Abstract
A fraction of fibrinogen contains a differently spliced γ chain called γ′, which presents itself mainly as heterodimer with the common γA chain as γA/γ′ fibrinogen. The γ′ chain differs from the γA chain in its C-terminus and has important functional implications for fibrinogen. The presence of the γ′ chain modulates thrombin and FXIII activity, influences clot architecture, and eliminates a platelet-binding site. Associations of γA/γ′ fibrinogen levels with arterial and venous thrombosis have been reported, indicating that the functional effects of γA/γ′ fibrinogen may contribute to the pathology of thrombosis. This review summarizes the key biologic aspects of this interesting variant of fibrinogen and discusses inconsistencies in current reports.
Introduction
Fibrinogen is a key component of the hemostatic system, playing a role in both primary and secondary response. Thrombin-catalyzed cleavage of fibrinopeptides (Fp) A and B converts fibrinogen into fibrin, which spontaneously polymerizes and forms double-stranded protofibrils that assemble into branched fibrin fibers, forming the fibrin clot.1,2 Fibrinogen molecules are elongated 45-nm structures consisting of a central E region connected by coiled-coil segments to 2 outer D regions. The molecule is a disulfide-bonded dimer each consisting of 3 polypeptides termed Aα, Bβ, and γ. The 3 polypeptides are encoded by separate genes, FGA, FGB, and FGG, clustered in a region of approximately 50 kb on chromosome 4q31.3.3
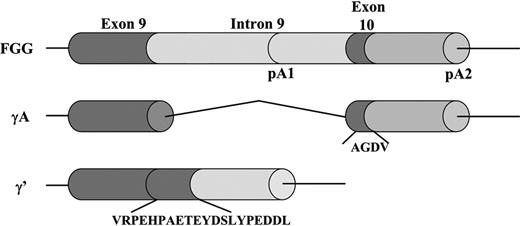
The fibrinogen γ-chain mRNA transcript is subject to alternative processing and polyadenylation (Figure 1).4 The main form, the γA chain, consists of 411 amino acids, composed of 10 exons and 9 introns. For this chain to arise, polyadenylation occurs at the polyadenylation signal downstream of exon 10 (pA2 in Figure 1). The alternative γ′ chain (also termed γB or γ57.55-7 ) arises when polyadenylation occurs at an alternative polyadenylation signal in intron 9 (pA1 in Figure 1). In this case, intron 9 is not spliced out, which leads to the translation of a unique 20-amino acid extension (γ′408-427; VRPEHPAETEYDSLYPEDDL) encoded by intron 9, substituting the 4 γA amino acids (γA408-411) of exon 10.8 A recent paper on the evolution of the fibrinogen γ′ chain shows evidence that the γ′ sequence is directly descended from a primitive unspliced version of the γ chain gene.9
Alternative FGG pre-mRNA processing. The γA chain arises when polyadenylation occurs at polyadenylation signal 2 (pA2) downstream of exon 10 and all 9 introns are removed. The alternative γ′ chain arises after polyadenylation at pA1 in intron 9. This leads to the translation of a polypeptide with a unique 20-amino acid extension encoded by intron 9, substituting the 4 γA amino acids of exon 10.
Alternative FGG pre-mRNA processing. The γA chain arises when polyadenylation occurs at polyadenylation signal 2 (pA2) downstream of exon 10 and all 9 introns are removed. The alternative γ′ chain arises after polyadenylation at pA1 in intron 9. This leads to the translation of a polypeptide with a unique 20-amino acid extension encoded by intron 9, substituting the 4 γA amino acids of exon 10.
Because of the overall negative charge of the γ′ carboxy-terminal 20 amino acids, plasma fibrinogen is separable by anion-exchange chromatography into 2 major peaks.10 Peak 1 fibrinogen is homodimeric with respect to its γ chains (γA/γA), whereas peak 2 fibrinogen molecules are heterodimeric (γA/γ′).8,11 Approximately 8% to 15% of a healthy person's total fibrinogen level is composed of γA/γ′ fibrinogen, whereas homodimeric γ′/γ′ fibrinogen accounts for less than 0.5% of total fibrinogen.8,12 In addition to γ′427L chains, a minor population exists of γ′ chains designated γ55 or γ′423P, which lack the carboxy-terminal 4 residues EDDL.13-15 This shortened γ′423P chain accounts for 3% to 34% of the γ′ chain and probably arises in plasma by postsecretory in vivo processing of γ′427L chains,15 although the exact process or enzyme responsible and its physiologic significance are currently unknown. The regulation of γA/γ′ fibrinogen levels in vivo has not yet been elucidated, but there is evidence that γ′ mRNA processing is a tissue-specific and regulated event because the γ′ chain is expressed only in hepatocytes and not by megakaryocytes or platelets.16,17
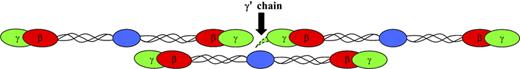
Replacement of the C-terminal residues of the γ chain has functional implications for the fibrinogen molecule. The γ′-chain extension protrudes from the D region and, depending on folding, could span up to 30 to 40 Å (Figure 2). The conformation of the γ′ chain in polymerizing fibrin is unknown, although it has been described that it is exposed or surface oriented in cross-linked fibrin.18 Before cross-linking, during initial polymerization, the γ′ chain may interact with the D-D interface. Considering a length of 30 to 40 Å, it is possible that the γ′ extension stretches to the E region of a neighboring molecule in the D-E-D complex. The γ′ chain is therefore positioned at or near an interaction area that forms the cornerstone of protofibril formation. Interactions of other coagulation proteins with the γ′ region positions these proteins in a functionally active area of fibrin formation. So far, the γ′ chain has been shown to bind to thrombin and copurifies with coagulation factor XIII (FXIII), both involved in the formation of the fibrin clot. In this review, we describe what is currently known about the influence of the γ′ chain in hemostasis. We illustrate that the γ′ chain may contribute to the pathology of thrombosis via modulating thrombin and FXIII activity, eliminating a platelet binding site, influencing clot architecture, and modifying thrombosis risk.
Fibrinogen molecules with location of the γ′ chain. The amino acid extension of the γ′ chain is located at the carboxy-terminus (black arrow) of the γ chain on the D region of the molecule. When fibrinogen is converted to fibrin by thrombin cleavage of fibrinopeptides in the E region, fibrin polymerizes through D-E-D interactions, with the γ′ extension at the D-D interface. The structure of the γ′ chain is unknown, but an extra 16 amino acid residues may extend up to 30 to 40 Å or more depending on folding. Considering this length, the γ′ extension could stretch to the E region of a neighboring molecule in the D-E-D complex.
Fibrinogen molecules with location of the γ′ chain. The amino acid extension of the γ′ chain is located at the carboxy-terminus (black arrow) of the γ chain on the D region of the molecule. When fibrinogen is converted to fibrin by thrombin cleavage of fibrinopeptides in the E region, fibrin polymerizes through D-E-D interactions, with the γ′ extension at the D-D interface. The structure of the γ′ chain is unknown, but an extra 16 amino acid residues may extend up to 30 to 40 Å or more depending on folding. Considering this length, the γ′ extension could stretch to the E region of a neighboring molecule in the D-E-D complex.
Thrombin binding
One of the main characteristics of the γ′ chain is its ability to interact with thrombin. Thrombin exosite II (residues encompassing R93, K236, K240, R101, and R23319 ) binds to the γ′ chain with high affinity (Kd value 0.20 μM).12,20 However, the highest affinity interaction occurs when both thrombin exosite I (residues encompassing K36, H71, R73, R75, Y76, R77a, and K109/11019 ) and II bind the fibrin(ogen) E region and γ′ chain simultaneously (Kd values of 107 ± 36 nM and 1.5 μM for the high- and low-affinity binding sites, respectively).21 Residues A414 to L427 of the γ′ chain have been reported to play a central role in thrombin binding because of the overall negative charge, and sulfation of residues Y418 and Y422 further enhances binding by increasing the net charge.12,20,22,23 Sabo et al reported that Y422-D425 forms a β-turn that is involved in exosite II binding, whereas further interactions occur between Y418 and Y422.24 Crystal structure data of the complex between thrombin and a γ′ V408-L427 peptide indicated that γ′ Y418 interacts with residues R126, K235, and K236 on thrombin and that γ′ Y422 binds to thrombin residue K240.25 These data were obtained with a synthetic γ′ peptide in which Y418 and Y422 were phosphorylated rather than sulfated. Although phosphorylation adds a negative charge similar to sulfation, and at least in vitro, tyrosine phosphorylation is functionally equivalent to sulfation, a note of caution may be required until confirmation is obtained with a sulfated polypeptide. The crystallography data also suggested that γ′ residues P413 and A414 play a role in thrombin binding.25 One study using nonsulfated peptides found that deletion of the C-terminal residues DDL425-427 reduces thrombin binding, suggesting that the γ′423P variant may not bind thrombin.12 This indicates that, although central thrombin interaction sites are located N-terminal to P423, residues between E424 and L427 may contribute to stabilizing the β-turn in the γ′ chain.
There are several functional consequences of thrombin binding to the γ′ chain. One of these involves inhibition of thrombin activity, which was first reported as antithrombin I more than 50 years ago by Seegers et al.26,27 For comprehensive reviews on this topic, see Mosesson.28,29 The thrombin clotting time was increased by addition of phosphorylated γ′ V408-L427 peptide in a purified system,30 but the γ′ peptide was added at a 80- to 320-fold molar excess over γA/γA fibrinogen, compared with a 0.1 molar ratio in vivo. Reconstitution of fibrinogen into fibrinogen-deficient plasma reduced thrombin generation as measured using a thrombin chromogenic substrate, and γA/γ′ fibrinogen showed more inhibition than γA/γA fibrinogen.23 Another study using a γ′ P410-L427 synthetic peptide found no effects on cleavage of a chromogenic substrate by thrombin, but a reduction in FVIII activation, suggesting selective inhibition of the intrinsic pathway.31 These data suggest that the γ′ chain may act as an inhibitor of the plasma consolidation (intrinsic) pathway for thrombin generation in the solution phase. The authors also demonstrated that this peptide inhibited thrombus formation in a primate thrombosis model.31 It has been suggested that allosteric effects of the binding of the γ′ chain to exosite II on the thrombin catalytic site may be responsible for the inhibitory activity of γA/γ′ fibrinogen.30
Recent reports suggest that the γ′ chain may also function as a reservoir for active thrombin in the fibrin clot. Thrombin bound to fibrin was shown to remain functionally active.32 Despite the higher affinity to γA/γ′ fibrin, the release of FpA from γA/γ′ fibrinogen by thrombin was similar compared with γA/γA fibrin,32 a finding supported by our data.33 In addition, Fredenburgh et al32 showed that binding of thrombin to the γ′ chain offers thrombin protection from inhibition by heparin in complex with heparin cofactor II or antithrombin, confirming a previous report by Pospisil et al.34 As exosite II provides the site of interaction for both heparin and the γ′ chain, it is probable that competitive inhibition for exosite II plays a role in the mechanism underpinning this.21,35,36 So although on one hand the γ′ chain is responsible for antithrombin I activity and reduction of intrinsic activation, FpA release on the other hand is normal and thrombin is afforded protection against serpin inactivation in the presence of glycosaminoglycans. It appears that the γ′ chain provides another example where exosite binding is involved in directing thrombin's activity,19,37 possibly by inhibiting thrombin generation in the solution phase and localizing thrombin to the fibrin surface.
Murine fibrinogen γ′ chains terminate at Y417 (position Y418 in human FGG) and do not bind thrombin. Recently, a transgenic mouse was studied where the wild-type sequence was replaced with the human γ′ sequence.38 This resulted in a murine fibrinogen containing chimeric human γ′ chains able to bind thrombin. In a femoral vein model, there were no differences in thrombus generation between wild-type and heterozygous mice carrying the human γ′ chain. However, mice heterozygous for both factor V Leiden (FVL) and human γ′ were protected against increased thrombus burden induced by FVL alone.38 FVL increases thrombin generation by limiting the inhibitory action of activated protein C. The antithrombotic effect of the γ′ chain may therefore occur at high levels of thrombin generation in the presence of FVL. However, interpretations need to be made with caution, as the human γ′ chain was placed in an environment of murine coagulation proteins, including thrombin. So far, there have been no reports of a relationship between FVL and the γ′ chain in humans.
Influence of the γ′ chain on platelet aggregation
Multiple studies on fibrinogen and platelet binding showed that the carboxy-terminal end of the γ chain is involved in binding to platelet integrin αIIbβ3.39-42 Subsequent studies showed that the γ′ chain does not promote platelet aggregation because it lacks the binding site required for platelet adhesion, γA residues 408 to 411.43-46 Recently, Lancellotti et al reported that the γ′ chain reduces thrombin-induced platelet aggregation through a combined mechanism.47 They showed that the γ′ chain, either in fragment D or the synthetic γ′ peptide, hinders both thrombin binding to GpIbα and thrombin cleavage of PAR1 on platelets. Lovely et al showed similar results in a study using synthetic γ′ peptides.48 These studies indicate that variations in the ratio of γA and γ′ fibrinogen could significantly modify platelet activation in vivo. The effect of the γ′ chain on platelet function should be considered largely antithrombotic.
Besides uptake from plasma, platelets are capable of synthesizing fibrinogen49 and other proteins through an intact translation machinery that lacks DNA but consists of mRNA, ribosomes, and amino acids.50 Interestingly, fibrinogen stored in the platelet α-granules completely consists of γA/γA fibrinogen.51,52 It is unknown why there is no γA/γ′ fibrinogen in platelets, but it is possible that because only the γA chain binds to αIIbβ3, this fibrinogen is taken up selectively through endocytosis of membrane vesicles that contain αIIbβ3 and γA/γA fibrinogen complexes. Alternatively, it could be that platelets only contain or activate the splicing machinery that produces γA fibrinogen, similar to the type of tissue-specific regulatory control described for interleukin-1β53 and tissue factor.54
FXIII binding and fibrinogen cross-linking
FXIII is a plasma transglutaminase that increases the resistance of the clot to fibrinolysis by forming covalent bonds between adjacent fibrin monomers55 and the cross-linking of plasmin inhibitor to fibrin. γA/γ′ fibrinogen has been shown to copurify with FXIII using anion-exchange chromatography.56 FXIII circulates in plasma as a tetramer (A2B2). Activation of FXIII occurs when thrombin cleaves the R37-G38 bond releasing a 4-kDa activation peptide from each A subunit, which is followed by dissociation of B subunits in the presence of calcium. Activated FXIII, FXIIIa, forms cross-links by producing isopeptide bonds between plasmin inhibitor and fibrin α chains, between 2 fibrin γ chains and 2 or several α chains, which strengthens the clot57 and increases its resistance to lysis.58,59
Evidence for an interaction between the γ′ chain and FXIII was reported as early as 1963, when Mosesson and Finlayson described that plasma FXIII copurified with fibrinogen and coeluted with γA/γ′ fibrinogen when separated on diethylaminoethyl-cellulose.56 In another study, mixtures of γA/γA fibrinogen with FXIII applied to a diethylaminoethyl-column eluted in 2 peaks, whereas a γA/γ′ fibrinogen/FXIII mixture eluted within the γA/γ′ fibrinogen peak.60 Interaction between γA/γ′ fibrinogen and FXIII has been reported to occur via FXIII-B subunits, as placental or platelet FXIII (A2), not containing B subunits, eluted separately from γA/γ′ fibrinogen. This suggests that the γ′ chain interacts with FXIII-B subunits and serves as a carrier for FXIII to increase the local concentration of FXIII in the fibrin clot.
Subsequent ultracentrifugation studies have shown that both γA/γA and γA/γ′ fibrinogen bind to FXIII in a 2 fibrinogen:1 FXIII molar ratio.61 Furthermore, FXIII binds to γA/γ′ fibrinogen with an approximate 20-fold higher affinity than γA/γA fibrinogen, and the interaction with γA/γ′ fibrinogen (but not γA/γA fibrinogen) is accompanied by a significant release of calcium. In addition, a γ′ peptide bound FXIII with the same molar ratio as full-length γA/γ′ fibrinogen and served as a competitive inhibitor of FXIII binding to γA/γ′ fibrinogen.61 In addition, a very recent study showed that FXIII binding to activated platelets is mediated via the γ′ chain of aIIbβ3-bound fibrinogen, suggesting a bridging function of the γ′ chain between FXIII and activated platelets.62 However, binding of FXIII to the γ′ chain has been put into question by a report from Gersh and Lord who used an enzyme-linked immunosorbent assay–based system with recombinant fibrinogens and found no difference in Kd for the binding of FXIII to γA/γA, γA/γ′, or γ′/γ′ fibrinogen.63 Further studies on FXIII and γA/γ′ fibrinogen will be required to clarify the interaction and whether it may modulate clot stability.
There are potential functional consequences of FXIII interaction with the γ′ chain, especially considering that thrombin also interacts with this area. The presence of polymerizing fibrin enhances FXIII activation by thrombin.64-66 The activation kinetics of FXIII by monitoring the FXIII activity to cross-link biotin-labeled pentylamine into a casein substrate was enhanced by both γA/γA and γA/γ′ fibrinogen, and the effect observed for γA/γ′ fibrinogen was greater than that for γA/γA fibrinogen.67 This suggests that γA/γ′ fibrinogen serves to enhance FXIII activation more than γA/γA fibrinogen. However, when kinetics of FXIII activation were assessed by monitoring cleaved FXIII-A subunits on sodium dodecyl sulfate–polyacrylamide gel electrophoresis, there appeared to be no distinction between the enhancement effect of γA/γA and γA/γ′ fibrin.67 These authors suggested that γA/γ′ fibrinogen may facilitate FXIII activation by enhancing the dissociation of the A and B subunits of FXIII, rather than the cleavage of the activation peptide by thrombin. In addition, more rapid and increased cross-linking of γA/γ′ fibrin by FXIIIa compared with γA/γA fibrin was found. In contrast, Siebenlist et al found slower initial γ chain cross-linking rates for γA/γ′ than for γA/γA fibrin, but no measurable differences after 1 minute.68 Their data further suggested that FXIII activation was slower in the presence of γA/γ′ fibrinogen than it was in the presence of γA/γA fibrinogen.30 FXIII activation peptide cleavage by Atroxin, a snake venom enzyme that cleaves FpA from fibrinogen but does not bind to the γ′ chain, was not affected by the presence of γA/γ′ fibrinogen, suggesting that the observed rate differences in the thrombin-catalyzed system were related to thrombin binding to the γ′ chain.
There are only a few studies that have investigated the influence of the γ′ chain on fibrinolysis. Falls and Farrell showed with both purified and whole plasma fibrinolysis assays that γA/γ′ fibrin clots are more resistant to fibrinolysis.69 Both the rate of clotting and the rate of lysis were significantly decreased in γA/γ′ fibrin clots compared with γA/γA fibrin clots, but only in the presence of FXIII. Similar results were obtained in a study using recombinant fibrinogens with the addition of purified FXIII, where the fiber lysis rate of γA/γA fibrin was approximately 10 times faster than γ′/γ′ fibrin.70 Further studies will be required to clarify the role of the γ′ chain with regards to resistance to fibrinolysis. It will be interesting to investigate whether the γ′ chain also affects the fibrinolysis rate of platelet-rich clots.
Effect of the fibrinogen γ′ chain on fibrin clot structure
The architecture of the fibrin clot can directly contribute to disease. Overwhelming evidence for this comes from dysfibrinogenemias, where alterations in structure caused by mutations in the fibrinogen genes have been shown to lead to either hemorrhagic or thrombotic tendencies. In-depth studies of the ultrastructure of some of the thrombophilic fibrinogens have found the formation of networks characterized by thin fibers and small pores, increased resistance to fibrinolysis, and increased clot stiffness.71-73 These structural features were also found in clots of plasma taken from men with myocardial infarction (MI) at a young age.74,75 It is therefore of interest to determine whether the γ′ chain could potentially influence clot architecture.
We have purified γA/γA and γA/γ′ fibrinogen from plasma and found that clots made from these variants displayed very different characteristics.33 The polymerization rate of γA/γ′ fibrin was significantly lower compared with γA/γA fibrin, regardless of thrombin concentration. Maximum absorbance, which at equal fibrin concentration reflects thickness of clot fibers, was lower in γA/γ′ fibrin, indicating an ultrastructure made from thinner fibers. This was confirmed by scanning electron microscopy, which revealed a dense network of thin fibers with small pores (Figure 3). Polymerization characteristics are partly determined by the rate at which thrombin removes the fibrinopeptides. We measured kinetics of FpA and FpB release by high-performance liquid chromatography and found that, whereas removal of FpA occurred at a comparable rate, FpB was cleaved at a slower rate from γA/γ′ fibrinogen. The timing of FpB release coincides with lateral aggregation of protofibrils and therefore could play a role in determining fiber thickness. However, the relationship between FpB release and lateral aggregation is not necessarily causal and not fully understood. We have hypothesized 2 possible models to explain the observed differences in fibrin structure. It could be that thrombin bound to the γ′ chain has its active site oriented in a way that cleavage of fibrinopeptides from the β but not the α chain is diminished, which in turn could lead to less lateral aggregation and consequently thinner fibers. Or possibly, the negatively charged extension of the γ′ chain directly interferes with D-E or D-D interactions. This could lead to reduced fiber growth and thinner fibers. The fibrin network that we observed cannot be explained by the antithrombin activity of the γ′ chain. The reason for this is that inhibition of thrombin is comparable with lowering the thrombin concentration, which associates with a fibrin phenotype of larger pores and thicker fibers.77 The fibrin structure we observed for γA/γ′ fibrinogen is the opposite of this and rather similar to that produced at a high thrombin concentration, supporting the findings that thrombin bound to the γ′ chain remains active.
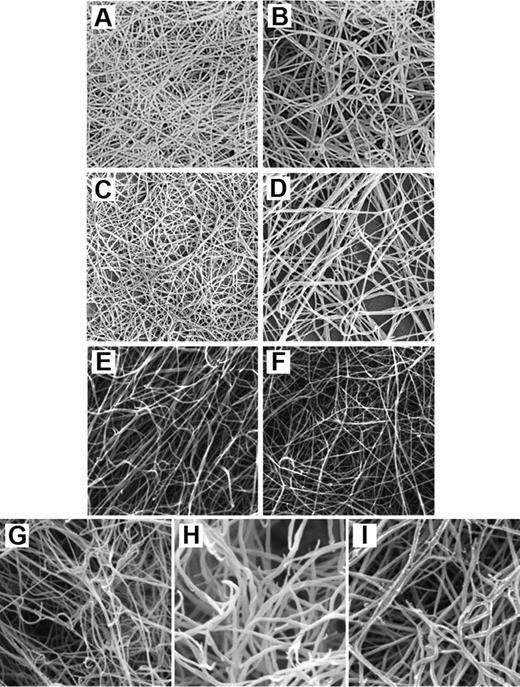
Scanning electron micrographs of purified and recombinant fibrinogen γ-chain variants. (A) Clot made from purified γA/γ′ fibrinogen. (B) Clot made from purified γA/γA fibrinogen. Both clots were made with final concentrations of 0.7 mg/mL fibrinogen and 1 IU/mL thrombin. Reprinted from Cooper et al33 with permission. (C) Clot made from purified γA/γ′ fibrinogen. (D) Clot made from purified γA/γA fibrinogen. These clots were made with final concentrations of 180 μg/mL fibrinogen and 0.1 U/mL thrombin. Reprinted from Siebenlist et al,30 with permission. (E) Clot made from recombinant γ′/γ′ fibrinogen. (F) Clot made from recombinant γA/γA fibrinogen. These clots were made with 10 μL of 4.4 μM fibrinogen and 0.9 IU/mL thrombin. Reprinted from Collet et al70 with permission. (G) Clot made from recombinant γ′/γ′ fibrinogen. (H) Clot made from recombinant γA/γ′ fibrinogen. (I) Clot made from recombinant γA/γA fibrinogen. These clots were made with 1.0 mg/mL fibrinogen and 0.1 U/mL thrombin. Reprinted from Gersh et al76 with permission.
Scanning electron micrographs of purified and recombinant fibrinogen γ-chain variants. (A) Clot made from purified γA/γ′ fibrinogen. (B) Clot made from purified γA/γA fibrinogen. Both clots were made with final concentrations of 0.7 mg/mL fibrinogen and 1 IU/mL thrombin. Reprinted from Cooper et al33 with permission. (C) Clot made from purified γA/γ′ fibrinogen. (D) Clot made from purified γA/γA fibrinogen. These clots were made with final concentrations of 180 μg/mL fibrinogen and 0.1 U/mL thrombin. Reprinted from Siebenlist et al,30 with permission. (E) Clot made from recombinant γ′/γ′ fibrinogen. (F) Clot made from recombinant γA/γA fibrinogen. These clots were made with 10 μL of 4.4 μM fibrinogen and 0.9 IU/mL thrombin. Reprinted from Collet et al70 with permission. (G) Clot made from recombinant γ′/γ′ fibrinogen. (H) Clot made from recombinant γA/γ′ fibrinogen. (I) Clot made from recombinant γA/γA fibrinogen. These clots were made with 1.0 mg/mL fibrinogen and 0.1 U/mL thrombin. Reprinted from Gersh et al76 with permission.
Siebenlist et al more recently performed a comparable study into the effects of the γ′ chain on fibrin structure.30 In agreement with our data, they found that thrombin-treated γA/γ′ fibrinogen produced finer, more branched networks than γA/γA fibrinogen and showed a slower release of FpB. In contrast, they found that FpA release was also delayed for γA/γ′ compared with γA/γA fibrinogen and that fibrin intermediates lacking one FpA (α-profibrin) were generated more slowly from γA/γ′ fibrinogen. However, their high-performance liquid chromatography analysis of fibrinopeptide release did not reach maximum; therefore, true kinetics of fibrinopeptide release could not be performed.
A limitation of these studies is the use of fibrinogen purified from plasma and therefore by nature characterized by some degree of heterogeneity. To minimize this, 2 groups conducted studies using recombinant fibrinogens. Collet et al expressed γA/γA and γ′/γ′ homodimers in baby hamster kidney cells and found that the morphology of clots formed from γ′ fibrin varied less and in a different way than previously observed.70 They showed that γ′/γ′ fibrin structure appeared less compact than γA/γA fibrin, with a 25% decrease in fiber density and a 7% increase in fiber diameter. Permeability of γ′/γ′ fibrin was increased by 20% compared with γA/γA fibrin, indicating a lower fiber density and larger pores. The authors also measured viscoelastic properties of clots in the presence and absence of FXIII. Whereas non–cross-linked γA/γA and γ′/γ′ fibrin did not vary in their storage modulus G′, which reflects clot stiffness, it was found that, once cross-linked, there was a 3-fold increase in G′ in the γ′/γ′ variant. Given the similar viscoelastic properties of both variants before cross-linking, the authors concluded that the only plausible explanation for this increase would be an increase of the extent of cross-linking. A limitation of this study is the use of homodimeric γ′/γ′ fibrinogen, which only exists in vivo at minimal concentrations. Gersh et al synthesized all 3 fibrinogens recombinantly: γA/γA homodimer, γA/γ′ heterodimer, and γ′/γ′ homodimer.76 In agreement with our study,33 they found that the rate of thrombin-catalyzed FpA release was similar for all 3 variants, whereas FpB release from γ′/γ′ fibrinogen was faster than from either γA/γ′ fibrinogen or γA/γA fibrinogen. Fibrin polymerization studies suggested that the γ′ chain slowed lateral aggregation and reduced fiber diameter. Contrary to the findings by Collet et al,70 scanning electron microscopy images showed that γ′/γ′ fibrin was composed of significantly thinner fibers than γΑ/γA fibrin and that γ′/γ′ fibrin was more tightly packed with smaller pores and more branching, although, in contrast to the uniform structure of γA/γA clots, the thin γ′/γ′ fibers appeared to loosely form into bundles.
Taken together, the majority of studies show that the presence of the γ′ chain produces a fibrin clot with smaller pores and thinner fibers, although the γ′/γ′ homodimer may behave differently. The effects on fibrin structure suggest that the γ′ chain may associate with a prothrombotic phenotype. The studies are clearly not always in agreement, probably because of experimental variations, for example, in thrombin and calcium. In addition, plasma-derived fibrinogen is a complex mixture of molecules resulting from posttranslational modifications, such as glycosylation, glycation, nitration, oxidation, sulfation, phosphorylation, and proteolytic degradation, and/or genetic differences among persons. Another layer of complexity is introduced by the probable presence of contaminating FXIII as debated by Farrell78 and Siebenlist.79 Studies with recombinant fibrinogens may overcome some of this variation but make it difficult to interpret the pathophysiologic significance of the data because other factors present in plasma that may be involved in clot formation or play a role in resistance to lysis are not included. No study has been conducted in which recombinant γA/γ′ fibrinogen was reconstituted into fibrinogen-deficient plasma to investigate interactions with other proteins.
Fibrinogen γ′ chain as modifier of thrombosis risk
As discussed, the effects of the γ′ chain on clot structure are clinically relevant because of potential predisposition to thrombotic risk,80-82 and it is therefore of interest to determine γA/γ′ fibrinogen levels in plasma. In a preliminary report, Drouet et al found that the ratio of γA/γ′ fibrinogen divided by total fibrinogen levels was increased in 335 patients with peripheral arterial disease, ischemic stroke, or MI, compared with 433 normal controls.80 In this study, a formal association by odds ratios was not calculated. Subsequently, Lovely et al showed that in a small study of coronary artery disease (CAD) γA/γ′ fibrinogen levels were significantly higher in 91 CAD patients than in 42 non-CAD patients with an odds ratio of 7.16 (95% confidence interval, 1.82-27.7) per γA/γ′ fibrinogen quartile.83 The authors found that the association between CAD and γA/γ′ fibrinogen level was independent of total fibrinogen because no significant association between total and γA/γ′ fibrinogen levels could be determined. Similarly, Mannila et al found that the γA/γ′ fibrinogen level was significantly higher in MI patients than in age- and sex-matched healthy controls; and although there was a weak correlation between γA/γ′ and total fibrinogen level in both patients and controls, they did not find a significant difference in the γ′ fibrinogen/total fibrinogen ratio between patients and controls.84
In contrast to the elevated plasma γA/γ′ fibrinogen levels found in patients with arterial disease, patients with deep venous thrombosis (DVT) have reduced γA/γ′ fibrinogen levels and reduced γ′ fibrinogen/total fibrinogen ratio compared with healthy controls.81 Persons with a γ′ fibrinogen/total fibrinogen ratio below the 10th percentile, as measured in the control group, had a 2.4-fold increased risk of DVT in the Leiden Thrombophilia Study.81 Furthermore, Mosesson et al measured fibrinogen γ′ content in thrombotic microangiopathy and found that both the total γ′ fibrinogen/total fibrinogen ratio (taking both γ′427L- and γ′423P chains into account) and the γ′427L-fibrinogen/total fibrinogen ratio were lower than those of their healthy counterparts.82
It appears, therefore, that the relationship between γA/γ′ fibrinogen level and thrombosis may depend on the type of vascular disease, whereby the γ′ chain associates with a prothrombotic risk in arterial disease but an antithrombotic effect in venous disease. Perhaps the reason for this is that the prothrombotic mechanisms of the γ′ chain, such as altered fibrin structure and elevated FXIII activity, may prevail in arterial disease, whereas the antithrombotic mechanisms of reduced thrombin generation and platelet activation may play a role in the relationship between the γ′ chain and venous disease. However, this notion is somewhat counterintuitive, as platelets are considered to play a larger role in arterial than in venous thrombosis. Possibly yet unidentified mechanisms contribute to some of these effects.
Clearly, the differences in fibrinogen γ′ content in arterial versus venous disease need further investigation. Prospective studies are needed to assess whether γA/γ′ fibrinogen levels already deviate from normal before the actual thrombotic event. Functionally, the involvement of the γ′ chain in clot formation might differ under shear stress conditions, in another vascular bed, or in the presence of platelets. These aspects of the γ′ chain need to be considered in future studies.
Determinants of the γA/γ′ fibrinogen level
Whereas genetic and environmental determinants of total fibrinogen levels are known,85,86 regulation and determinants of γA/γ′ fibrinogen level are only beginning to emerge. Cheung et al observed elevated γA/γ′ fibrinogen levels and γ′ fibrinogen/total fibrinogen ratios in ischemic stroke patients in the acute phase, whereas these were reduced in the convalescent phase.87 It is possible that the fibrinogen γ′ alternative processing mechanism is affected by the acute phase of the disease, as suggested for other genes.88-90 Another explanation could be that the clearance of γA/γA and γA/γ′ fibrinogen from the circulation is different during different stages of disease.
To date, 2 genetic determinants of γA/γ′ fibrinogen levels have been reported.81 By scanning the genes of the fibrinogen cluster using haplotype-tagging single nucleotide polymorphisms (SNPs), 2 FGG haplotypes, FGG-H2 tagged by SNP 10034C→T [rs2066865], and FGG-H3 tagged by SNP 9340T→C [rs1049636], were associated with γA/γ′ fibrinogen levels. SNP 10034C→T was associated with reduced γA/γ′ fibrinogen levels, which was later confirmed in other studies.82,87,91 This SNP associated with an increased DVT risk in a Dutch population,81 in an Austrian population,92 and in a white population in the United States.93 A recent German study showed that the same H2 was significantly more prevalent in VT patients and significantly associated with thromboembolic stroke.91 No association of this SNP was found with risk of MI.94 SNP 9340T→C was associated with increased γA/γ′ fibrinogen levels, again confirmed in other studies,84,87,91 and a slight decrease in DVT risk.81 In addition, 9340T→C was associated with a reduced risk in a Swedish MI study,94 a Dutch study on ischemic stroke,87 but not in a Dutch study on MI.95 In addition, this SNP was recently shown to be less prevalent in German VT patients.91 As with most common polymorphisms in the hemostatic system, however, the relationship between SNPs and disease is not always consistent, even if the SNPs have biologic plausibility as a disease modifier.96,97
The 3 fibrinogen chains are clustered on the same chromosome, and there is a very high degree of linkage disequilibrium within and between the fibrinogen genes. Because FGG-H2 SNPs 10034C→T and 9615C→T [rs2066864] are completely linked, the functionality of these SNPs on γA/γ′ fibrinogen expression was investigated using mini-gene constructs.98 This study concluded that 10034C→T is responsible for the reduction in γA/γ′ fibrinogen level since this SNP is located in a GT-rich downstream sequence element, which composes a putative cleavage stimulation factor binding site and regulates the usage of the 2 polyadenylation signals in FGG. How FGG-H3 influences γA/γ′ fibrinogen expression is still unknown, but as SNP 9340T→C is situated in intron 9, it may be that this variation somehow also regulates the 2 polyadenylation signals in FGG.
An important note that might have been overlooked in previous studies is that FGA-H2 SNP 6534A→G [rs6050] is very strongly linked to FGG-H2 SNP 10034C→T. This FGA polymorphism codes for a threonine to alanine substitution at position 312 of the Aα chain, in a region important for FXIIIa-dependent cross-linking.99 Compared with clots made of Thr312 fibrinogen, clots made of Ala312 fibrinogen have been shown to be more rigid and less porous, with thicker fibers and more extensive α-chain cross-linking,100 which agrees with clots made of γA/γA fibrinogen (Figure 3). Because of the linkage, it is probable that the risk reported to be associated with this polymorphism101 is indeed the result of linkage with FGG 10034C→T and subsequently with reduced γA/γ′ fibrinogen levels.
So far, only a few studies have reported environmental determinants of γA/γ′ fibrinogen. Drouet et al showed that the γ′ fibrinogen/total fibrinogen ratio followed a non-Gaussian distribution in a young population (18-45 years); whereas in the elderly population (range, 65-85 years), they found a more normal distribution, suggesting that age might have an influence on γA/γ′ fibrinogen level.80 In contrast, Lovely et al found no significant association of γA/γ′ fibrinogen levels with age (range, 41-80 years) or sex in a population of 120 normal blood donors.83 Correspondingly, Mosesson et al showed that in healthy persons, expression of the γ′427L or γ′423P chains was not significantly influenced by gender, age, and ethnicity.82 The first real attempt to identify genetic and environmental determinants of the plasma γA/γ′ fibrinogen level was presented by Mannila et al.84 In control persons, they showed that FGG 9340T→C (10.4%), fibrinogen (3.4%), and FGA 2224G→A (2.2%) emerged as independent predictors, together accounting for 16% of the variation in γA/γ′ fibrinogen level. In post-MI patients, fibrinogen (9.2%), FGG 9340T→C (5.9%), FGA 2224G→A (3.9%), high-density lipoprotein-cholesterol (1.4%), insulin (1.1%), and sex (0.9%) emerged as independent determinants, together accounting for 22.4% of variation in γA/γ′ fibrinogen. Future studies are required to determine whether there are other variables that influence γA/γ′ fibrinogen regulation and expression.
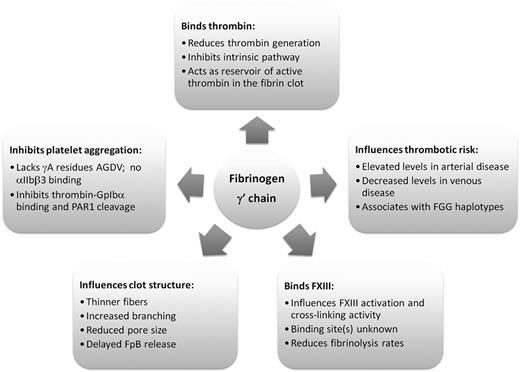
In conclusion, the C-terminus of the fibrinogen γ chain is modified by differential splicing and alternative polyadenylation to produce a common variant that has several important functions in hemostasis. However, the functions of the fibrinogen γ′ chain, as summarized in Figure 4, potentially counteract each other with regards to overall hemostatic “potential.” Binding of the γ′ chain to thrombin and FXIII modulates their activity, resulting in a variant structure of the fibrin clot. With thinner fibers and smaller pores, these clots are more resistant to fibrinolysis and therefore influence thrombosis risk. Interesting associations between γA/γ′ fibrinogen level and thrombosis risk have been reported, which appear different in arterial thrombosis (increased γA/γ′ fibrinogen levels) and venous thrombosis (reduced γA/γ′ fibrinogen levels). Future investigations including prospective studies will need to address whether one or more of the pleiotropic functions that the fibrinogen γ′ chain displays in hemostasis may play a role in regulating arterial or venous thrombosis.
Authorship
Contribution: S.U.d.W. designed and wrote the manuscript; K.F.S. and H.P. cowrote and revised the manuscript; and R.A.S.A. helped in the design and cowrote and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shirley Uitte de Willige, Division of Cardiovascular & Diabetes Research, Section on Mechanisms of Thrombosis, LIGHT Laboratories, University of Leeds, Clarendon Way, Leeds LS2 9JT, United Kingdom; e-mail: s.uittedewillige@leeds.ac.uk.