Immune regulatory CD4+CD25+ cells play a vital role in the induction and maintenance of self-tolerance and the prevention of autoimmunity. Recently, CD4+CD25+ cells have been shown to be required for the ex vivo induction of tolerance to alloantigen via costimulatory blockade and to inhibit allogeneic skin graft rejection. Data presented here demonstrate that CD4+CD25+ cells play an important role in graft-versus-host disease (GVHD) generation. Depletion of CD4+CD25+ cells from the donor T-cell inoculum or in vivo CD25-depletion of the recipient before transplantation resulted in increased GVHD mediated by CD4+or whole T cells in several strain combinations irrespective of the total body irradiation conditioning regime. The infusion of freshly purified donor CD4+CD25+ cells modestly inhibited GVHD when administered in equal numbers with whole CD4+ cells. Because CD4+CD25+ cells only account for 5% to 10% of the total CD4+ population, the administration of high numbers of fresh donor CD4+CD25+ cells may not be clinically practical. However, we found that large numbers of CD4+CD25+ cells can be obtained by ex vivo activation and expansion. Cultured CD4+CD25+ cells, administered in equal numbers with CD4+ T cells or CD25-depleted whole T cells, resulted in significant inhibition of rapidly lethal GVHD. To our knowledge, this study is the first to demonstrate that activated, cultured CD4+CD25+ cells can offer substantial protection in a relevant in vivo animal model of disease. These data have important ramifications for clinical bone marrow and solid organ transplantation. CD4+CD25+ cells warrant consideration as an exciting new modality of cellular therapy for the inhibition of undesirable autologous and allogeneic responses.
Introduction
Immune regulatory CD4+CD25+ cells are essential for the induction and maintenance of self-tolerance and for the prevention of autoimmunity. These thymically derived professional regulatory cells prevent the activation and proliferation of autoreactive T cells that have escaped thymic deletion or recognize extrathymic antigens. Elegant studies by several investigators have elucidated their vital role in T-cell homeostasis and immune regulation.1-9 Sakaguchi et al1 found that the transfer of CD4+CD25− T cells into nude mice led to the development of autoimmune disorders that could be prevented by the cotransfer of CD4+CD25+ T cells. Suri-Payer et al3 found that susceptible mouse strains made CD4+CD25+ deficient by neonatal thymectomy developed a wide spectrum of organ-specific autoimmunities that could be prevented by the transfer of CD4+CD25+ T cells by 10 to 14 days of age. They also found that CD4+CD25+ T cells could inhibit autoimmunity induced by autoantigen-specific T-cell clones.3 Data also indicate that the role of CD4+CD25+ cells is not limited to self-tolerance and the prevention of autoimmunity. Studies indicate that depletion of these regulatory cells led to increased tumor-specific immune responses and eradication of tumors in otherwise nonresponding animals.10,11 Fewer studies have addressed the role of CD4+CD25+ T cells in alloresponses or in transplantation. Sakaguchi et al1 found that nude mice rejected allogeneic skin grafts faster if transferred lymphocytes were first depleted of CD25+cells.1 We found that CD4+CD25+cells were an essential requirement for the ex vivo induction of tolerance to alloantigen via costimulatory blockade.12
The data presented here indicate that CD4+CD25+cells play an important role in alloresponses in vivo, specifically graft-versus-host disease (GVHD) generation. Ex vivo depletion of CD4+CD25+ cells from the donor T-cell inoculum or in vivo CD25 depletion of the recipient before transplantation resulted in increased GVHD responses. These findings were observed irrespective of the strain combinations, total body irradiation (TBI) conditioning regime, and whether GVHD was mediated by CD4+T cells or both CD4+ and CD8+ T cells. The infusion of ex vivo–activated and –expanded donor CD4+CD25+ cells resulted in significant inhibition of rapidly lethal GVHD. These data are the first to evaluate the therapeutic efficacy of ex vivo–activated and –expanded CD4+CD25+ regulatory cells in an in vivo animal model of disease. We suggest that CD4+CD25+cellular therapy warrants clinical consideration for the inhibition of GVHD. Collectively, our and others' data indicate that CD4+CD25+ immune regulatory cells will have an exciting and expanding role in many areas of clinical immunology.
Materials and methods
Mice
B6.C-H2bm12/KhEg (termed bm12) (H2b) and B10.BR (H2k) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6 (termed B6) (H2b), BALB/c (H2d), and BALB/c severe combined immune deficient (SCID) mice were purchased from the National Institutes of Health (Bethesda, MD). B6 and bm12 (both H2b) differ at 3 amino acids due to mutations in the class II IA region. Mice were used at 8 to 12 weeks of age. All mice were housed in a specific pathogen-free facility in microisolator cages.
Cell enrichments and depletions
To purify whole or CD4+ T cells, axillary, mesenteric, and inguinal lymph nodes were mashed, and single-cell suspensions were passed through a wire mesh and collected in phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS; Hyclone, Logan, UT). Cell preparations were depleted of natural killer (NK) cells (hybridoma PK136, rat IgG2a) and CD8+ T cells (for CD4+ cell purification; hybridoma 2.43, rat IgG2b) by incubation with monoclonal antibody (mAb), followed by passage through a goat antimouse and goat antirat Ig-coated column (Cellect Cell Enrichment Immunocolumns, Cedarlane, Hornby, ONT, Canada). The final composition of purified T cells was determined by flow cytometric analysis to be at least 94% whole or CD4+ T cells. Where indicated, CD25+ immune regulatory cells were depleted by incubation with anti-CD25 mAb (hybridoma 3C7, rat IgG2b; BD Pharmingen, San Diego, CA) and sheep antirat Dynabeads (Dynal, Lake Success, NY) and determined to be more than 95% depleted. To enrich for CD4+CD25+ cells, purified CD4+cells were incubated with anti-CD25 biotin (hybridoma 7D4, rat IgM), followed by streptavidin-phycoerythrin (PE; both from BD Pharmingen). After incubation with MACS anti-PE MicroBeads, cells were positively selected on an MS or VS MACS separation column (both Miltenyi Biotec, Auburn, CA). Cells were determined to be more than 90% CD4+CD25+.
CD25+ ex vivo activation protocols
Enriched CD25+ cells were suspended at a final concentration of 0.5 × 106 cells/mL in 24-well plates (Costar, Acton, MA) and cultured for 1 week. Culture media was Dulbecco modified Eagle medium (DMEM; Biowhittaker, Walkersville, MD) supplemented with 10% FBS (Hyclone), 50 mM 2-mercaptoethanol (Sigma, St Louis, MO), 10 mM Hepes buffer, 1 mM sodium pyruvate (Life Technologies, Grand Island, NY), amino acid supplements (1.5 mMl-glutamine, l-arginine, andl-asparagine; Sigma), and antibiotics (penicillin, 100 U/mL; streptomycin, 100 mg/mL; Sigma). Several conditions were tested. Condition 1 used soluble anti-CD3 (0.5 μg/mL; hybridoma 145-2C11, hamster IgG; BD Pharmingen) and recombinant human interleukin 2 (IL-2; 5.0 ng/mL; Amgen, Thousand Oaks, CA). Condition 2 used immobilized anti-CD3 (5.0 μg/mL and IL-2, 100 U/mL). Cells were removed from antibody-coated plates on day 3 and transferred to fresh plates and fed with IL-2–containing media. Condition 3 used irradiated allogeneic, host-type splenic stimulators to trigger the T-cell receptor (TCR) for activation and high-dose IL-2 (100 U/mL). Condition 4 was similar to condition 3 except that IL-2 was reduced to 10 U/mL and recombinant human transforming growth factor-β2(TGF-β2) was added as an additional growth factor (1.0 ng/mL; R & D Systems, Minneapolis, MN).
GVHD induction
In some experiments, bm12 recipients were sublethally irradiated by exposing mice to 6.0 Gy TBI from a 137Cs source at a dose rate of 85 cGy/min 4 hours prior to cell infusion. B6 CD4+ T cells were administered intravenously at the indicated cell number. In other experiments, recipients were lethally irradiated by x-ray on the day prior to transplantation with allogeneic, T cell–depleted bone marrow (BM) and either whole spleen or purified whole lymph node T cells or CD4+ T cells administered intravenously at the indicated cell dose. Where indicated, donor-type CD25+ cells were infused by separate intravenous injection. Where indicated, anti-CD25 mAb (hybridoma 7D4) was administered intravenously on day −10, −7, and −4 relative to day of transplantation (0.5 mg antibody/injection). This dose and schedule resulted in depletion of 60% to 70% of CD4+CD25+ cells in lymph node and spleen when examined 3 days after the last injection. Anti-CD25 mAb was partially purified by ammonium sulfate precipitation of ascites produced in nude mice. BALB/c SCID recipients were not irradiated but were depleted of NK cells by intraperitoneal injection of 25 μL antiasialo GM1 (Wako Chemicals, Richmond, VA) 2 and 4 days prior to allogeneic T-cell transfer. Mice were monitored daily for survival and weighed twice weekly as well as examined for the clinical appearance of GVHD. Survival data were analyzed by life-table methods, and actuarial survival rates are shown. Group comparisons were made by log-rank test statistics. P ≤ .05 was considered significant.
Results
Depletion of immune regulatory CD4+CD25+cells results in acceleration of GVHD mortality in vivo
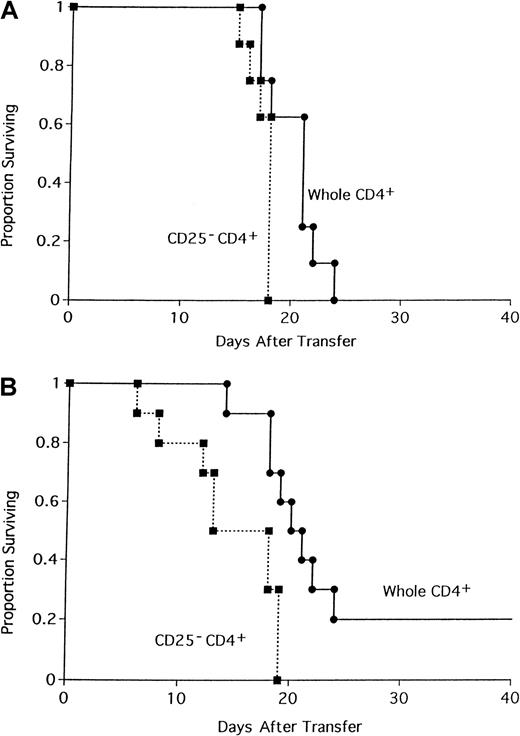
In prior studies we demonstrated that CD4+CD25+ immune regulatory cells are required for the ex vivo induction of tolerance to alloantigen via costimulatory blockade.12 Furthermore, the addition of graded numbers of freshly purified B6 CD4+CD25+ cells resulted in the dose-dependent suppression of alloresponses in a mixed lymphocyte reaction composed of B6 CD4+CD25−responders and irradiated bm12 stimulators, whereas CD25 depletion of CD4+ T cells resulted in a heightened response.12 Those studies led us to further investigate the potential role of these professional suppressor cells in regulating T-cell responses to alloantigen and in generating GVHD. To determine if depletion of CD25+ cells in a T-cell donor inoculum would result in accelerated or increased GVHD mortality in vivo, 105 whole B6 CD4+ T cells or CD25-depleted B6 CD4+ T cells were administered to sublethally irradiated bm12 recipients (Figure1A). Recipients of CD25-depleted CD4+ T cells died of GVHD 1 week earlier than recipients of whole CD4+ cells (P = .024). Because 105 cells result in a rapid and highly lethal GVHD, the experiment was repeated with a lower cell dose in an attempt to magnify the difference in survival (Figure 1B). All recipients of 0.5 × 105 CD25-depleted CD4+ T cells succumbed to GVHD by 19 days after infusion of cells. In contrast, onset of GVHD was slower in recipients of whole CD4+ T cells with 20% of mice surviving long-term (Figure 1B;P = .0068).
Depletion of CD4+CD25+ cells accelerates GVHD lethality.
The 105 (A) or 50 000 (B) whole CD4+ or CD25-depleted CD4+ B6 T cells were transferred into sublethally irradiated bm12 recipients. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group; P = .024 for panel A;P = .0068 for panel B).
Depletion of CD4+CD25+ cells accelerates GVHD lethality.
The 105 (A) or 50 000 (B) whole CD4+ or CD25-depleted CD4+ B6 T cells were transferred into sublethally irradiated bm12 recipients. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group; P = .024 for panel A;P = .0068 for panel B).
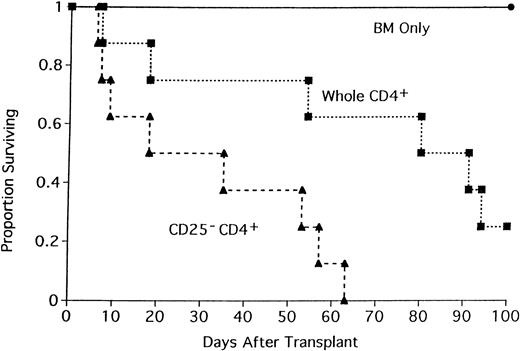
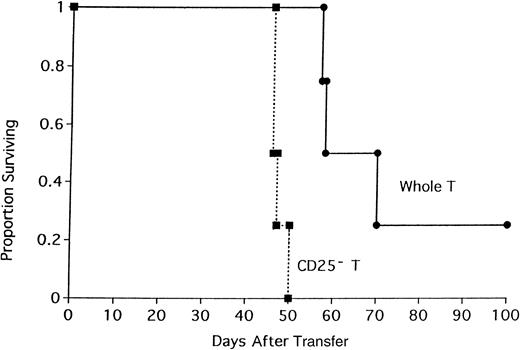
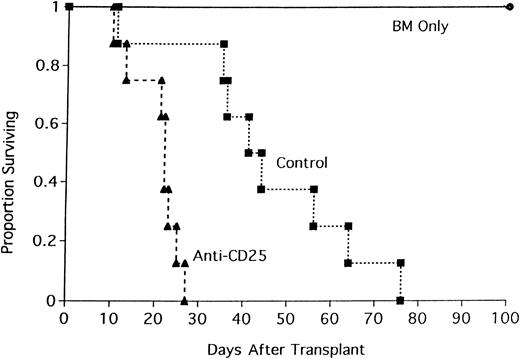
To determine if the acceleration in GVHD mortality was either unique to this strain combination or operative only under sublethal TBI conditions, lethally irradiated BALB/c mice were recipients of B6 BM and either whole CD4+ T cells or CD25-depleted CD4+ T cells (Figure 2). All recipients of CD25-depleted CD4+ T cells died by day 63 after transplantation (median survival = 35 days). In contrast, 25% of mice receiving whole CD4+ T cells survived to day 100 (median survival = 91 days; Figure 2; P = .016). The effect of CD25 depletion on GVHD generation was tested in 3 different strain combinations where GVHD was mediated by both CD4+and CD8+ T cells. In the first GVHD model, nonirradiated, NK-depleted BALB/c SCID mice received whole T cells or CD25-depleted T cells (Figure 3). CD25 depletion of the T cells resulted in an acceleration of GVHD mortality (Figure 3;P = .021) indicating that CD4+CD25+ cells play a role in GVHD mediated by both CD4+ and CD8+ T cells in the absence of TBI conditioning. In a different strain combination, lethally irradiated B10.BR mice received B6 BM and either whole B6 spleen or CD25-depleted B6 spleen (Figure 4). Recipients of CD25-depleted spleen succumbed to GVHD mortality 10 days earlier than recipients of whole spleen (P = .055). In a third strain combination, B6 recipient mice were thymectomized prior to transplantation to prevent the emergence of donor BM-derived CD4+CD25+ immune regulatory cells after transplantation. Additionally, anti-CD25 mAb was administered to these adult-thymectomized recipients before transplantation to deplete host CD4+CD25+ regulatory cells in vivo. Anti-CD25 mAb-treated or control thymectomized B6 mice were lethally irradiated and given BALB/c BM and whole spleen, and survival was monitored (Figure 5). Mice treated with anti-CD25 mAb in vivo only prior to transplantation had a significantly lower median survival rate as compared to controls (22 versus 44 days). All anti-CD25 mAb-treated recipients succumbed to GVHD mortality by 28 days after transplantation, 58 days earlier than control recipients (Figure5; P = .0063). Collectively, these data indicate that CD4+CD25+ immune regulatory cells play a significant inhibitory role in GVHD generation regardless of strain combination, pretransplant conditioning regime, or whether GVHD is mediated by CD4+ T cells or by both CD4+ and CD8+ T cells.
Depletion of CD4+CD25+ cells accelerates GVHD lethality in a different strain combination.
Lethally irradiated BALB/c mice were recipients of B6 BM and either 2 × 106 whole CD4+ T cells or CD25-depleted CD4+ T cells. On the x-axis are days after transplantation. On the y-axis is the proportion of recipients (n = 8/group;P = .016).
Depletion of CD4+CD25+ cells accelerates GVHD lethality in a different strain combination.
Lethally irradiated BALB/c mice were recipients of B6 BM and either 2 × 106 whole CD4+ T cells or CD25-depleted CD4+ T cells. On the x-axis are days after transplantation. On the y-axis is the proportion of recipients (n = 8/group;P = .016).
Depletion of CD25+ cells from a whole T-cell inoculum accelerates GVHD in a nonirradiated SCID GVHD model.
The 106 whole or CD25-depleted B6 T cells were infused into nonirradiated BALB/c SCID mice previously NK-depleted with antiasialo GM1. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 4/group;P = .021).
Depletion of CD25+ cells from a whole T-cell inoculum accelerates GVHD in a nonirradiated SCID GVHD model.
The 106 whole or CD25-depleted B6 T cells were infused into nonirradiated BALB/c SCID mice previously NK-depleted with antiasialo GM1. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 4/group;P = .021).
Depletion of CD25+ cells from whole spleen results in an acceleration of GVHD mortality.
Lethally irradiated B10.BR mice were recipients of B6 BM and either 15 × 106 whole spleen or CD25-depleted spleen. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group;P = .055).
Depletion of CD25+ cells from whole spleen results in an acceleration of GVHD mortality.
Lethally irradiated B10.BR mice were recipients of B6 BM and either 15 × 106 whole spleen or CD25-depleted spleen. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group;P = .055).
Pretransplantation in vivo depletion of CD25+ cells accelerates GVHD.
Anti-CD25 mAb-treated or control-treated thymectomized B6 mice were lethally irradiated and received transplants of BALB/c BM and 15 × 106 spleen. Anti-CD25 mAb was administered at a dose of 0.5 mg/injection on days −10, −7, and −4 relative to day of transplantation. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group;P = .0063).
Pretransplantation in vivo depletion of CD25+ cells accelerates GVHD.
Anti-CD25 mAb-treated or control-treated thymectomized B6 mice were lethally irradiated and received transplants of BALB/c BM and 15 × 106 spleen. Anti-CD25 mAb was administered at a dose of 0.5 mg/injection on days −10, −7, and −4 relative to day of transplantation. On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group;P = .0063).
Infusion of ex vivo–activated and –expanded CD4+CD25+ immune regulatory cells ameliorates GVHD
We hypothesized that the GVHD-protective effect of CD4+CD25+ cells could be clinically exploitable for the inhibition of GVHD lethality. However, previous data indicated that freshly purified CD4+CD25+ cells had only a very modest protective effect on GVHD when administered in a 1:1 ratio with whole CD4+ cells into sublethally irradiated major histocompatibility complex (MHC) class II–disparate recipients.12 Because CD4+CD25+cells account for only 5% to 10% of the total CD4+population in both mice and humans,13,14 the administration of sufficient numbers of freshly purified immune regulatory cells to be of significant therapeutic benefit may not be clinically practical. Because data indicate that CD4+CD25+ cells can become more potent suppressor cells on activation,15 we hypothesized that the ex vivo activation and expansion of CD4+CD25+cells may make immune regulatory cellular therapy clinically more feasible. Initial attempts used ex vivo incubation of purified CD4+CD25+ cells with soluble anti-CD3 mAb, syngeneic antigen-presenting cells (APCs), and high-dose IL-2 (100 U/mL) as reported by others.15 Although cells expanded 10- to 15-fold with this protocol, suppressor function as measured after adoptive transfer in vivo was significantly impaired. Expanded activated CD4+CD25+ cells did not suppress GVHD when combined with equal numbers of fresh GVHD-inducing CD4+ T cells (data not shown). Additionally, in contrast to freshly isolated CD4+ T cells, control CD4+CD25− cells expanded under the same ex vivo activation protocol failed to mediate lethality when injected into allogeneic recipients, indicating that this expansion and activation protocol resulted in a general loss of function in vivo (data not shown). Therefore, we elected to modify the activation protocol to use immobilized rather than soluble anti-CD3 mAb. After 3 days, cells were removed from the antibody-coated plates to permit TCR re-expression, and expanded in IL-2–containing media for an additional 4 days. This protocol resulted in a 15- to 20-fold expansion of CD4+CD25+ cells. These expanded CD25+ cells were evaluated in vivo for their capacity to inhibit GVHD generation. Two million freshly purified B6 CD4+ T cells were infused into nonirradiated, NK-depleted BALB/c SCID recipients. Cohorts of mice received a separate injection of 2 × 106 activated CD4+CD25+cells or CD4+CD25− cells, and survival and weights were monitored (Figure 6 and data not shown). The infusion of ex vivo–expanded CD25+ cells significantly increased the median survival time from 10 days to 72 days (Figure 6; P = .022). Survival in mice receiving supplemental expanded CD25− cells was not significantly different from control mice receiving only fresh CD4+ T cells (Figure 6; P = .285), indicating that the protective effect was specific to the CD25+ population. Although the administration of activated and expanded CD25+ cells significantly prolonged survival, mice had substantial clinical manifestations of GVHD (20% weight loss, diarrhea, hunched posture, rough poor hair coat, and generalized erythema) and did eventually die of GVHD. These data indicated that although CD25+ cells could be expanded considerably ex vivo to obtain sufficient numbers to significantly inhibit GVHD, additional improvements in the activation and expansion protocol would need to be made to increase the anti-GVHD effects.
Ex vivo expanded and activated CD25+ cells inhibit GVHD.
Two million freshly purified naı̈ve B6 CD4+ T cells were infused into nonirradiated, NK-depleted BALB/c SCID recipients. Cohorts of mice received a separate injection of 2 × 106activated CD4+CD25+ cells or CD4+CD25− cells. Cells were activated and expanded by immobilized anti-CD3 mAb and high-dose IL-2 for 1 week as described in “Materials and methods.” On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group; P = .022 for CD4+versus CD4+ + Act.CD25+).
Ex vivo expanded and activated CD25+ cells inhibit GVHD.
Two million freshly purified naı̈ve B6 CD4+ T cells were infused into nonirradiated, NK-depleted BALB/c SCID recipients. Cohorts of mice received a separate injection of 2 × 106activated CD4+CD25+ cells or CD4+CD25− cells. Cells were activated and expanded by immobilized anti-CD3 mAb and high-dose IL-2 for 1 week as described in “Materials and methods.” On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 8/group; P = .022 for CD4+versus CD4+ + Act.CD25+).
In the next experiment, 3 different methods of activating and culturing B6 CD25+ cells were compared. Condition 2, activation via immobilized anti-CD3 and high-dose IL-2 (100 U/mL), as described above, was used as a standard for comparison. Because immobilized antibody can result in strong TCR signaling and activation-induced cell death,16-19 a less potent and global means of activation was tested. Thus, in condition 3, irradiated BALB/c splenic stimulators were added to purified B6 CD25+ cells (at a 2:1 ratio), to induce more physiologic levels of TCR signaling and activation, and cultured in the presence of high-dose IL-2 (100 U/mL). For condition 4, we hypothesized that although a relatively high dose of IL-2 might be required for optimal expansion, withdrawal from high-dose IL-2 could be contributing to poor cell survival on transfer in vivo thereby potentially resulting in less than optimal GVHD protection.16 To test this hypothesis, condition 4 used irradiated BALB/c splenic stimulators and low-dose IL-2 (10 U/mL), and TGF-β (1.0 ng/mL), which has been reported to be a growth factor for CD4+ immune regulatory cells.20
An important part of the evaluation of the culturing conditions was the recovery data because the clinical feasibility of this approach would be dependent on being able to infuse sufficient numbers of activated CD25+ regulatory cells. The culture protocol using immobilized anti-CD3 and high-dose IL-2 resulted in a 12-fold expansion of cells in 1 week. Irradiated allogeneic stimulators and high-dose IL-2 led to only a 1.5-fold expansion of cells. The culture condition using allogeneic splenic stimulators, low-dose IL-2, and TGF-β resulted in the lowest recovery with only 31% of input cells recovered at 1 week. All cultures were more than 95% viable.
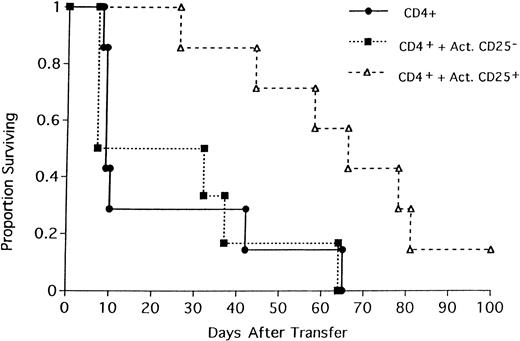
All 3 types of cultured CD25+ cells were evaluated for their ability to inhibit GVHD mediated by both CD4+ and CD8+ T cells (Figure 7). BALB/c SCID mice received 106 CD25-depleted whole T cells to induce GVHD. Two separate cohorts of mice also received 106 CD25+ cells cultured under each of the first 2 conditions (anti-CD3/IL-2 or allo-APCs/IL-2). A third cohort received 106 CD25-depleted whole T cells and 0.5 × 106 CD25+ cells cultured with irradiated BALB/c splenocytes, low-dose IL-2, and TGF-β (insufficient recovery in this group did not permit the infusion of 106 CD25+ cells). All recipients of CD25− T cells died 8 days after transfer of cells (Figure7). In contrast, the infusion of cultured CD25+ cells significantly inhibited GVHD mortality regardless of culture protocol. Fifty percent (3 of 6) of recipients of fresh CD25− T cells and CD25+ cells expanded by immobilized anti-CD3 mAb and high-dose IL-2 were alive 2 months after transfer of cells. However, clinical appearance and examination of weight curves revealed that survivors had significant manifestations of GVHD as indicated by lethargy, hunched posture, generalized erythema, poor hair coat, and a 25% reduction in mean weights from pretransplant levels (data not shown). Survival was prolonged in recipients of CD25− T cells and CD25+ cells cultured with BALB/c splenocytes and high-dose IL-2, but all mice died of GVHD by day 54 (median survival of 31 days; Figure 7). The best GVHD inhibition was mediated by CD25+ cells cultured with irradiated BALB/c splenocytes, low-dose IL-2, and TGF-β. Despite the lower cell number infused, CD25+ cells cultured under this protocol protected 5 of 6 recipients against GVHD lethality for 2 months. Additionally, 4 of the 5 survivors did not have any obvious clinical signs of GVHD and had weights identical to their pretransplant values (data not shown). These data indicate that CD4+CD25+cells can easily be expanded ex vivo in sufficient numbers and mediate significant protection against rapidly lethal GVHD.
CD25+ cells cultured under different conditions inhibit GVHD.
One million CD25-depleted B6 T cells were infused into nonirradiated, NK-depleted BALB/c SCID recipients. A second group of mice received a separate injection of 106 CD25+ cells expanded as for Figure 6, with immobilized anti-CD3 mAb and high-dose IL-2 (open boxes). A third group of mice received a separate injection of 106 CD25+ cells cultured with irradiated BALB/c splenocytes and high-dose IL-2 (open triangles). A fourth group of mice received a separate injection of 0.5 × 106CD25+ cells cultured with irradiated BALB/c splenocytes, low-dose IL-2 and TGFβ as described in “Materials and methods” (star). On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 6/group; allP values ≤ .016 compared to control group [closed circle]).
CD25+ cells cultured under different conditions inhibit GVHD.
One million CD25-depleted B6 T cells were infused into nonirradiated, NK-depleted BALB/c SCID recipients. A second group of mice received a separate injection of 106 CD25+ cells expanded as for Figure 6, with immobilized anti-CD3 mAb and high-dose IL-2 (open boxes). A third group of mice received a separate injection of 106 CD25+ cells cultured with irradiated BALB/c splenocytes and high-dose IL-2 (open triangles). A fourth group of mice received a separate injection of 0.5 × 106CD25+ cells cultured with irradiated BALB/c splenocytes, low-dose IL-2 and TGFβ as described in “Materials and methods” (star). On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 6/group; allP values ≤ .016 compared to control group [closed circle]).
Discussion
Although CD4+CD25+ immune regulatory cells are important regulators of in vivo homeostasis and are required for the prevention of autoimmunity, the role of these professional suppressor cells in alloresponses has been less well studied. The data presented here indicate that these professional cells also play an important role in the regulation of GVHD generation. This was demonstrated by the acceleration of GVHD and by the increase in lethality that occurred when CD25+ cells were depleted ex vivo from the donor T-cell inoculum or in vivo in the recipients before transplantation with anti-CD25 mAb infusion. Depletion of CD25+ cells resulted in an increase in GVHD regardless of whether donor antihost responses were mediated by CD4+ T cells or both CD4+ and CD8+ T cells. This is consistent with data by others that also found that CD4+CD25+ immune regulatory cells could inhibit CD8+ T-cell responses.21 22 Additionally, CD25+ cell depletion accelerated GVHD in several strain combinations irrespective of intensity of conditioning, indicating that even in a high proinflammatory cytokine milieu, CD25+ cells were functioning as suppressors of alloresponses.
The depletion data implicated the role of CD25+ cells in inhibition of alloresponses and suggested that the infusion of CD25+ cells could prevent or ameliorate GVHD. Our previous data indicate that fresh naı̈ve CD4+CD25+ cells did not mediate GVHD lethality alone and had only a modest protective effect when infused with GVHD-inducing T cells at a 1:1 ratio into sublethally irradiated MHC class II–disparate recipients.12 Because CD25+ cells constitute 5% to 10% of the total CD4+ T-cell population in human peripheral blood mononuclear cells,13,14 it would be difficult to infuse sufficient numbers of purified regulatory cells to be of significant therapeutic benefit. However, data by Thornton and Shevach indicate that CD4+CD25+ cells become more potent suppressor cells on ex vivo activation and furthermore cells can be significantly expanded during culture.15 Although the experiments presented in this paper used cells that were cultured for only 1 week, CD4+CD25+ cells can be expanded to an even greater degree with longer culture duration. A 67-fold expansion of CD4+CD25+ cells was obtained in 8 days in a recent experiment and cells have been maintained in culture for over 4 weeks (P. A. Taylor, unpublished data, 2001). The in vivo efficacy of such long-term cultured cells is yet to be determined.
Data presented in this study indicate that different ex vivo activation protocols led to varying recovery or expansion of CD4+CD25+ cells, but all protocols resulted in cells that significantly inhibited GVHD albeit to varying degrees. The ex vivo activation protocols investigated in these studies were meant to demonstrate proof-of-principle and not to be an exhaustive list of potential strategies for expansion and activation. Clearly, additional studies designed to develop new activation and expansion approaches and to determine which of these approaches is the most effective in GVHD prevention are warranted. For example, CD4+CD25+ immune regulatory cells are a very heterogeneous population. Therefore, it seems likely that different methods of activation and expansion may result in distinct populations of cells with potentially different suppressor/effector function. Although the best protection (despite the lower number of infused cells) was mediated by the culture method that resulted in the lowest recovery (allogeneic splenocytes, low-dose IL-2, and TGF-β), it seems likely that culture protocols can be modified to optimize both expansion and suppressor function. Allogeneic splenocytes did not result in sufficient expansion of CD25+ cells even in the presence of high-dose IL-2 under these conditions to be clinically feasible if the mouse conditions directly translate to the human setting. However, more potent APCs such as activated dendritic cells may result in better expansion and superior function through the delivery of multiple physiologic signals that may also aid cell survival. Because CD4+CD25+ cells do not require activation via alloantigen per se to inhibit alloantigen-reactive CD25− T cells, it may be desirable to achieve maximal activation (and expansion) by using polyclonal inducers of TCR signaling as long as activation-induced cell death does not completely mitigate the beneficial effect. Withdrawal from high-dose IL-2 can also result in the death of cells on transfer in vivo.16 Although there is significant inhibition of GVHD with CD25+ cells cultured with immobilized anti-CD3 mAb and high-dose IL-2, it is possible that poor survival of CD4+CD25+ cells in vivo from IL-2 withdrawal or activation-induced cell death is limiting the anti-GVHD effect. The inclusion of TGF-β in this activation protocol may be warranted because data indicate that TGF-β is a growth factor for CD4+ regulatory cells and, additionally, renders them more resistant to activation-induced cell death.20,23However, TGF-β added to a low IL-2 concentration was less effective in supporting the expansion/survival of CD4+CD25+ cells as compared to a high IL-2 concentration. Therefore, further modifications of this protocol that resulted in the highest anti-GVHD effect will be required to increase CD4+CD25+ cell yield. In that regard, potentially attractive candidate cytokines for testing in the culture include IL-4 and IL-7, which have been shown to increase survival of T cells,24 IL-10, which has been shown to be responsible for the generation of regulatory T cells,25 and IL-15, which has been shown to synergize with low-dose IL-2 to induce vigorous proliferation of human CD4+CD25+cells.13
An important contribution of these data is that the various GVHD models used allow for the evaluation of the in vivo therapeutic efficacy of activation and expansion protocols of CD4+CD25+cells in a relevant animal model of disease. Because of the known potential deficits of activated cultured cells in homing, migration, survival, and function in vivo, it is important that the regulatory function of ex vivo activated and expanded regulatory cells be evaluated in vivo as well as in vitro. For example, although CD4+CD25+ cells can be activated and expanded via culture with soluble anti-CD3 mAb, APCs, and IL-2 (the current “gold standard” in ex vivo expansion protocols) and maintain in vitro suppressor function, their ability to suppress alloresponses was lost on in vivo transfer15 (data not shown).
It is also important to note that although multiple cell doses were not examined in these studies, the ease of expansion of CD4+CD25+ cells will readily permit the infusion of much higher cell doses than those used for these experiments. Additionally, the infusion of expanded CD25+cells as pretransplantation conditioning to inhibit either GVHD generation or graft rejection or the administration of multiple infusions after BM transplantation may also be of clinical benefit. Future experiments will address these issues.
The mechanism by which activated CD25+ cells inhibit GVHD remains unknown, but the literature suggests several possibilities that are not necessarily mutually exclusive. Data from Nakamura et al23 indicate that a culture protocol including immobilized anti-CD3 mAb and anti-CTLA4 mAb, soluble anti-CD28 mAb, and IL-2 led to the highest production of cell surface-bound TGF-β, which was responsible for the cell contact-dependent immunosuppression mediated by CD4+CD25+ cells in an in vitro system. Thornton and Shevach showed that CD4+CD25+ T cells block the induction of IL-2 production by the CD4+CD25− cells at the level of RNA transcription.26 The relative paucity of IL-2 could inhibit the activation, proliferation, and effector function of both CD4+CD25− and CD8+ T cells. Other data indicate that the constitutive expression of CTLA-4 on CD4+CD25+ cells may play an important role in their regulatory function.9 27
If we accept that CD4+CD25+ cells have a regulatory role in immune responses to foreign or alloantigen as well as in autoimmunity, the increase in GVHD lethality resulting from CD25 depletion of the donor T-cell inoculum might be anticipated. However, interpretation of the in vivo depletion data in which anti-CD25 mAb is administered to the recipient is potentially more complicated and suggests some intriguing possibilities that might be of clinical relevance. Anti-CD25 mAb was administered to the recipient before transplantation in an attempt to avoid depletion of host-reactive donor T cells that would up-regulate CD25 as an activation marker during GVHD. Although CD25 depletion after BM transplantation has been shown to ameliorate GVHD,28-31 CD25 depletion of the recipient before transplantation accelerated GVHD implicating host CD25+ cells as involved in dampening the GVHD response. Although the goal was to deplete host CD25+ cells and the last anti-CD25 mAb injection was given 4 days prior to transplantation, we cannot exclude the possibility that there was sufficient circulating antibody to deplete both host and donor CD25+ cells including donor CD4+CD25+ regulatory cells. However, because GVHD was worsened by the infusion of anti-CD25 mAb before transplantation, we hypothesize that residual host CD25+ cells remaining after radiation may also inhibit the generation of GVHD by the donor T-cell inoculum via a host antidonor resistance mechanism. This raises the rather intriguing speculation that either donor or host-type CD25+ cells could be used to inhibit GVHD responses and further suggests that maintenance of host CD25+ cells would be clinically desirable. Future studies will address these issues. These data also suggest that the clinical targeting of activated CD25+ donor T cells as a means to prevent GVHD may be complicated by the fact that potentially beneficial CD25+ immune regulatory cells could also be depleted.
In summary, we show that CD4+CD25+ cells play an important role in alloresponses in vivo and in the generation of GVHD. Ex vivo expanded and activated immune regulatory cells significantly inhibited rapidly lethal GVHD. Because human CD4+CD25+ regulatory cells have been reported to inhibit in vitro alloresponses of both naı̈ve and memory CD4+ T cells and can be expanded in vitro with maintenance of suppressor function,13,14 32 these preclinical data may be translatable. Collectively, these studies have important clinical ramifications in many areas of clinical immunology and transplantation biology.
The authors thank Drs Angela Thornton and Ethan Shevach for helpful advice on the purification and expansion of CD4+CD25+ T cells.
Supported by National Institutes of Health grants R01 AI34495, R37 HL56067, and PO1 AI 35225 to B.R.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Bruce R. Blazar, University of Minnesota Cancer Center and Department of Pediatrics, Division of Bone Marrow Transplantation, Minneapolis, MN 55455; e-mail: blaza001@tc.umn.edu.


![Fig. 7. CD25+ cells cultured under different conditions inhibit GVHD. / One million CD25-depleted B6 T cells were infused into nonirradiated, NK-depleted BALB/c SCID recipients. A second group of mice received a separate injection of 106 CD25+ cells expanded as for Figure 6, with immobilized anti-CD3 mAb and high-dose IL-2 (open boxes). A third group of mice received a separate injection of 106 CD25+ cells cultured with irradiated BALB/c splenocytes and high-dose IL-2 (open triangles). A fourth group of mice received a separate injection of 0.5 × 106CD25+ cells cultured with irradiated BALB/c splenocytes, low-dose IL-2 and TGFβ as described in “Materials and methods” (star). On the x-axis are days after transfer of cells. On the y-axis is the proportion of recipients surviving (n = 6/group; allP values ≤ .016 compared to control group [closed circle]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/10/10.1182_blood.v99.10.3493/6/m_h81022550007.jpeg?Expires=1763461668&Signature=GJAEPlqkUc6vweyoPWFXn54eSM9uwD2ISuC-40XfzI8FdGVJtJwo-aJ8JF0By6IwgCOW3s-wLjJ~Y8LBAFoS87-uPDhK34owI8HRBHS7mf-32FYeanPVkLyWrAJ8xdub3AX2IDbx6Az4CpJdIHnVAw-bz95-8GcdeVkGa3qoMYbhEa3cnCVFkg5yJVAfGi3m8STBIc-S-RyXaOZCmSdu15v-EqMtM6oROAdvd~Kw4Qdsc37OYXfTvIoE-2SjbB-GMZ7vu-3e8NExmJK1DQ0057kH1exs4YLSk3DpMBuS45ytXvuvW5MKppe72m~qJ5KHqaUwQK8sPXf4Iu8ZYnEXXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal