Abstract
Gene transfer of a factor VIII (FVIII) plasmid into hemophilia A (HemA) mice achieved supraphysiologic FVIII expression, but triggered production of high-titer FVIII-specific antibodies and loss of functional FVIII activity. To test whether FVIII-specific regulatory T cells (Tregs) can modulate immune responses against FVIII, we developed a HemA mouse model in which all T cells overexpressed Foxp3 (HemA/Foxp3-Tg). FVIII plasmid therapy did not induce antibody production in HemA/Foxp3-Tg mice. CD4+Foxp3+ T cells isolated from plasmid-treated HemA/Foxp3-Tg mice significantly suppressed proliferation of FVIII-stimulated CD4+ effector T cells. The percentage of CD4+ T cells expressing CD25, glucocorticoid-induced tumor necrosis factor receptor, and cytotoxic T lymphocyte antigen 4 increased significantly in spleen and peripheral blood for 9 weeks. Mice receiving adoptively transferred Tregs from FVIII-exposed HemA/Foxp3-Tg mice produced significantly reduced antibody titers compared with controls after initial challenge with FVIII plasmid and second challenge 16 weeks after first plasmid treatment. Adoptively transferred Tregs engrafted and distributed at 2% to 4% in the Treg compartment of blood, lymph nodes, and spleens of the recipient mice and induced activation of endogenous Tregs; the engraftment decreased to negligible levels over 8 to 12 weeks. Antigen-specific Tregs can provide long-lasting protection against immune responses in vivo and limit recall responses induced by a second challenge via infectious tolerance.
Introduction
The formation of neutralizing inhibitory antibodies against human factor VIII (hFVIII) poses a significant challenge for the development of innovative hemophilia A gene therapy. Approximately 25% to 30% of hemophilia A patients produce inhibitory antibodies in response to FVIII protein replacement therapy,1 limiting the effectiveness of treatment. Potential gene therapy methods used in murine models to treat hemophilia A have resulted in a significant humoral immune response. Previous work demonstrated that naked DNA transfer of a liver-specific, high-expressing plasmid (pBS-HCRHPI-hFVIIIA) in immunocompetent, FVIII-deficient hemophilia A (HemA) mice produced a supraphysiologic level of hFVIII; however, a strong humoral immune response followed shortly,2 leading to a complete loss of functional hFVIII activity.3
Thus, establishment of long-term immune tolerance to FVIII is essential to ensure the success of gene therapy for hemophilia A. Development of tolerance is a dynamic process involving several mechanisms, including the limitation or deletion of alloreactive T-cell pools and immune regulation.4 CD4+CD25+ Forkhead boxes P3 (FOXP3)+ regulatory T cells (Tregs) play a critical role in the regulation and suppression of autoimmune and alloimmune responses.5-9 T-cell homeostasis can be achieved by balancing Treg and effector T-cell (Teff) populations. Regulation of T-cell function and tolerance induction can be accomplished by inducing a shift in the balance between effector and regulatory cells.
CD4+CD25+FOXP3+ Tregs are generated in the thymus as fully differentiated suppressor cells that regulate immune homeostasis via a cell-cell contact-dependent mechanism.10,11 Antigen-specific Tregs appear to proliferate selectively upon exposure to antigen in the periphery.12-14 The transcription factor, FOXP3, is essential for Treg generation and survival. FOXP3 is expressed preferentially in CD4+CD25+ cells, and lack of FOXP3 protein leads to lack of functional Tregs.15-17 Expression of Foxp3 in naive murine CD4+CD25− T cells is sufficient to convert these cells to a Treg-like phenotype regardless of CD25 expression.16,18-20 The success of immunosuppressive therapy used in transplantation and autoimmune diseases has suggested a correlation with the induction of Tregs. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent onset autoimmune diabetes by inducing antigen-specific Tregs.13 In addition, agents interfering with costimulatory pathways (eg, anti-CD40L) can prevent allograft rejection and induce tolerance by functional dominance of CD4+CD25+ Tregs.21 Thus, Treg activation appears to play an important role in the establishment of antigen-specific immune tolerance.
Recent gene therapy studies have demonstrated that tolerance induction after hepatic gene transfer is associated with induction of Tregs.22 Furthermore, adoptive transfer of CD4+CD25+ Tregs from tolerized mice treated with an anti-inducible costimulatory molecule antibody and FVIII plasmid could transiently protect recipient mice from inhibitory antibody formation.23 In contrast to strong inhibitory responses induced in HemA mice, we show in this study that nonviral gene transfer of FVIII plasmid into HemA mice that concurrently overexpress Foxp3 within the T lineage fails to elicit an inhibitory response. Our findings indicate that dominance of Foxp3+ Tregs in this transgenic murine model effectively suppresses functional Teffs. Furthermore, adoptive transfer of antigen-specific CD4+Foxp3+ cells from FVIII plasmid-treated HemA/Foxp3-transgenic mice protected recipient mice from antigen-specific immune responses for prolonged periods of time. Our combined findings suggest that Treg therapy may provide a novel and effective strategy to limit unwanted, antigen-specific, antibody responses mounted against therapeutic proteins in the setting of gene therapy.
Methods
Animal experiments
Animals were kept according to the National Institutes of Health guidelines for animal care and the guidelines of Seattle Children's Research Institute, and animal protocols were approved by the Institutional Animal Care and Use Committee at Seattle Children's Research Institute. All mouse experiments were conducted using the C57BL/6 strain. Mixed background HemA mice (exon 16 deleted),24 obtained from Drs Rita Sarkar and Haig Kazazian Jr (University of Pennsylvania), were crossed with C57BL/6 wild-type (wt) mice for 8 generations to yield a HemA mouse model of C57BL/6 genetic background. Transgenic mice constitutively producing Foxp3 in CD4+ T cells (Foxp3-Tg) in the C57BL/6 background were generated in Dr Steve Ziegler's laboratory.18 HemeA/Foxp3-Tg mice were developed by cross-breeding HemA and Foxp3-Tg mice. CD45.1+HemA mice were generated by cross-breeding CD45.2+HemA mice with CD45.1+ C57BL/6 mice (The Jackson Laboratory). Animals were housed under specific pathogen-free conditions at the vivarium of Seattle Children's Research Institute.
Delivery of plasmid DNA
The methods of plasmid DNA preparation and DNA infusion have been described previously.2 Briefly, 100 μg of high-expressing, liver-specific pBS-HCRHPI-hFVIIIA2plasmids in 2 mL of phosphate-buffered saline was injected into the tail vein of 20 to 24 g mice over 6 to 8 seconds. pBS-HCRHP-FIXIA was used as a control plasmid.25,26
Assays for measuring hFVIII activity
Scheduled blood samples were taken from the retro-orbital plexus of experimental mice and collected in a 3.8% sodium citrate solution. Plasma samples isolated from blood were analyzed using a modified activated partial thromboplastin time assay (APTT) and FVIII-deficient plasma. hFVIII levels were calculated from a standard curve generated by serial dilutions of normal human pooled plasma. The measurements were carried out in a Coag Screener hemostasis analyzer (American Labor). APTT values were confirmed by a chromogenic assay (COATEST, measuring factor Xa generation; diaPharma).
Assay for anti-hFVIII antibodies
Inhibitory antibodies were measured by hFVIII Bethesda inhibitor assay, as previously described.27
Immunization of mice with bacteriophage Φx174
Bacteriophage Φx174 was prepared, as previously described.28 The stock solution of 1011 plaque-forming units (PFU) per milliliter was diluted and injected intraperitoneally into mice at a dose of 1010 PFU/kg (2 × 108 PFU/mouse). A secondary immunization was carried out 4 weeks after the primary immunization. Approximately 200 μL of peripheral blood was collected before immunization and at 1, 2, and 4 weeks after each immunization. Sera were analyzed for phage-neutralizing antibody activity expressed as the rate of phage inactivation (Kv) using a standard formula.28,29 Antibody resistant to 2-mercaptoethanol was considered to be of the immunoglobulin (Ig)G isotype.30
Harvest and purification of splenic T cells
Murine spleens were removed aseptically, and single-cell suspensions were prepared in RPMI 1640 medium (Invitrogen) containing 2 mM glutamine, 50 μM 2-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal calf serum. Red blood cells were lysed with sterile 17 mM Tris and 140 mM NH4Cl buffer, pH 7.4. CD4+ and CD4+CD25+ T cells were collected by depletion of non-CD4+ cells using the magnetic-activated cell sorting (MACS) CD4+ T Cell Isolation Kit (Miltenyi Biotec) and positive selection of CD25+ cells using the MACS CD25+ MicroBead Kit (Miltenyi Biotec).
Proliferation assay
Splenic cells from treated mice were collected from C57BL/6 wt, HemA, Foxp3-Tg, and HemA/Foxp3-Tg strains 30 to 90 days after plasmid injection. For assessment of proliferation, triplicates of CD4+ T cells (105 cells/200 μL/well) were stimulated with 5 μg/mL plate-bound anti-CD3 antibody (BD Pharmingen) in cell-harvesting media. To assess FVIII-specific proliferation, CD4+ T cells from both plasmid-injected and control HemA mice were stimulated with 10 U/mL hFVIII protein (Advate; Baxter) in the presence of Ag-presenting cells (APCs). After 72 hours of incubation, [3H]thymidine was added to the cell culture media for additional 18 hours before harvest of cells. [3H]Thymidine incorporation was measured by scintillation counter, and results of triplicates were expressed as mean counts per minute (cpm). The data presented (Δcpm) are mean cpm minus background cpm.
Suppressive assay
Splenic wt CD4+CD25+ cells, Foxp3-Tg CD4+ T cells, and HemA/Foxp3-Tg CD4+ T cells were isolated, as described, and added to cultures of CD4+ Teffs (105 cells/200 μL/well) in vitro at a ratio of 1:8 and stimulated with anti-CD3 antibody (100 μL of 5 μg/mL anti-CD3/well; BD Pharmingen).
For FVIII-specific suppression, CD4+ splenic T cells collected from FVIII plasmid-transferred HemA mice were used as Teffs. HemA/Foxp3-Tg CD4+ T cells were isolated 14 days after plasmid injection and added at a ratio of 1:1 to CD4+ Teffs stimulated with 10 U/mL hFVIII protein in the presence of irradiated APCs. CD4+ splenic T cells from naive C57BL/6 were used as negative control of suppressor cells. After 72 hours of incubation, [3H]thymidine was added to the cell culture media for an additional 18 hours before harvest of cells. Suppression of the proliferative response to hFVIII stimulation by Tregs in vitro in different ratios to Teffs (1:1 to 1:32) was assessed in a separate experiment (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Adoptive transfer of hFVIII-specific Tregs
Spleens were removed aseptically from HemA/Foxp3-Tg mice at different time points after plasmid injection. CD4+ splenic T cells were harvested, as described above. CD4+ T cells from untreated HemA and HemA/Foxp3-Tg mice were used as separate controls. A total of 3 × 106 cells suspended in 300 μL of phosphate-buffered saline was injected into the tail vein of HemA recipients over 30 seconds. Twenty-four hours after transfer, 100 μg of pBS-HCRHPI-hFVIIIA plasmid in 2 mL of phosphate-buffered saline was injected into the tail vein of recipient mice over 6 to 8 seconds. At 4 months after transfer, a second 100 μg dose of pBS-HCRHPI-hFVIIIA plasmid was administered via an identical injection. Plasma samples collected at various time points were analyzed for FVIII content and anti-FVIII antibody titer by APTT and Bethesda inhibitor assays. For evaluation of engraftment efficiency of Tregs, 3 × 106 Tregs isolated from CD45.2+ HemA/Foxp3-Tg mice were adoptively transferred to CD45.1+ HemA mice.
Staining of Treg markers after plasmid transfer
Spleens, lymph nodes, and peripheral blood were harvested at different time points from FVIII plasmid-treated HemA and HemA/Foxp3-Tg donor mice before adoptive transfer or from FVIII plasmid-treated HemA recipient mice after adoptive transfer. Single-cell suspensions were prepared, as described above, and stained with anti–CD4-Alexa 700, CD45.1-PECy7, CD45.2-allophycocyanin, CD25-PECy5, and glucocorticoid-induced tumor necrosis factor receptor–related gene (GITR)–PECy7. Splenic cells were also permeabilized and stained with anti-Foxp3 fluorescein isothiocyanate and cytotoxic T lymphocyte antigen 4 (CTLA4) phycoerythrin (PE). Stained cells were analyzed by flow cytometry using a LSRII instrument (BD Biosciences), and the data processed by FlowJo software (TreeStar). CD4+ cells were gated and analyzed for percentage and mean fluorescence intensity of CD45.1, CD45.2, CD25, GITR, CTLA4, and Foxp3 expression.
Results
Establishment of an experimental model to assess Foxp3 T cells in regulating anti-hFVIII immune responses
Previous studies developed and characterized a mouse model for Foxp3 overexpression.18 Foxp3-Tg mice were generated using a genomic copy of the Foxp3 gene; nearly all CD4+ T cells isolated from these mice express Foxp3 and are functional Tregs. To test whether overexpression of Foxp3 in HemA mice can modulate the immune responses against FVIII after gene transfer, we generated a HemA/Foxp3-Tg mouse strain by cross-breeding HemA mice and Foxp3-Tg mice. Intracellular staining of splenic T cells indicated that 10% to 15% of the CD4+ T-cell population are Foxp3+ in HemA and wt mice, whereas 70% to 90% of the CD4+ splenic T-cell population stained positive for intracellular Foxp3 in the Foxp3-Tg and HemA/Foxp3-Tg models (Figure 1A). Approximately 25% of the transgenic CD4+Foxp3+ cells are CD25+; however, it has been reported that all the transgenic CD4+Foxp3+ populations have suppressive activity irrespective to CD25 expression.16,18-20 To further characterize the immunoreactive nature of each mouse strain, we measured the CD4+ splenic T-cell responses to stimulation with anti-CD3 antibody in an in vitro proliferation assay. Cells isolated from HemA and wt strains proliferated robustly, whereas cells isolated from Foxp3-Tg and HemA/Foxp3-Tg models exhibited little or no proliferation (Figure 1B).
Assessment of immune competence of HemA, Foxp3-Tg, HemA/Foxp3-Tg, and wt mice. (A-C) Characterization of splenic CD4+ T cells isolated from the 4 strains of mice. (A) Foxp3 staining of CD4+ T cells. (B) Proliferation assay after in vitro stimulation of CD4+ T cells by anti-CD3 antibody. Splenic CD4+ T cells were stimulated with plate-bound anti-CD3 antibody. CD4+ cells were incubated for 3 days. Proliferation was estimated by [3H]thymidine incorporation. (C) Suppression of proliferative response to anti-CD3 stimulation by the addition of Tregs to the cultures. CD4+ responder T cells were isolated from spleens of wt mice, and stimulated with 5 μg/mL anti-CD3 antibody (i). CD4+CD25+ Tregs from wt mice, and CD4+ Tregs from Foxp3-Tg and HemA/Foxp3-Tg mice were added to proliferating responder cells at a ratio of 1:8 (ii-iv). Wild-type CD4+CD25+ T cells, Foxp3-Tg CD4+ T cells, and HemA/Foxp3-Tg CD4+ T cells were also stimulated with anti-CD3 antibody to check for Treg proliferation (v-vii). Each column represents the percentage of proliferation relative to that of control CD4+ responder cells. (D) Antibody response to immunization with bacteriophage Φx174. HemA and Hem/AFoxp3-Tg mice (n = 4/group) were challenged twice 4 weeks apart with the neoantigen bacteriophage Φx174 (2 × 108 PFU/each challenge). Phage-neutralizing antibody activity was expressed as the rate of phage inactivation (Kv) using a standard formula. Mice not receiving bacteriophage did not produce neutralizing antibody (data not shown).
Assessment of immune competence of HemA, Foxp3-Tg, HemA/Foxp3-Tg, and wt mice. (A-C) Characterization of splenic CD4+ T cells isolated from the 4 strains of mice. (A) Foxp3 staining of CD4+ T cells. (B) Proliferation assay after in vitro stimulation of CD4+ T cells by anti-CD3 antibody. Splenic CD4+ T cells were stimulated with plate-bound anti-CD3 antibody. CD4+ cells were incubated for 3 days. Proliferation was estimated by [3H]thymidine incorporation. (C) Suppression of proliferative response to anti-CD3 stimulation by the addition of Tregs to the cultures. CD4+ responder T cells were isolated from spleens of wt mice, and stimulated with 5 μg/mL anti-CD3 antibody (i). CD4+CD25+ Tregs from wt mice, and CD4+ Tregs from Foxp3-Tg and HemA/Foxp3-Tg mice were added to proliferating responder cells at a ratio of 1:8 (ii-iv). Wild-type CD4+CD25+ T cells, Foxp3-Tg CD4+ T cells, and HemA/Foxp3-Tg CD4+ T cells were also stimulated with anti-CD3 antibody to check for Treg proliferation (v-vii). Each column represents the percentage of proliferation relative to that of control CD4+ responder cells. (D) Antibody response to immunization with bacteriophage Φx174. HemA and Hem/AFoxp3-Tg mice (n = 4/group) were challenged twice 4 weeks apart with the neoantigen bacteriophage Φx174 (2 × 108 PFU/each challenge). Phage-neutralizing antibody activity was expressed as the rate of phage inactivation (Kv) using a standard formula. Mice not receiving bacteriophage did not produce neutralizing antibody (data not shown).
We also assessed the activity of Foxp3-Tg and HemA/Foxp3-Tg CD4+ T cells using an in vitro suppression assay. CD4+ T cells were isolated from Foxp3-Tg mice and HemA/Foxp3-Tg mice, and their in vitro suppressive activities were evaluated using CD4+ Teffs isolated from either C57BL/6 wt mice or HemA mice, respectively. CD4+ T cells (70%-90% Foxp3+) derived from either Foxp3-Tg or HemA/Foxp3-Tg mice strongly suppressed the proliferative response of wt CD4+ Teffs to anti-CD3 stimulation in the presence of APCs (Figure 1C). This suppressive activity was comparable with that of CD4+CD25+ Tregs isolated from wt mice (P > .5, no statistically significant difference). Neither CD4+ T cells from Foxp3-Tg or HemA/Foxp3-Tg mice nor CD4+CD25+ Tregs from wt mice proliferated in response to anti-CD3, consistent with previous studies of the functional responses of Tregs (Figure 1C). Similar results were observed with CD4+ effector cells isolated from HemA mice (data not shown).
We subsequently challenged HemA mice and HemA/Foxp3-Tg mice with the T-dependent antigen, bacteriophage Φx174,28,29 to examine their ability to produce phage-neutralizing antibody. The HemA and HemA/Foxp3-Tg mice (n = 4 per group) were immunized twice, 4 weeks apart. As shown in Figure 1D, HemA mice displayed normal primary and secondary antibody responses to bacteriophage Φx174 immunization with a strong amplification of antibody titers and isotype switching (100% IgG) after secondary immunization, identical to the antibody responses observed in untreated wt mice (data not shown). HemA/Foxp3-Tg mice exhibited greatly reduced antibody responses (< 10% of HemA mice) with weak amplification. All transgenic mice were able to isotype switch (100% IgG) after secondary immunization, except one HemA/Foxp3-Tg mouse that showed partial isotype switching (70% IgM, 30% IgG).
Naked FVIII plasmid transfer into Foxp3-Tg and control mouse strains
Previously, we have shown that hydrodynamics-based injection of pBS-HCRHPI-hFVIIIA plasmid into FVIII-deficient mice results in a supraphysiologic expression of hFVIII, followed by a humoral response characterized by the generation of high titer anti-FVIII inhibitors and a drastic reduction in the levels of functional FVIII protein. HemA mice treated with a control pBS-HCRHP-FIXIA plasmid25,26 yielded neither FVIII activity nor anti-FVIII inhibitors. To investigate whether increasing the number of CD4+ Tregs can down-regulate this response and lead to higher levels of circulating FVIII protein, we treated mice from each of the 4 mouse strains (HemA, Foxp3-Tg, HemA/Foxp3-Tg, and wt; n = 5 per group) with 100 μg of plasmid, and followed each group over a 6-mo period. As expected, HemA mice developed significant titer of hFVIII-specific antibody between 2 and 4 weeks after plasmid injection. Antibody formation inversely corresponded to a proportional decrease in measurable FVIII, which eventually fell to undetectable levels (Figure 2A-B). The wt mice with normal levels of endogenous murine FVIII did not respond robustly to plasmid (containing hFVIII) treatment, yet still developed low-titer inhibitory antibody at approximately 4 weeks. Similarly, a corresponding moderate decrease in FVIII levels was observed. In contrast, FVIII gene expression was maintained at high levels for > 180 days in both the Foxp3-Tg and HemA/Foxp3-Tg groups of mice, and inhibitory antibodies could not be detected. These findings strongly suggest that the higher ratio of CD4+Foxp3+ Tregs to Teffs in Foxp3-Tg and HemA/Foxp3-Tg mice was directly responsible for preventing the antibody response.
Gene transfer of FVIII plasmid into experimental mouse strains. (A-B) FVIII levels and inhibitory antibody activity were assessed after delivery of pBS-HCRHPI-hFVIIIA into the HemA (■), Foxp3-Tg (▴), HemA/Foxp3-Tg (X), and wt mouse (♦) strains (n = 8/group). Mice were bled at regular intervals beginning on day 3, after plasmid injection. (A) Circulating FVIII activity was measured via a modified clotting assay and confirmed by a COATEST assay. (B) Inhibitory antibody titers were evaluated by Bethesda assay and expressed as Bethesda units/mL. (C-D) In vitro proliferation and suppression assay after stimulation of CD4+ T cells with hFVIII. (C) CD4+ splenic T cells isolated from wt, HemA, Foxp3-Tg, and HemA/Foxp3-Tg mice (n = 3/group) 4 weeks after FVIII plasmid treatment. Cells from untreated mice of each strain were used as controls. Triplicates of isolated CD4+ T cells isolated were stimulated with 4 U/mL hFVIII protein in the presence of APCs for 72 hours. Each dataset represents mean Δcpm. (D) Suppression of the proliferative response to hFVIII stimulation by Tregs in vitro. CD4+ T cells from spleens of FVIII plasmid-treated HemA mice were used as Teffs, and CD4− cells were used as APCs. CD4+CD25+ Tregs from untreated and FVIII plasmid-treated HemA mice and CD4+ Tregs from untreated and FVIII plasmid-treated HemA/Foxp3-Tg mice (n = 3/group) were cocultured with the Teffs at a ratio of 1:1 Tregs to Teffs in the presence of APCs and 10 U/mL hFVIII for 3 days. [3H]Thymidine was subsequently added and cultures maintained for another 18 hours. Data are shown by percentage of suppression compared with effector cells only (without the presence of Tregs). *P < .05 between the group of effector cells only and with Tregs from Foxp3-Tg mice.
Gene transfer of FVIII plasmid into experimental mouse strains. (A-B) FVIII levels and inhibitory antibody activity were assessed after delivery of pBS-HCRHPI-hFVIIIA into the HemA (■), Foxp3-Tg (▴), HemA/Foxp3-Tg (X), and wt mouse (♦) strains (n = 8/group). Mice were bled at regular intervals beginning on day 3, after plasmid injection. (A) Circulating FVIII activity was measured via a modified clotting assay and confirmed by a COATEST assay. (B) Inhibitory antibody titers were evaluated by Bethesda assay and expressed as Bethesda units/mL. (C-D) In vitro proliferation and suppression assay after stimulation of CD4+ T cells with hFVIII. (C) CD4+ splenic T cells isolated from wt, HemA, Foxp3-Tg, and HemA/Foxp3-Tg mice (n = 3/group) 4 weeks after FVIII plasmid treatment. Cells from untreated mice of each strain were used as controls. Triplicates of isolated CD4+ T cells isolated were stimulated with 4 U/mL hFVIII protein in the presence of APCs for 72 hours. Each dataset represents mean Δcpm. (D) Suppression of the proliferative response to hFVIII stimulation by Tregs in vitro. CD4+ T cells from spleens of FVIII plasmid-treated HemA mice were used as Teffs, and CD4− cells were used as APCs. CD4+CD25+ Tregs from untreated and FVIII plasmid-treated HemA mice and CD4+ Tregs from untreated and FVIII plasmid-treated HemA/Foxp3-Tg mice (n = 3/group) were cocultured with the Teffs at a ratio of 1:1 Tregs to Teffs in the presence of APCs and 10 U/mL hFVIII for 3 days. [3H]Thymidine was subsequently added and cultures maintained for another 18 hours. Data are shown by percentage of suppression compared with effector cells only (without the presence of Tregs). *P < .05 between the group of effector cells only and with Tregs from Foxp3-Tg mice.
The wt, but not Foxp3-Tg mice generate antigen-specific Teffs after FVIII gene transfer
CD4+ T cells were isolated from 4 strains of mice that were injected with plasmid pBS-HCRHPI-FVIIIA, including wt, HemA, Foxp3-Tg, and HemA/Foxp3-Tg mice. Antigen-specific proliferation was assessed by culturing CD4+ T cells in the presence of hFVIII. CD4+ T cells isolated from the same 4 strains of untreated mice and from HemA mice treated with pBS-HCRHP-FIXIA plasmid were used as controls. As shown in Figure 2C, only CD4+ T cells isolated from FVIII plasmid-treated HemA mice that had developed inhibitors proliferated in response to hFVIII stimulation. In contrast, CD4+ T cells isolated from untreated HemA mice (Figure 2C) or HemA mice treated with a nonspecific control FIX plasmid (no FVIII inhibitors; data not shown) failed to proliferate. Similarly, CD4+ T cells isolated from plasmid-injected wt mice controls that exhibited only very low titers of inhibitory antibodies did not proliferate in response to hFVIII. Proliferative responses to hFVIII were not observed in CD4+ T cells isolated from either plasmid-injected or untreated Foxp3-Tg and HemA/Foxp3-Tg mice, consistent with the observation that no inhibitory antibodies were generated in these 2 strains of mice with overexpression of the Foxp3 gene. Thus, FVIII-specific CD4+ T cells were detectable only in HemA mice that exhibit high levels of inhibitory antibodies.
We next investigated the relative suppressive activities of CD4+ Tregs derived from wt versus HemA/Foxp3-Tg mice against CD4+ Teffs isolated from FVIII plasmid-treated HemA mice using an in vitro proliferation assay based on hFVIII stimulation in the presence of host APCs. CD4+ T cells (enriched in vivo for Foxp3+ Tregs, as noted above) were isolated from untreated and from plasmid-treated HemA/Foxp3-Tg mice. Similarly, CD4+CD25+ T cells were isolated by MACS in vitro from untreated, FVIII plasmid, and control FIX plasmid-treated HemA mice. CD4+Foxp3+ T cells derived from plasmid-treated HemA/Foxp3-Tg mice demonstrated strong suppressive activity toward CD4+ Teffs isolated from plasmid-treated HemA mice. This suppressive activity was comparable with that of CD4+CD25+ Tregs isolated from FVIII plasmid-treated HemA mice (Figure 2D). In contrast, neither CD4+Foxp3+ T cells derived from untreated HemA/Foxp3-Tg mice nor CD4+CD25+ Tregs obtained from untreated (Figure 2D) or control FIX plasmid-treated (data not shown) HemA mice exhibited statistically significant suppressive activity toward educated Teffs stimulated by FVIII, indicating the suppressive activity of Tregs from FVIII plasmid-treated mice is antigen-specific.
Adoptive transfer of Tregs regulates hFVIII-specific immune responses
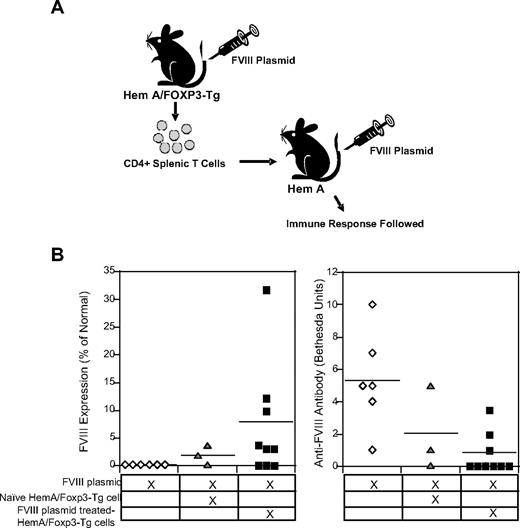
Having shown that antigen-specific Tregs are capable of down-regulating immune responses to hFVIII in vitro, we next evaluated the in vivo immunosuppressive potential of adoptively transferred hFVIII-specific Tregs in groups of mice treated with FVIII plasmid. Tregs were isolated from either unmanipulated or tolerized HemA/Foxp3-Tg mice 2 weeks after injection of 100 μg of pBS-HCRHPI-FVIIII. We hypothesized that Treg populations derived from tolerized mice were enriched for FVIII-specific Tregs, in contrast to those isolated from untreated control mice. Based on this hypothesis, we adoptively transferred control versus tolerized Treg (2 × 106 cells) into naive HemA mice (Figure 3A). The 2 groups of Treg-treated HemA mice and the group of control HemA mice were challenged with 100 μg of pBS-HCRHPI-FVIIIA 1 day after the adoptive transfer. At 3 weeks after FVIII plasmid injection, control HemA mice that did not receive adoptively transferred Tregs had no detectable hFVIII levels, whereas 6 of 9 HemA mice receiving Tregs from hFVIII-tolerized HemA/Foxp3-Tg mice had therapeutic plasma levels of hFVIII. Consistent with these data, none of these 6 Treg recipient animals produced anti-hFVIII antibodies. In contrast, all control HemA mice produced inhibitory antibodies and none exhibited hFVIII activity in the plasma (Figure 3B). Importantly, HemA mice that received CD4+ T cells derived from naive HemA/Foxp3-Tg mice without FVIII plasmid pretreatment had lower serum levels of FVIII activity and showed less protection from inhibitory antibody formation. In a separate experiment, adoptive transfer of 4 × 106 cells and 8 × 106 cells isolated from FVIII plasmid-treated HemA/Foxp3-Tg mice (n = 4/group) protected all the recipient mice from antibody production at 3 weeks after plasmid transfer. Together, these data indicate that FVIII-specific Tregs can suppress the hFVIII-specific immune responses after adoptive transfer.
Modulation of immune responses induced by FVIII plasmid injection after adoptive transfer of CD4+Foxp3+ T cells from FVIII plasmid-treated HemA/Foxp3-Tg mice into naive HemA mice. CD4+ T cells were isolated from the spleens of HemA/Foxp3-Tg mice 2 weeks after FVIII plasmid treatment (n = 9), and from untreated HemA/Foxp3-Tg mice (n = 3), and adoptively transferred into naive HemA mice. Recipient mice as well as a control group of naive HemA mice (n = 6) were subsequently challenged with FVIII plasmid injection 1 day after adoptive transfer. (A) Flow chart showing overview of these adoptive transfer experiments. (B) FVIII activities and anti-FVIII antibody titers were measured (as described in Figure 2) 3 weeks after adoptive transfer and FVIII plasmid injection.
Modulation of immune responses induced by FVIII plasmid injection after adoptive transfer of CD4+Foxp3+ T cells from FVIII plasmid-treated HemA/Foxp3-Tg mice into naive HemA mice. CD4+ T cells were isolated from the spleens of HemA/Foxp3-Tg mice 2 weeks after FVIII plasmid treatment (n = 9), and from untreated HemA/Foxp3-Tg mice (n = 3), and adoptively transferred into naive HemA mice. Recipient mice as well as a control group of naive HemA mice (n = 6) were subsequently challenged with FVIII plasmid injection 1 day after adoptive transfer. (A) Flow chart showing overview of these adoptive transfer experiments. (B) FVIII activities and anti-FVIII antibody titers were measured (as described in Figure 2) 3 weeks after adoptive transfer and FVIII plasmid injection.
Evidence for Treg activation in HemA/Foxp3-Tg mice after FVIII plasmid treatment
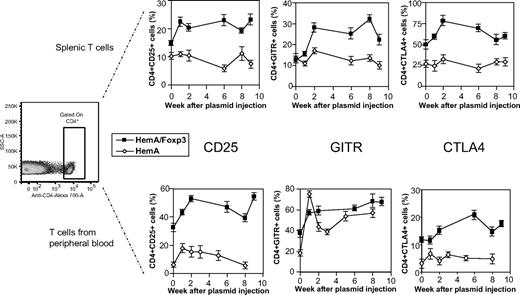
As a potential approach to identify and isolate effective antigen-specific Treg population from FVIII plasmid-treated mice, we assessed CD4+ T cells isolated from the spleen and peripheral blood of plasmid-treated mice for markers characteristic for Tregs at different time points after FVIII plasmid injection. In FVIII plasmid only-treated HemA mice, no significant changes were detected in the relative percentages of CD25+, GITR+, or CTLA4+ cells in the splenic CD4+ T-cell compartment. In peripheral blood, minor increases were observed in the percentage of CD25+ and CTLA4+CD4+ cells; GITR+ cells increased significantly at 1 week after FVIII plasmid injection and remained at moderately increased levels compared with untreated mice. Compared with naive HemA mice, untreated HemA/Foxp3-Tg mice exhibited higher basal levels of CTLA4+ and CD25+ cells, but similar levels of GITR+ cells on both splenic and peripheral blood CD4+ T cells. After FVIII plasmid injection into HemA/Foxp3-Tg mice, the percentage of CD25+, GITR+, and CTLA4+ cells in the CD4 compartment of both spleen and peripheral blood increased significantly at 1 week after treatment and remained elevated for at least 9 weeks (Figure 4), and the mean fluorescence intensity of these marker expressions on the positive cells also increased (supplemental Figure 2), suggesting that significant numbers of Tregs were activated after FVIII plasmid injection. These results are consistent with the increased in vitro suppressive activity of transgenic CD4+Foxp3+ Tregs after antigen stimulation, as shown in Figure 2D, and absence of immune responses to FVIII in HemA/Foxp3-Tg mice (Figure 2B). Furthermore, these data suggest that activated Tregs can be identified and harvested during a 1- to 9-week window after antigen exposure for potential use in adoptive therapy to regulate antigen-specific immune responses.
Staining of Treg markers in FVIII plasmid-treated HemA and HemA/Foxp3-Tg mice over time. Single-cell suspensions were prepared from spleens and peripheral blood of FVIII plasmid-treated HemA and HemA/Foxp3-Tg mice (n = 2/group) at different time points and stained for CD4, CD25, GITR, and CTLA4 expression. The cells were first gated for CD4 expression. The percentages of CD4+CD25+, CD4+GITR+, and CD4+CTLA4+ cells were determined over time; splenic cells are shown in the top panel, and peripheral blood lymphocytes in the bottom panel.
Staining of Treg markers in FVIII plasmid-treated HemA and HemA/Foxp3-Tg mice over time. Single-cell suspensions were prepared from spleens and peripheral blood of FVIII plasmid-treated HemA and HemA/Foxp3-Tg mice (n = 2/group) at different time points and stained for CD4, CD25, GITR, and CTLA4 expression. The cells were first gated for CD4 expression. The percentages of CD4+CD25+, CD4+GITR+, and CD4+CTLA4+ cells were determined over time; splenic cells are shown in the top panel, and peripheral blood lymphocytes in the bottom panel.
Transferred Tregs mediate long-term protection from inhibitor development after repeat FVIII plasmid challenge
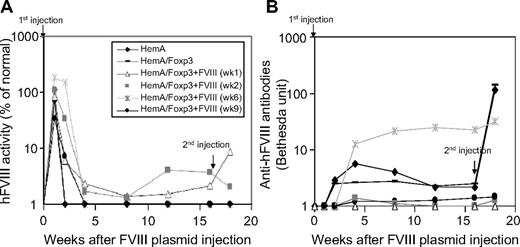
Based upon the prolonged activation window identified above, we performed in vivo adoptive transfer experiments using 3 × 106 transgenic CD4+Foxp3+ Tregs harvested at different time points after FVIII plasmid injection (weeks 1, 2, 6, and 9). CD4+Foxp3+ Tregs were adoptively transferred into naive HemA mice (n = 3/group, total of 4 groups). In addition, we included 2 control groups of mice (n = 3/group) receiving CD4+ T cells from untreated HemA/Foxp3-Tg mice or naive HemA mice, respectively. One day after adoptive transfer, recipient mice were challenged with 100 μg of FVIII plasmid. All plasmid-treated mice had high-level FVIII gene expression for the initial 2 weeks, at which point FVIII levels began to rapidly decrease to undetectable levels, except for HemA mice that received CD4+Foxp3+ Tregs harvested from HemA/Foxp3-Tg mice at week 1 or 2 after FVIII plasmid treatment; these mice maintained low-level FVIII gene expression for 16 weeks (Figure 5A). Furthermore, mice that received transgenic CD4+Foxp3+ Tregs from FVIII plasmid-treated HemA/Foxp3-Tg mice produced lower levels of inhibitory antibodies compared with both control groups, except for one animal from the week 6 group that developed high-titer antibody (Figure 5B). Notably, recipients of week 1 or week 2 Tregs generated only transient low-level inhibitory antibody at week 4 and 8 after FVIII plasmid injection, and the antibody dropped to undetectable levels after week 12 after plasmid injection, correlated with the increase of FVIII activity.
Long-term protective effect of CD4+Foxp3+ Tregs in FVIII plasmid-treated HemA mice. CD4+ T cells were isolated from HemA/Foxp3-Tg mice 1, 2, 6, and 9 weeks after FVIII plasmid treatment (n = 3/group) and adoptively transferred to naive HemA mice. Recipient mice, a control group of naive HemA mice (n = 3), and a control group receiving CD4+ T cells derived from untreated HemA/Foxp3-Tg mice were challenged with FVIII plasmid injection 1 day after adoptive transfer. All treated mice were then subjected to a second challenge with FVIII plasmid injection 16 weeks after the first injection. (A) FVIII activity and (B) anti-FVIII antibody titers were evaluated over time, as described in Figure 2.
Long-term protective effect of CD4+Foxp3+ Tregs in FVIII plasmid-treated HemA mice. CD4+ T cells were isolated from HemA/Foxp3-Tg mice 1, 2, 6, and 9 weeks after FVIII plasmid treatment (n = 3/group) and adoptively transferred to naive HemA mice. Recipient mice, a control group of naive HemA mice (n = 3), and a control group receiving CD4+ T cells derived from untreated HemA/Foxp3-Tg mice were challenged with FVIII plasmid injection 1 day after adoptive transfer. All treated mice were then subjected to a second challenge with FVIII plasmid injection 16 weeks after the first injection. (A) FVIII activity and (B) anti-FVIII antibody titers were evaluated over time, as described in Figure 2.
To determine whether the partial protective effect observed in Treg recipients might be sustained despite repeated plasmid therapy, each of these animal cohorts was rechallenged with a second dose of FVIII plasmid at week 16 after initial therapy. Interestingly, the recipients of CD4+ cells derived from untreated HemA/Foxp3-Tg and HemA mice mounted a robust secondary antibody response to repeat FVIII plasmid treatment. In striking contrast, mice that had received CD4+ cells from FVIII plasmid-treated HemA/Foxp3-Tg mice exhibited very weak or no response to the secondary challenge; and recipients of week 1 or week 2 Treg continued to produce detectable circulating levels of hFVIII. These results suggest that Tregs exhibit the greatest suppressive and protective activity against FVIII antigen-specific immune responses at 1 or 2 weeks after antigen challenge. Most notably, these cell populations can mediate long-lasting protection against FVIII antigen-specific immune responses. Furthermore, we challenged the recipient mice (n = 4) 6 months after the adoptive transfer of Tregs and FVIII plasmid injection, the HemA mice receiving Tregs mounted normal responses against bacteriophage Φx174, indicating that protective effect by Tregs is antigen specific.
Adoptively transferred Tregs persisted only short-term; however, they induced significant activation of endogenous Tregs
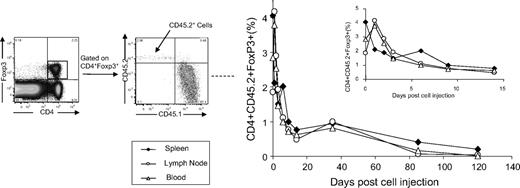
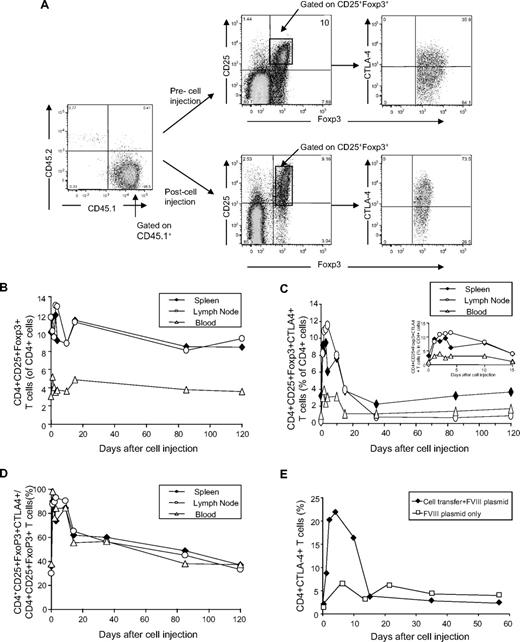
Next, we evaluated whether adoptively transferred Tregs persisted in recipient mice to exert long-term protective effect against antigen-specific immune responses. A total of 3 × 106 CD4+Foxp3+ Tregs isolated from CD45.2+HemA/Foxp3-Tg mice 2 weeks after FVIII treatment was adoptively transferred into CD45.1+HemA mice. The recipient mice were challenged 1 day after adoptive transfer. CD45.2+Tregs engrafted in the spleen, lymph node, and blood with 4%, 3%, and 2% frequency in the Treg compartment of the recipient mice 1 day after adoptive transfer, respectively. The engraftment frequencies fell rapidly to less than 1% within 14 days, then slowly declined to negligible levels over 8 to 12 weeks (Figure 6).
Engraftment of CD45.2+ Tregs isolated from FVIII plasmid-treated HemA/Foxp3-Tg mice in CD45.1+HemA mice. A total of 3 × 106 T cells was isolated from CD45.2+HemA/Foxp3-Tg mice (n = 22 with 2 mice per time point) 2 weeks after FVIII plasmid treatment, and adoptively transferred into CD45.1+HemA mice at day 0. The recipient mice were subsequently challenged with 100 μg of FVIII plasmid by hydrodynamic injection at day 1. Single-cell suspensions were prepared from spleens, lymph nodes, and peripheral blood of recipient mice after adoptive transfer at different time points. The cells were stained with CD4, CD45.1, CD45.2, CD25, Foxp3, and CTLA4 markers. The percentages of engrafted CD4+Foxp3+CD45.2+T cells were evaluated over 120 days. Insert shows the time curve over 14 days (spleen, ♦; lymph node, ○; peripheral blood, Δ).
Engraftment of CD45.2+ Tregs isolated from FVIII plasmid-treated HemA/Foxp3-Tg mice in CD45.1+HemA mice. A total of 3 × 106 T cells was isolated from CD45.2+HemA/Foxp3-Tg mice (n = 22 with 2 mice per time point) 2 weeks after FVIII plasmid treatment, and adoptively transferred into CD45.1+HemA mice at day 0. The recipient mice were subsequently challenged with 100 μg of FVIII plasmid by hydrodynamic injection at day 1. Single-cell suspensions were prepared from spleens, lymph nodes, and peripheral blood of recipient mice after adoptive transfer at different time points. The cells were stained with CD4, CD45.1, CD45.2, CD25, Foxp3, and CTLA4 markers. The percentages of engrafted CD4+Foxp3+CD45.2+T cells were evaluated over 120 days. Insert shows the time curve over 14 days (spleen, ♦; lymph node, ○; peripheral blood, Δ).
Furthermore, endogenous CD45.1+ T cells isolated from the spleen, lymph node, and peripheral blood of plasmid-treated mice were stained for markers characteristic for Tregs at different time points after FVIII plasmid injection. In both FVIII plasmid only- and adoptive transfer plus FVIII plasmid-treated HemA mice, no significant changes were detected in the relative percentages of CD25+Foxp3+ Tregs in the CD4+ T-cell compartment of spleen, lymph node, and blood (Figure 7A-B). However, the relative percentages of CD25+Foxp3+CTLA4+ cells in the CD4+ T cells and CTLA4+ cells in the CD4+CD25+Foxp3+ T cells significantly increased in the initial 2 weeks after adoptive transfer, then declined to the normal levels of the untreated mice (Figure 7C-D), indicating induction of endogenous Treg activation. As shown in Figure 7E, Treg activation is induced only in mice that received adoptively transferred Tregs and FVIII plasmid treatment, not in mice treated with FVIII plasmid only.
Staining of Treg markers on endogenous CD45.1+T cells in Treg recipient mice. Adoptive transfer of Tregs into HemA mice, FVIII plasmid challenge, and preparation of single-cell suspensions from spleens, lymph nodes, and peripheral blood of the recipient mice were described in Figure 6. The plasmid-only–treated HemA mice without adoptive transfer were used as controls. The cells were stained with CD4, Foxp3, CD45.1, CD45.2, CD25, and CTLA4 markers. (A) Representative dot plots of stained cells. The percentages of (B) Foxp3+CD25+ cells and (C) Foxp3+CD25+CTLA4+ cells in CD4+T cells and (D) CTLA4+ cells in CD4+Foxp3+CD25+T cells isolated from Treg recipient mice were evaluated over time (spleen, ♦; lymph node, ○; peripheral blood, Δ). (E) The percentages of CD4+CTLA4+ T cells in plasmid-treated HemA control (□) and Treg recipient mice (♦) over time.
Staining of Treg markers on endogenous CD45.1+T cells in Treg recipient mice. Adoptive transfer of Tregs into HemA mice, FVIII plasmid challenge, and preparation of single-cell suspensions from spleens, lymph nodes, and peripheral blood of the recipient mice were described in Figure 6. The plasmid-only–treated HemA mice without adoptive transfer were used as controls. The cells were stained with CD4, Foxp3, CD45.1, CD45.2, CD25, and CTLA4 markers. (A) Representative dot plots of stained cells. The percentages of (B) Foxp3+CD25+ cells and (C) Foxp3+CD25+CTLA4+ cells in CD4+T cells and (D) CTLA4+ cells in CD4+Foxp3+CD25+T cells isolated from Treg recipient mice were evaluated over time (spleen, ♦; lymph node, ○; peripheral blood, Δ). (E) The percentages of CD4+CTLA4+ T cells in plasmid-treated HemA control (□) and Treg recipient mice (♦) over time.
Discussion
A range of observations demonstrates the potential of Tregs to modulate immune responses.5-9 Adoptive transfer of Tregs aimed at modulating antigen-specific responses has several advantages over conventional treatments, including protection through antigen-specific regulation in the absence of broader immunosuppression; induction of long-lasting physiologic regulation in vivo; and the potential for customized therapy with limited side effects for individual patients. Several pilot clinical trials designed to induce tolerance in humans are currently underway under the auspices of the Immune Tolerance Network (http://www.immunetolerance.org/).31
The formation of inhibitory antibodies against FVIII after treatment of hemophilia patients with either protein replacement or gene therapy represents a major problem. In this study, we explored the possibility that CD4+Foxp3+ Tregs can modulate anti-hFVIII immune responses. Because it is technically difficult to obtain large homogeneous populations of CD4+Foxp3+ cells from wt mice, we first established a HemA/Foxp3 transgenic mouse model. In this model, 70% to 90% of the CD4+ T cells express Foxp3 that possess suppressive activity comparable with naturally occurring Tregs derived from wt mice. In addition, we showed that HemA/Foxp3-Tg mice immunized with bacteriophage Φx174 have significantly down-regulated antibody responses characterized by depressed antibody titers and a lack of amplification, but intact isotype switching. These data indicate that HemA/Foxp3-Tg mice are significantly immune suppressed with regard to their responses to a T-dependent neoantigen.
When we injected FVIII plasmid into different mouse strains, HemA and to a lesser extent wt mice developed anti-hFVIII antibodies, whereas none of the HemA/Foxp3-Tg and Foxp3-Tg mice developed antibodies. This is in accordance with our previous observation that the generation of the hFVIII-specific inhibitor is driven by a Th2-induced antibody response3 expected to be regulated by the ratio of Teffs and Tregs. This concept is supported by our finding that CD4+ T cells (enriched in Foxp3+ cells) isolated from tolerized HemA/Foxp3-Tg mice can significantly suppress proliferative activity from FVIII-specific Teffs when stimulated in vitro with hFVIII, whereas CD4+ T cells isolated from untreated HemA/Foxp3 mice cannot. These data provide the direct evidence that antigen-specific Foxp3-expressing Tregs play an important role in modulating anti-FVIII antibody responses in vivo and in vitro. During the 2-week timeframe that the CD4+ cells were isolated, both antigen-specific Teffs and Tregs are produced. In the case of HemA mice, the balance shifts to higher ratios of Teffs to Tregs to induce FVIII-specific immune responses, whereas in HemA/Foxp3-Tg mice, Tregs are much more prevalent than Teffs, therefore suppressing the production of antibody responses. It has also been demonstrated that Tregs are important in tolerance induction by liver-directed gene transfer.32 In addition, retroviral gene transfer to activated B cells induced tolerance by stimulating an endogenous population of Tregs.33 Neonatal gene transfer into HemA mice34 and mucopolysaccharidosis I mice with transient immunomodulation35 prevented antibody production with increase in Treg population.
Thus, we next tested the hypothesis that adoptive transfer of antigen-specific Foxp3-expressing Tregs isolated from transgenic mice can modulate immune responses in plasmid-treated HemA mice. Because we postulated that Tregs are most critical during the initial period after the antigen challenge in modulating antigen-specific immune responses, we transferred Foxp3-expressing Tregs 1 day before plasmid injection. This procedure clearly demonstrated a protective effect of the adoptively transferred Tregs in preventing an immune response against hFVIII. Moreover, mice that received CD4+Foxp3+ Tregs derived from HemA/Foxp3-Tg mice 1 or 2 weeks after FVIII plasmid treatment maintained therapeutic levels of FVIII gene expression for 16 weeks. These results indicate that adoptive Treg therapy is a potential tool to down-regulate FVIII-specific immune responses.
Nevertheless, the protection against FVIII-specific immune responses by adoptively transferred Tregs is not sufficient to allow persistent, high-level FVIII gene expression as we have previously achieved using several immunosuppressive regimens.23,29,36,37 Anti-inducible costimulatory molecule or anti-CD3 induced both depletion of Teffs and increase in the percentage of Tregs in the CD4+ T-cell population, tilting the balance toward a regulatory milieu.23,29,36,37 It has been recognized recently that the ratio of Tregs:Teffs is critical for eliciting immune responses versus immune tolerance.38,39 Our less than optimal results could simply be due to the insufficient numbers of Tregs that engrafted after adoptive transfer. This interpretation is supported in part by our observation that transfer of larger numbers of Tregs into plasmid-treated HemA mice led to better protection. In addition, our data and those of others show that antigen-specific Tregs are more potent than nonspecific polyclonal Tregs in mediating/suppressing antigen-specific immune responses.5-9 We confirmed the superiority of antigen-specific Tregs in vitro using a suppression assay (Figure 2D) and in vivo by adoptive transfer experiments of transgenic Foxp3+ Tregs (Figures 3 and 5). However, polyclonal CD4+CD25+ Tregs used in this study contained only a small portion of in vivo induced hFVIII-specific Tregs. We hypothesize that enrichment of antigen-specific Tregs will further enhance the protective effect toward the antigen-specific immune responses. Other factors to consider are the survival time of suppressive Tregs in the host after adoptive transfer, and the length of time that the antigen-specific Treg phenotype will persist in vivo. By considering these various factors, more refined protocols are likely to be developed to generate antigen-specific Tregs capable of optimal suppression of antibody production and induce long-term antigen-specific tolerance after adoptive transfer. A more potent regulatory effect may be achieved by increasing the efficiency of Treg engraftment and improving the relative homoestatic expansion of antigen-specific Tregs.
The adoptive transfer experiments described in this study indicate that CD4+Foxp3+ T cells isolated from transgenic mice at 1 and 2 weeks after FVIII plasmid treatment were most effective in protecting recipient mice from anti-hFVIII immune responses, suggesting that Tregs are most effective suppressors, and/or that antigen-specific Tregs are relatively more abundant during this early period. By assessing Treg-specific markers over time after FVIII plasmid injection, we could show that the percentage of CD25+, GITR+, and CTLA4+ cells in the CD4+ T-cell compartment of both spleen and peripheral blood (as well as the mean fluorescence intensity of these markers; data not shown) increased significantly and remained at elevated levels for at least 9 weeks in plasmid-treated HemA/Foxp3-Tg mice. These findings contrasted with minimal changes observed in plasmid-treated HemA control mice. Expression of these markers has previously been shown to increase after the activation of Tregs in vitro and in vivo.38,39 Thus, our results imply that in plasmid-treated HemA/Foxp3-Tg mice, significant numbers of antigen-specific Tregs remain activated for an extended period of time and are likely to be responsible for induction and maintenance of tolerance to hFVIII.
Interestingly, adoptive transfer of partially antigen-specific transgenic CD4+Foxp3+ Tregs at the time of first plasmid transfer not only reduced the quantity of anti-FVIII antibodies after the first plasmid injection, but also suppressed the markedly increased antibody response observed in control mice after the second plasmid challenge, given 16 weeks after the initial plasmid injection. A widely accepted hypothesis postulates that the long-term protective effect of Tregs on antigen-specific immune responses is the direct result of a mechanism known as infectious tolerance.38,40 Based on in vivo transfer experiments, it has been proposed that one population of suppressor T cells can create a regulatory milieu that promotes, in a transforming growth factor-β–dependent manner, the outgrowth of additional populations of functional Tregs with distinct antigen specificities.40 In the settings of transplantation and type 1 diabetes, this hypothesis explains that even after the loss or removal of the original antigen-specific Tregs, the tolerant state is maintained.41-43 Recent reports also demonstrated that antigen expression in the liver promoted the generation of antigen-specific Tregs for the treatment of autoimmune diseases by transforming growth factor-β–dependent peripheral conversion from conventional non-Tregs.32,44 We showed that in nonlymphopenic HemA mice, exogenously introduced antigen-specific Tregs engrafted and redistributed into blood, spleen, and lymph node of the recipient mice. However, the initially engrafted Tregs (2%-4% in the Treg compartment) are short-lived, with levels decreasing to less than 1% within 2 weeks and then to negligible in 8 to 12 weeks. Therefore, there were very little adoptively transferred Tregs present when we performed second plasmid challenge 16 weeks after plasmid injection. In contrast, we found that the adoptively transferred Tregs induced significant activation of endogenous Tregs after adoptive transfer plus FVIII plasmid treatment, suggesting that exogenously added antigen-specific Tregs induced generation of activated endogenous Tregs with antigen specificity. Thus, the Treg-mediated generation of new functional FVIII-specific CD4+Foxp3+ cells may be the major mechanism by which Tregs expand their suppressive abilities to maintain long-term protective effect against FVIII-specific immune tolerance.
To date, few strategies have been developed to effectively suppress or reduce a pre-existing inhibitory antibody response against FVIII. Our approach to explore the mechanisms by which Tregs modulate anti-FVIII immune responses provides new opportunities to develop innovative strategies to eliminate already established antibody responses, and to induce long-term immune tolerance against specific antigens.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Peiqing Ye and Elizabeth Ocheltree for technical assistance.
This work was supported by R01 grants from National Institutes of Health/National Heart, Lung, and Blood Institute (HL69049 and HL82600).
National Institutes of Health
Authorship
Contribution: C.H.M. designed and performed research, analyzed data, and wrote the paper; B.R.H. performed research and analyzed data; S.F.Z. provided helpful ideas and provided Foxp3-Tg mice; B.C.Y. performed research and analyzed data; T.T. provided helpful suggestions; L.C., R.J.Y., and B.P. performed research; A.R.T. provided helpful suggestions; H.D.O. provided helpful ideas and revised the paper; and D.J.R. provided helpful suggestions and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carol H. Miao, Department of Pediatrics, University of Washington and Seattle Children's Research Institute, 1900 Ninth Ave, C9S-7, Seattle, WA 98101; e-mail: miao@u.washington.edu.

![Figure 1. Assessment of immune competence of HemA, Foxp3-Tg, HemA/Foxp3-Tg, and wt mice. (A-C) Characterization of splenic CD4+ T cells isolated from the 4 strains of mice. (A) Foxp3 staining of CD4+ T cells. (B) Proliferation assay after in vitro stimulation of CD4+ T cells by anti-CD3 antibody. Splenic CD4+ T cells were stimulated with plate-bound anti-CD3 antibody. CD4+ cells were incubated for 3 days. Proliferation was estimated by [3H]thymidine incorporation. (C) Suppression of proliferative response to anti-CD3 stimulation by the addition of Tregs to the cultures. CD4+ responder T cells were isolated from spleens of wt mice, and stimulated with 5 μg/mL anti-CD3 antibody (i). CD4+CD25+ Tregs from wt mice, and CD4+ Tregs from Foxp3-Tg and HemA/Foxp3-Tg mice were added to proliferating responder cells at a ratio of 1:8 (ii-iv). Wild-type CD4+CD25+ T cells, Foxp3-Tg CD4+ T cells, and HemA/Foxp3-Tg CD4+ T cells were also stimulated with anti-CD3 antibody to check for Treg proliferation (v-vii). Each column represents the percentage of proliferation relative to that of control CD4+ responder cells. (D) Antibody response to immunization with bacteriophage Φx174. HemA and Hem/AFoxp3-Tg mice (n = 4/group) were challenged twice 4 weeks apart with the neoantigen bacteriophage Φx174 (2 × 108 PFU/each challenge). Phage-neutralizing antibody activity was expressed as the rate of phage inactivation (Kv) using a standard formula. Mice not receiving bacteriophage did not produce neutralizing antibody (data not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/19/10.1182_blood-2009-06-228155/4/m_zh89990943800001.jpeg?Expires=1765964033&Signature=AyrdJ9jadxrme6fm1Fng~GLqTT42wrJu1H9B~s63C0vC1NqBbSSaKtpo6f6T4VTGw-xUuZdZM-r98kCzMjdzFW11zUarK6yCL88q5UX4V3hwuah2RaOzzMyKY613Y67CYjcMa9MebHgZIEvFsGAjl2Adw7Njc03XYS2O38wQwF-4UK-zLPgKcZPYaBeGskBygMaSqbMMqlosisTGi2tH1opxDJcIV1I6kuYzURhh4Qkac5ezSZoTZ~LfBBk37a3BamHBnVlFSZskpbEfZe1yeWBzvntoW3T8MGyu9gcnH09JPo1-i0HeJ~ktdWBhws8x0T4GhbDM9D7X9bi7TS9DgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Gene transfer of FVIII plasmid into experimental mouse strains. (A-B) FVIII levels and inhibitory antibody activity were assessed after delivery of pBS-HCRHPI-hFVIIIA into the HemA (■), Foxp3-Tg (▴), HemA/Foxp3-Tg (X), and wt mouse (♦) strains (n = 8/group). Mice were bled at regular intervals beginning on day 3, after plasmid injection. (A) Circulating FVIII activity was measured via a modified clotting assay and confirmed by a COATEST assay. (B) Inhibitory antibody titers were evaluated by Bethesda assay and expressed as Bethesda units/mL. (C-D) In vitro proliferation and suppression assay after stimulation of CD4+ T cells with hFVIII. (C) CD4+ splenic T cells isolated from wt, HemA, Foxp3-Tg, and HemA/Foxp3-Tg mice (n = 3/group) 4 weeks after FVIII plasmid treatment. Cells from untreated mice of each strain were used as controls. Triplicates of isolated CD4+ T cells isolated were stimulated with 4 U/mL hFVIII protein in the presence of APCs for 72 hours. Each dataset represents mean Δcpm. (D) Suppression of the proliferative response to hFVIII stimulation by Tregs in vitro. CD4+ T cells from spleens of FVIII plasmid-treated HemA mice were used as Teffs, and CD4− cells were used as APCs. CD4+CD25+ Tregs from untreated and FVIII plasmid-treated HemA mice and CD4+ Tregs from untreated and FVIII plasmid-treated HemA/Foxp3-Tg mice (n = 3/group) were cocultured with the Teffs at a ratio of 1:1 Tregs to Teffs in the presence of APCs and 10 U/mL hFVIII for 3 days. [3H]Thymidine was subsequently added and cultures maintained for another 18 hours. Data are shown by percentage of suppression compared with effector cells only (without the presence of Tregs). *P < .05 between the group of effector cells only and with Tregs from Foxp3-Tg mice.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/114/19/10.1182_blood-2009-06-228155/4/m_zh89990943800002.jpeg?Expires=1765964033&Signature=JRUv1idPSs6iDmxbx5E~TstFB-Jwiz8dPVM2hdQ2pNMzD3XgD0yrpMMz58hH9wOhaGKPH-OSIY5bISCzMegWMujFPcFg0LPk27UgZOu11Qs0MrpMTYloQ6aIWHDv2bzR9wC8PgJnqP-4cCujg8MJcunARbgcrUtF3HGwyZziDcXOPA~cxN83R6dTouuNqnJCIAbpVAb1n2o1VwcaAp9MuxQq14PlAonGyrTe7qz3ZTkIM4XUG9Gp3Op-TjoSbnH-Dl5nL-9DZK1HDRvj4ZRvKPC0XkC2GLz3VYrMQB8l4bpvIXrnWl8SBDNcBYwv-1XOURrpqzhOWTzvSvMclt3oSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal