen.
Abnormal erythroid development in aging PolgA mice. Polg+ and PolgA mice were humanely killed for analysis at the indicated ages. Single-cell suspensions were prepared from bone marrow (BM) and spleen (Spleen) without hypotonic lysis, then stained for surface expression of CD71 (transferrin receptor) and the erythroid-specific antigen Ter119. In panels A and B, the individual graphs presented are representative of 3 independent experiments for each age group. In each independent experiment, one PolgA mouse was compared with one or 2 Polg+ littermates. The percentages shown with each region represent the mean frequency of that population, as a percentage of all singlet events, across all 3 experiments for the indicated age group. Each region was analyzed for statistically significant differences in frequency between genotypes using the Student t test (*P < .05, **P < .01). (A) All events remaining after exclusion of debris and nonsinglet events (80%-90% of all events; data not shown) were analyzed on the basis of expression of CD71 and Ter119. (B) The regions indicated in panel A were further analyzed on the basis of FSC, a surrogate for cell size relative to expression of CD71. (C) After the exclusion of both lineage+ and nonviable cells (see “Flow cytometric analysis” in supplemental Methods) as well as gating as described in panel A, cells from 5 populations were sorted by use of the indicated regions. Sorted cells were then spun on slides, stained with Wright-Giemsa, and visualized. All images were acquired at identical magnification; bar represents 10 microns. (D) After preparation and analysis as described in panel A, spleen and bone marrow from 6-month-old β-thalassemia (bottom left) and WT littermate control (top left) mice were analyzed as in panel B in parallel with samples from 10-month-old Polg+ (top right) and PolgA (bottom right) mice. These results from a single parallel experiment are representative of results obtained during independent analysis of β-thalassemia and Polg D257A samples (3 and 7 samples, respectively). (E) Whole bone-marrow cell suspensions from 10-month-old Polg+ (left) and PolgA (right) mice prepared as in (C) demonstrate the presence of abnormally hemoglobinized orthochromatic normoblasts (arrows) in PolgA marrow only and normal polychromatic normoblasts (arrowheads) in both Polg+ and PolgA marrow. All images were acquired at identical magnification; bar represents 10 microns.
Abnormal erythroid development in aging PolgA mice. Polg+ and PolgA mice were humanely killed for analysis at the indicated ages. Single-cell suspensions were prepared from bone marrow (BM) and spleen (Spleen) without hypotonic lysis, then stained for surface expression of CD71 (transferrin receptor) and the erythroid-specific antigen Ter119. In panels A and B, the individual graphs presented are representative of 3 independent experiments for each age group. In each independent experiment, one PolgA mouse was compared with one or 2 Polg+ littermates. The percentages shown with each region represent the mean frequency of that population, as a percentage of all singlet events, across all 3 experiments for the indicated age group. Each region was analyzed for statistically significant differences in frequency between genotypes using the Student t test (*P < .05, **P < .01). (A) All events remaining after exclusion of debris and nonsinglet events (80%-90% of all events; data not shown) were analyzed on the basis of expression of CD71 and Ter119. (B) The regions indicated in panel A were further analyzed on the basis of FSC, a surrogate for cell size relative to expression of CD71. (C) After the exclusion of both lineage+ and nonviable cells (see “Flow cytometric analysis” in supplemental Methods) as well as gating as described in panel A, cells from 5 populations were sorted by use of the indicated regions. Sorted cells were then spun on slides, stained with Wright-Giemsa, and visualized. All images were acquired at identical magnification; bar represents 10 microns. (D) After preparation and analysis as described in panel A, spleen and bone marrow from 6-month-old β-thalassemia (bottom left) and WT littermate control (top left) mice were analyzed as in panel B in parallel with samples from 10-month-old Polg+ (top right) and PolgA (bottom right) mice. These results from a single parallel experiment are representative of results obtained during independent analysis of β-thalassemia and Polg D257A samples (3 and 7 samples, respectively). (E) Whole bone-marrow cell suspensions from 10-month-old Polg+ (left) and PolgA (right) mice prepared as in (C) demonstrate the presence of abnormally hemoglobinized orthochromatic normoblasts (arrows) in PolgA marrow only and normal polychromatic normoblasts (arrowheads) in both Polg+ and PolgA marrow. All images were acquired at identical magnification; bar represents 10 microns.
Abnormal erythroid development in aging PolgA mice
Despite the development of anemia as early as 6 months of age (Figure 1A), no differences in either marrow or splenic erythropoiesis were observed in 6-month-old mice (data not shown). Likewise, the marrow of 8-month-old PolgA mice was essentially identical to that of littermate Polg+ mice, demonstrating similar frequencies of total Ter119+ cells (Figure 2A, 8 months, BM) as well as a preponderance of both reticulocytes and polychromatophilic normoblasts (Figure 2B, 8 months, BM). Consistent with the stress erythropoietic response observed in thalassemic mice (Figure 2D), analysis of 8-month-old PolgA mice demonstrated a significant increase in the frequency of Ter119+ splenocytes (mean frequencies of 51.6% in PolgA mice vs. 18.6% in control mice, P < .001), with a significant expansion in both polychromatophilic normoblasts and reticulocytes (Figure 2B, 8 months, spleen, P < .001) that was nearly identical to that observed in the spleen of thalassemic mice (Figure 2D). Upon analysis of splenocytes from 10-month-old PolgA mice (Figure 2A-B, 10 months, spleen) a pattern consistent with stress erythropoiesis was again observed, but a statistically significant increase in relative frequency was only observed in polychromatophilic normoblasts (P = .017). In the bone-marrow compartment, although the total number of Ter119+ events was largely similar between the genotypes (Figure 2A, 10 months, BM, P = .88), analysis of the Ter119+ population on the basis of cell size versus CD71 expression (Figure 2B, 10 months, BM) demonstrated a significant expansion of polychromatophilic normoblasts (P = .017) at the expense of both reticulocytes (nonsignificant decrease, P = .052) and mature RBCs (significant decrease, P = .012). Furthermore, a fifth population of erythroblasts, characterized by intermediate cell size and low-to-negative expression of CD71, also was identified and shown to be significantly expanded in the marrow of 10-month-old PolgA mice (P = .015).
To further characterize this abnormal erythroblast population, we next sorted erythroblasts from 10-month-old Polg+ and PolgA mice, gating on the regions indicated in Figure 2C, and then performed cytospins followed by Wright-Giemsa staining. Morphologic analysis of the abnormal population of CD71 low cells of intermediate size sorted from region number 5 demonstrated a large population of orthochromic erythroblasts with abundant, fully hemoglobinized cytoplasm (Figure 2C column 5, PolgA). This finding is an example of the nuclear-cytoplasmic dyssynchrony that is characteristic of megaloblastic morphologic changes. Hemoglobinized orthochromatic erythroblasts were readily appreciated, although less frequent, in other marrow erythroblast populations of PolgA mice (Figure 2D bottom row), as well as in cytospins of unfractionated marrow from PolgA mice beginning at 8 months of age (Figure 2E arrows). Similar megaloblastic changes were never observed in cytospins of either sorted cells (Figure 2C, top row) or of unfractionated marrow and spleen (Figure 2E) from the Polg+ littermates. Consistent with the absence of this megaloblastic population in other models of anemia,21,24 parallel analysis of both thalassemic and PolgA mice demonstrated these megaloblastic changes in the marrow of the 10-month-old PolgA mouse only, despite a more severe anemia in the thalassemic mouse (Hgb 7.8 g/dL vs 10.2 g/dL). Of note, iron staining of sorted erythroblasts and whole bone marrow did not demonstrate ringed sideroblasts (data not shown).
Abnormal lymphoid development in aging PolgA mice
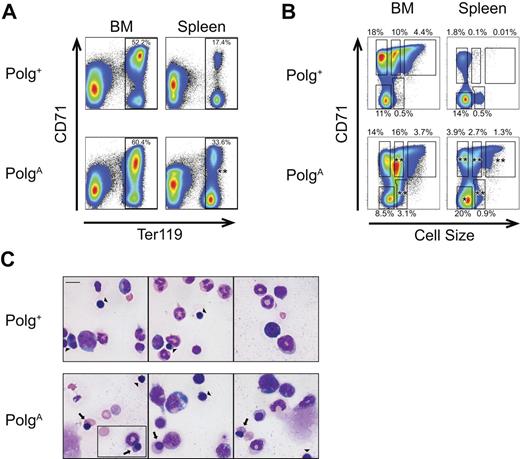
To further characterize the progressive B-cell and T-cell defects of aging PolgA mice, the relative frequency of various lymphoid precursor populations were assessed in 8- to 13-month-old mice. Consistent with the previously reported decrease in overall thymic mass of PolgA mice,18 PolgA mice demonstrated a significant decrease in total viable thymocytes compared with Polg+ littermates (mean 2.0 × 105 vs 5.6 × 107, P = .006), with a significant decrease in the relative frequency of CD4/CD8 double-positive cells (P = .012, Figure 3A). We next analyzed the frequency of pro-B, immature B/pre-B, and mature B-cell populations in bone marrow. Consistent with the peripheral-blood lymphopenia described previously, the analysis of the bone marrow of PolgA mice (Figure 3B) demonstrated a significant decrease in mature B cells (P = .022). These defects were not limited to more mature B-cell compartments because there were also significant defects in the generation of both pro-B (P = .009) and immature B/pre-B cells (P = .007; Figure 3B).
Abnormal lymphoid development in aging PolgA mice. Polg+ and PolgA mice were humanely killed for analysis at 9 to 13 months of age. Single-cell suspensions were prepared from thymus and bone marrow (BM), stained as indicated for each panel, and then incubated with the viability stain 4′,6-diamidino-2-phenylindole immediately before analysis. The individual graphs presented are representative of 5 independent experiments, in which a total of 6 PolgA and 5 Polg+ mice were analyzed. The percentages shown with each region represent the mean frequency of that population, as a percentage of viable singlet events, across all 4 experiments. *P < .05, **P < .01, as assessed by Student t test, for regions with statistically significant differences in frequency between genotypes. (A) Thymus: The mean frequencies of CD4+CD8+ (double-positive) cells (oval) and CD4−CD8− (double-negative) cells (rectangle) are shown. (B) Bone marrow: The mean frequencies of mature B cells (B220hiCD43−, top left region), immature B/pre B cells (B220intCD43−, bottom left region) and B220+CD43+ cells (oval), are presented. The B220+CD43+ region was further analyzed for expression of AA4.1 and CD19 (right panels). The mean frequency of B220+CD43+AA4.1+CD19+ pro-B cells as a percentage of viable singlet BM cells is presented.
Abnormal lymphoid development in aging PolgA mice. Polg+ and PolgA mice were humanely killed for analysis at 9 to 13 months of age. Single-cell suspensions were prepared from thymus and bone marrow (BM), stained as indicated for each panel, and then incubated with the viability stain 4′,6-diamidino-2-phenylindole immediately before analysis. The individual graphs presented are representative of 5 independent experiments, in which a total of 6 PolgA and 5 Polg+ mice were analyzed. The percentages shown with each region represent the mean frequency of that population, as a percentage of viable singlet events, across all 4 experiments. *P < .05, **P < .01, as assessed by Student t test, for regions with statistically significant differences in frequency between genotypes. (A) Thymus: The mean frequencies of CD4+CD8+ (double-positive) cells (oval) and CD4−CD8− (double-negative) cells (rectangle) are shown. (B) Bone marrow: The mean frequencies of mature B cells (B220hiCD43−, top left region), immature B/pre B cells (B220intCD43−, bottom left region) and B220+CD43+ cells (oval), are presented. The B220+CD43+ region was further analyzed for expression of AA4.1 and CD19 (right panels). The mean frequency of B220+CD43+AA4.1+CD19+ pro-B cells as a percentage of viable singlet BM cells is presented.
Transplantation of PolgA marrow into Polg+ recipients recapitulates age-related macrocytic anemia and lymphopenia of PolgA mice
Given the prominent aging-related intestinal pathology observed in PolgA mice,18 we next transplanted PolgA marrow into Polg+ hosts to exclude cell-extrinsic effects, eg, malabsorption of B12 or folate, as the cause of these megaloblastic changes. For all experiments, 2-month-old congenic B6.SJL mice were lethally irradiated and reconstituted with either Polg+/+, Polg+/A, or PolgA/A marrow (5 B6.SJL hosts per group). As described previously for intact mice, no differences were observed between recipients of Polg+/+ and Polg+/A marrow, and all data from these mice have been combined and referred to as Polg+. In our initial experiments, 2-month-old mice of all 3 genotypes were used as marrow donors, whereas in subsequent experiments 8-momth-old mice (Polg+/+ and PolgA/A genotypes only) were used. Complete engraftment of donor hematopoiesis in all recipients was confirmed through CD45B6 versus CD45SJL analysis of peripheral-blood leukocytes (data not shown). Although anemia did not become apparent in whole PolgA mice until 6 months of age (Figure 1A), mice transplanted with 2-month-old PolgA marrow demonstrated significantly lower Hgb levels compared with mice transplanted with Polg+ marrow as early as 2 months after transplantation, or 4 months of age (Figure 4A, P < .001). Although the MCVs of both genotypes were initially lower than normal, both the MCV (Figure 4B squares) and reticulocyte MCV (Figure 4B, diamonds) of mice transplanted with PolgA marrow were significantly greater than that of mice transplanted with Polg+ marrow as early as 5 months of age (P < .001). Although aging-related increases in platelet counts were observed in both whole PolgA and whole Polg+ mice starting between 10 and 12 months of age (Figure 1C), a statistically significant increase in platelet counts was observed in the recipients of PolgA marrow as early as 8 months of age (Figure 4C, P < .01). Despite the clear difference in platelet counts between the 2 genotypes, upon review of marrow histology no significant differences in megakaryocyte morphology were appreciated (data not shown). In parallel with the findings in whole PolgA mice (Figure 1D), lymphopenia also was observed in the recipients of PolgA marrow (Figure 4D).
Transplantation of PolgA marrow into Polg+ recipients recapitulates age-related macrocytic anemia and leukopenia of PolgA mice. Two-month-old B6.SJL hosts were lethally irradiated and then reconstituted with 5 × 106 nucleated marrow cells from 2-month-old donor mice. Peripheral-blood samples were analyzed as in Figure 1 from 2 to 8 months after transplantation (4-10 months of age). Each point represents the mean ± SEM of recipients of Polg+ marrow (n = 9, solid lines) and recipients of PolgA marrow (n = 5, dotted lines). (A) Hemoglobin levels. (B) Mean corpuscular volume of all RBCs (□) and of reticulocytes only (◇). (C) Platelet count. (D) White blood cell (WBC) count. (E) Representative Wright-Giemsa–stained images of RBCs and lymphocytes from peripheral blood smears of 15-month-old mice. Bars represent 10 microns. * P < .05 (statistical significance) by Student t test; **P < .01
Transplantation of PolgA marrow into Polg+ recipients recapitulates age-related macrocytic anemia and leukopenia of PolgA mice. Two-month-old B6.SJL hosts were lethally irradiated and then reconstituted with 5 × 106 nucleated marrow cells from 2-month-old donor mice. Peripheral-blood samples were analyzed as in Figure 1 from 2 to 8 months after transplantation (4-10 months of age). Each point represents the mean ± SEM of recipients of Polg+ marrow (n = 9, solid lines) and recipients of PolgA marrow (n = 5, dotted lines). (A) Hemoglobin levels. (B) Mean corpuscular volume of all RBCs (□) and of reticulocytes only (◇). (C) Platelet count. (D) White blood cell (WBC) count. (E) Representative Wright-Giemsa–stained images of RBCs and lymphocytes from peripheral blood smears of 15-month-old mice. Bars represent 10 microns. * P < .05 (statistical significance) by Student t test; **P < .01
Transplantation of PolgA marrow into Polg+ recipients recapitulates megaloblastic features of PolgA mice
To determine whether isolation of the PolgA mutation to the hematopoietic compartment likewise recapitulated the megaloblastic changes observed in PolgA mice, we next repeated the studies described in Figure 2 on bone marrow and spleen from 8-, 9-, and 10-month-old transplant recipients. Although both the marrow and spleen of recipients of PolgA marrow demonstrated similar changes at 8 and 9 months of age (data not shown), these changes were most prominent at 10 months of age (Figure 5). Analysis of splenocytes from 10-month-old recipients of PolgA marrow (Figures 5A-B, bottom right panels) demonstrated statistically significant increases in all Ter119+ populations, consistent with both stress erythropoiesis as well as the profound lymphopenia observed in these mice (Figure 4D). Analysis of the Ter119+ population in marrow of these mice (Figure 5B bottom left panel) demonstrated a statistically significant increase in the frequency of both polychromatophilic normoblasts (P < .001) and FSCint/CD71lo erythroblasts (P < .001) that closely matched the changes observed in the marrow of whole PolgA mice (Figure 2B, 10 months, BM). Upon morphologic examination, megaloblastic changes were again readily identified in whole marrow (Figure 5C, bottom row, arrows) as well as sorted erythroblast populations (data not shown).
Transplantation of PolgA marrow into Polg+ recipients recapitulates megaloblastic features of PolgA mice. Cells from the bone marrow and spleen of 10-month-old transplant recipient mice were prepared and analyzed as described in Figure 2. The individual graphs presented are representative of 3 independent experiments, in which a total of 3 PolgA and 5 Polg+ mice were analyzed. The percentages shown with each region represent the mean frequency of that population, as a percentage of all singlet events, across all 3 experiments. *P < .05, **P < .01, as assessed by Student t test, for regions with statistically significant differences in frequency between genotypes. (A) All events remaining after exclusion of debris and nonsinglet events (80%-90% of all events; data not shown), were analyzed on the basis of expression of CD71 and Ter119. The frequency of Ter119+ cells, as a percentage of all singlet events, is presented. (B) The regions indicated in panel A were further analyzed on the basis of cell size relative to expression of CD71. (C) Whole bone-marrow cell suspensions from 10-month-old recipients of Polg+ (top panels) and PolgA (bottom panels) marrow prepared as in Figure 2 demonstrate the presence of abnormally hemoglobinized orthochromatic normoblasts (arrows) in PolgA marrow only and normal polychromatic normoblasts (arrowheads) in both Polg+ and PolgA marrow. All images were acquired at identical magnification; bar represents 10 microns.
Transplantation of PolgA marrow into Polg+ recipients recapitulates megaloblastic features of PolgA mice. Cells from the bone marrow and spleen of 10-month-old transplant recipient mice were prepared and analyzed as described in Figure 2. The individual graphs presented are representative of 3 independent experiments, in which a total of 3 PolgA and 5 Polg+ mice were analyzed. The percentages shown with each region represent the mean frequency of that population, as a percentage of all singlet events, across all 3 experiments. *P < .05, **P < .01, as assessed by Student t test, for regions with statistically significant differences in frequency between genotypes. (A) All events remaining after exclusion of debris and nonsinglet events (80%-90% of all events; data not shown), were analyzed on the basis of expression of CD71 and Ter119. The frequency of Ter119+ cells, as a percentage of all singlet events, is presented. (B) The regions indicated in panel A were further analyzed on the basis of cell size relative to expression of CD71. (C) Whole bone-marrow cell suspensions from 10-month-old recipients of Polg+ (top panels) and PolgA (bottom panels) marrow prepared as in Figure 2 demonstrate the presence of abnormally hemoglobinized orthochromatic normoblasts (arrows) in PolgA marrow only and normal polychromatic normoblasts (arrowheads) in both Polg+ and PolgA marrow. All images were acquired at identical magnification; bar represents 10 microns.
Transplantation of old PolgA marrow into young Polg+ recipients accelerates the development of age-related macrocytic anemia and lymphopenia
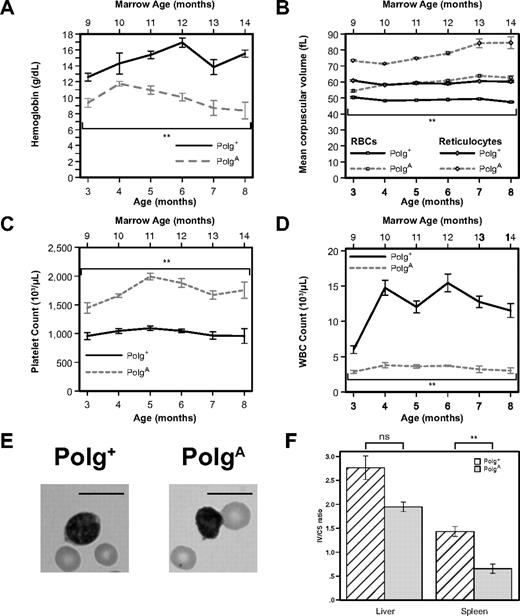
To differentiate effects related to the age of the marrow recipient from effects related to the age of the marrow donor, we next performed transplants in which 8-month-old marrow (either Polg+/+ or PolgA/A) was used to reconstitute 2-month-old hosts. As early as 1 month after transplantation, at a marrow age of 9 months, statistically significant anemia (Figure 6A, P < .001) and macrocytosis (Figure 6B squares, P < .001) were readily appreciated in recipients of PolgA marrow. When RBCs generated by the host marrow before transplantation were excluded through analysis of reticulocyte MCV, the maximal increase in reticulocyte size was observed in recipients of 8-month-old PolgA marrow as early as 1 month after transplantation (Figure 6B diamonds), compared with 6 months after transplantation of 2-month-old PolgA marrow (Figure 4B diamonds). Likewise, although both platelet counts and white blood cells initially were identical in recipients of 2-month-old Polg+ and PolgA marrow (Figure 4C and 4D, respectively), statistically significant differences in both platelet counts (P < .001) and white blood cells (P < .001) were observed in recipients of 8-month-old PolgA marrow at the first measurement after transplantation, corresponding to a marrow age of 9 months (Figure 6C and 6D, respectively). Taken together, the results obtained after transplantation of marrow from older donors imply that the cell-intrinsic and aging-associated hematopoietic phenotype of Polg exonuclease deficiency is associated with the age of the bone marrow donor, rather than the age of the transplant recipient.
Transplantation of old PolgA marrow into young Polg+ recipients accelerates the development of age-related macrocytic anemia and leukopenia and induces dysfunction in splenic mitochondria. Two-month-old B6.SJL hosts were lethally irradiated and then reconstituted with 5 × 106 nucleated marrow cells from 8-month-old donor mice. Peripheral-blood samples were analyzed as in Figure 1 from 1 to 6 months after transplantation (3-8 months of age and 9-14 months of marrow age). Each point represents the mean ± SEM of recipients of Polg+ marrow (n = 5, solid lines) and recipients of PolgA marrow (n = 5, dotted lines). (A) Hemoglobin levels. (B) Mean corpuscular volume of all RBCs (□) and of reticulocytes only (◇). (C) Platelet count. (D) White blood cell (WBC) count. (E) Representative Wright-Giemsa stained images of RBCs and lymphocytes from peripheral blood smears of 5-month-old transplant recipients (11 months marrow age). Bars represent 10 microns. (F) The relative activity of the mtDNA-dependent mitochondrial enzyme COX was compared with that of the nuclear DNA-dependent mitochondrial enzyme CS in mitochondria isolated from the livers and spleens of Polg+ transplanted with either Polg+ or PolgA bone marrow. Each bar represents the mean ± SEM of mitochondria isolated from liver or spleen, respectively, of mice transplanted with Polg+ marrow (n = 6, cross-hatched) and PolgA marrow (n = 3, solid gray). **P < .01 (statistical significance) by Student t test; ns, nonsignificant.
Transplantation of old PolgA marrow into young Polg+ recipients accelerates the development of age-related macrocytic anemia and leukopenia and induces dysfunction in splenic mitochondria. Two-month-old B6.SJL hosts were lethally irradiated and then reconstituted with 5 × 106 nucleated marrow cells from 8-month-old donor mice. Peripheral-blood samples were analyzed as in Figure 1 from 1 to 6 months after transplantation (3-8 months of age and 9-14 months of marrow age). Each point represents the mean ± SEM of recipients of Polg+ marrow (n = 5, solid lines) and recipients of PolgA marrow (n = 5, dotted lines). (A) Hemoglobin levels. (B) Mean corpuscular volume of all RBCs (□) and of reticulocytes only (◇). (C) Platelet count. (D) White blood cell (WBC) count. (E) Representative Wright-Giemsa stained images of RBCs and lymphocytes from peripheral blood smears of 5-month-old transplant recipients (11 months marrow age). Bars represent 10 microns. (F) The relative activity of the mtDNA-dependent mitochondrial enzyme COX was compared with that of the nuclear DNA-dependent mitochondrial enzyme CS in mitochondria isolated from the livers and spleens of Polg+ transplanted with either Polg+ or PolgA bone marrow. Each bar represents the mean ± SEM of mitochondria isolated from liver or spleen, respectively, of mice transplanted with Polg+ marrow (n = 6, cross-hatched) and PolgA marrow (n = 3, solid gray). **P < .01 (statistical significance) by Student t test; ns, nonsignificant.
Transplantation of old PolgA marrow into young Polg+ recipients results in impaired mitochondrial function of splenic mitochondria
To determine whether the mitochondrial dysfunction observed in PolgA mice also was observed in hematopoietic tissues of Polg+ mice transplanted with PolgA marrow, we isolated mitochondria, as explained previously, from Polg+ recipients of older Polg+ and PolgA marrow. As expected, given the presence in the liver of marrow-derived cells such as macrophages, a slight but nonsignificant decrease in the COX/CS ratio of mitochondria isolated from the livers of recipients of PolgA marrow was observed. When the analysis was confined to the spleen, the relative decrease in the COX/CS ratio of mitochondria isolated from recipients of PolgA marrow was highly significant (P < .005), which is consistent with a cell-intrinsic, transplantable decrease in mitochondrial function after homozygous loss of Polg proofreading activity.
Discussion
We have demonstrated that mitochondrial dysfunction in the hematopoietic compartment can generate key features of human MDS and have further demonstrated that the effect of mitochondrial dysfunction on hematopoiesis is cell intrinsic. PolgA mice maintain normal blood counts through 4 months of age, and then develop a progressive, cell-intrinsic megaloblastic anemia that ultimately results in death between 15 and 16 months of age. This megaloblastic anemia is accompanied by a profound defect in de novo B and T lymphopoiesis. At 8 months of age, the sole abnormality of erythropoietic development in PolgA mice is an expansion of splenic erythropoiesis, which is consistent with a stress erythropoietic response. However, by 10 months of age PolgA mice demonstrate megaloblastic morphologic changes in their erythroblasts, characterized by nuclear-cytoplasmic maturation dyssynchrony. These changes are not found in thalassemic mice, indicating that this abnormality is not simply an effect of stress erythropoiesis. When mitochondrial dysfunction is confined to the hematopoietic compartment, all of the erythropoietic and lymphopoietic abnormalities of this model system are recapitulated. Specifically, Polg+ recipients of PolgA marrow, but not of Polg+ marrow, demonstrate an aging-associated megaloblastic anemia. Finally, when marrow from 8-month-old, rather than 2-month-old, donors is transplanted into 2-month-old Polg+ hosts, the aging-associated abnormalities of recipients of PolgA marrow occur earlier, aligning with the age of the marrow donor rather than the age of the host.
Clinically, our impression is that PolgA mice die as a direct result of their profound anemia because they invariably appear profoundly fatigued at the end of life. When symptomatic PolgA mice are analyzed, their hemoglobin levels can be as low as 3.3 g/dL; when the assessment is delayed, they succumb before their hemoglobin levels can be assessed. However, because PolgA mice accumulate mitochondrial abnormalities in all tissues, it is possible that anemia is only one contributor to their demise. Of note, we have so far not observed this degree of anemia in Polg+ recipients of PolgA marrow, suggesting that the profound anemia of PolgA mice is in part the result of potentiation of the observed hematopoietic phenotype by additional effects of mitochondrial dysfunction in nonhematopoietic tissues.
Several critical features of this experimental system must be considered in evaluating these results. First, given the late onset of disease in both primary PolgA mice and PolgA bone-marrow recipients, one must recognize that the abnormalities generated by the Polg D257A allele are not constant. Given the absence of mtDNA proofreading in the cells of these mice, abnormalities of mtDNA may accumulate throughout the lifespan of those cells, whether they are in intact PolgA mice or in the marrow of Polg+ mice after transplantation with PolgA marrow. The resulting aging-associated development of anemia in these mice is therefore quite consistent with the aging-associated development of anemia25 and MDS26-28 in humans. Second, it is curious that the (presumably random) alterations in mtDNA that accumulate in PolgA produce abnormalities that are strikingly consistent, both in severity as well as in the age at onset of affected mice. This commonality of phenotype suggests some final common pathway through which disparate alterations in mtDNA generate their pathology. Confirmation of the existence of such a pathway, and elucidation of the underlying mechanism, will require direct experimental verification. However, if such a common pathway to pathology were confirmed, it would provide an explanation for the curious absence of mutational “hotspots” in the MDS bone marrow mtDNA sequencing results reported by Wulfert et al.16 Finally, it should be noted that isolated mutations in the exonuclease domain with decreased proofreading and normal mtDNA replication rate have not been reported in human patients.29 Furthermore, the clinical syndromes associated with Polg mutations generally are not associated with anemia or macrocytosis.30,31 Overall, an experimental model in which a mitochondrial disease progresses to death at 15 months of age is unlikely to provide an exact phenocopy of the decades-long course of human MDS.
The anemia of PolgA mice is associated with megaloblastic erythropoiesis, and PolgA mice develop profound defects of lymphopoiesis. Similar erythroid changes are the most common form of dyserythropoiesis observed in the marrow of patients with MDS.32 Furthermore, patients with MDS frequently demonstrate disruptions of B lymphopoiesis33-36 and T-cell homeostasis.37 Although the confirmation of direct equivalence between this model and human MDS may require the demonstration of altered mitochondrial function in marrow-derived cells of patients with MDS, the mice described here satisfy criteria for murine MDS as defined in 2002.38 Further investigation of the underlying mechanisms responsible for the cell-intrinsic megaloblastic changes described here may finally unlock a longstanding clinical mystery regarding the similarities of the megaloblastic changes caused by both MDS and vitamin deficiencies.39 Despite the similarities in morphologic appearance, MDS and megaloblastic anemia caused by vitamin deficiency are 2 distinct pathophysiologic entities40 that in some cases can occur simultaneously.41,42 The clear mechanistic association of altered nucleotide pools with megaloblastic anemia43-45 suggests that other disruptions of the nucleotide pool available for rapid DNA synthesis in erythroblasts could be responsible for the megaloblastic changes observed in PolgA mice. One mechanism whereby mitochondrial dysfunction could result in alterations in nucleotide pools is the mitochondrial dependence of a key enzyme in de novo pyrimidine biosynthesis, dihydroorotate dehydrogenase.9 Direct pharmacologic inhibition of the electron transport chain results in decreased pyrimidine biosynthesis,46 and a pharmacologic inhibitor of dihydroorotate dehydrogenase, leflunomide, has been reported to cause an anemia with megaloblastic features.47 Furthermore, the demonstrated clinical efficacy of leflunomide as a potent lymphotoxin48 suggests that an impairment of de novo pyrimidine biosynthesis may also underlie the impaired lymphopoiesis of both PolgA mice and patients with MDS.
Although this model system meets established criteria for the diagnosis of MDS in a murine model,38 it lacks some features commonly observed in patients with MDS. First, these mice do not develop ringed sideroblasts, despite the prominent appearance of ringed sideroblasts in the pediatric mtDNA disease Pearson syndrome,5 and so it will be important to determine whether correction of the megaloblastic process in these mice permits the formation of ringed sideroblasts. Second, we have not observed leukemic transformation in any PolgA mice. The absence of leukemic transformation may simply reflect insufficient time for the selection of a leukemic clone. Because the majority of MDS patients die before ever converting to acute leukemia,49 similar results in this model system are not surprising. Polg+ recipients of PolgA marrow demonstrate an attenuated anemia, suggesting that these mice may tolerate their anemia long enough for leukemic transformation to occur. Third, we have not yet determined whether PolgA mice develop clonal hematopoiesis. Although the absence of allelic variation in this highly inbred strain of mice limits the utility of X-inactivation patterns in the analysis of clonality, the induced variability of mtDNA in this model system may provide an “internal” marker of clonality. Future studies of mtDNA sequences at the single-cell level may provide insight into clonality as well as the specific mechanisms, whereby alterations in mtDNA in the marrow result in mitochondrial dysfunction and megaloblastic anemia. Finally, we have not yet determined whether PolgA mice develop chromosomal abnormalities as their anemia progresses. Routine Giemsa banding techniques are difficult to interpret in mice, and so it may prove necessary to apply molecular techniques such as spectral karyotyping or array comparative genomic hybridization to detect the presence of recurrent cytogenetic abnormalities.
The results presented here demonstrate that targeted disruption of the mitochondrial genome, either in whole animals or confined to the hematopoietic compartment, recapitulates the megaloblastic changes frequently observed in human MDS. Studies9,46 currently are underway to explore the role of decreased de novo pyrimidine biosynthesis in the development of this phenotype.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mitch Weiss for the β-globin+/th−3 and β-globin+/+ mice. We also thank Elizabeth Hexner, Dan Vogl, Mitch Weiss, and Paul Wallace for their insightful review of the manuscript.
This research was supported by grants from the American Federation for Aging Research and the J. P. McCarthy Foundation. J.E.T. was supported by grant K08-HL73977 from the National Heart, Lung, and Blood Institute (NHLBI). M.K. is supported by the American Society of Hematology, the Burroughs Wellcome Fund, and grant K08-HL084199 from the NHLBI. S.G.S. was supported by grant K08-HL075246 from the NHLBI.
National Institutes of Health
Authorship
Contributions: M.A.S., M.K, M.C., and J.E.T. designed experiments; M.L.C., T.D.L., M.L.H., S.G.S., X.Y., M.A.S., and J.E.T. performed experiments; G.E.W. designed statistical analyses; W.T. performed statistical analyses; G.E.W., M.A.S., M.K., and J.E.T. analyzed data; G.C.K. and T.A.P. provided essential reagents; M.C. and J.E.T. provided funding; J.E.T. wrote the manuscript; and M.L.C., S.G.S., X.Y., G.E.W., M.K., M.C., and J.E.T. edited the manuscript.
Conflict-of-interest disclosure: G.C.K. and T.A.P. hold US Patent 7 126 040 related to the murine model discussed in this article. The remaining authors declare no competing financial interests.
Correspondence: James E. Thompson, Roswell Park Cancer Institute, Elm & Carlton Sts, Buffalo, NY 14263; e-mail: james.thompson@roswellpark.edu.