Abstract
Epstein-Barr virus (EBV)–associated B-cell lymphoproliferation is a life-threatening complication after hematopoietic stem cell or solid organ transplantation resulting from outgrowth of EBV-infected B cells that would normally be controlled by EBV-cytotoxic T cells. During the past decade, early detection strategies, such as serial measurement of EBV-DNA load in peripheral blood samples, have helped to identify high-risk patients and to diagnose early lymphoproliferation. Treatment options include manipulation of the balance between outgrowing EBV-infected B cells and the EBV cytotoxic T lymphocyte response and targeting the B cells with monoclonal antibodies or chemotherapy. Major challenges remain for defining indications for preemptive therapies and integrating novel and conventional therapies.
Introduction
Epstein-Barr virus (EBV) lymphoproliferative disease (LPD) is the result of the outgrowth of EBV-infected B cells that would normally be controlled by an effective EBV-specific cytotoxic T-cell response. LPD may occur during both primary and secondary immune deficiencies and even in some persons without documented immunodeficiency. In this article, I focus on EBV type III latency B-cell lymphoproliferation, which occurs during the immunosuppression that follows hematopoietic stem cell transplantation (HSCT) or solid organ transplantation (SOT). To understand the etiology of these lymphoproliferations and how manipulation of the immune system may be a treatment option, it is important to first understand the biology of EBV.
Biology of EBV
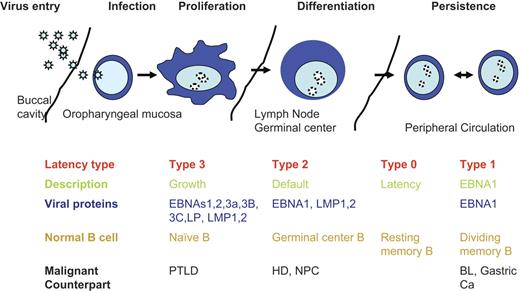
EBV is a latent γ-herpesvirus that infects more than 90% of the world's population. Primary lytic infection occurs in the oropharynx and may be asymptomatic or present as infectious mononucleosis.1 EBV is highly immunogenic; and during primary infection, normal persons mount a vigorous humoral and cellular immune response with the cellular component consisting of CD4+ and CD8+ T cells, which control both primary infection and the periodic reactivations that occur in all EBV-seropositive persons.2 Indeed, analyses using multimers to enumerate EBV-specific T cells have shown that up to 1% to 5% of circulating T cells in a normal EBV-seropositive person may be specific for EBV.3,4 After clearance of primary infection, EBV persists as an episome in infected B cells, establishing latent infection characterized by the expression of only a limited array of subdominant EBV antigens. There are 4 types of latency, distinguished by the pattern of EBV antigen expression in infected memory B cells (Figure 1).
EBV latent life cycle. Virus enters though mucosal routes (shown is the buccal cavity), then infects normal naive B cells circulating through mucosal sites. Virus expresses type 3 latency, which drives B-cell proliferation and expands the infected memory pool. B-cell differentiation into the memory compartments occurs in germinal centers driven by type 2 latency proteins. Infected memory B cells exiting the germinal center down-regulate viral proteins and are invisible to the immune response. EBNA1 is expressed during homeostatic proliferation to maintain the latent viral episome. Virus replication is induced at mucosal sites, and virus is released into the saliva. PTLD indicates posttransplantation lymphoproliferative disease; HD, Hodgkin disease; NPC, nasopharyngeal cancer, and BL, Burkitt lymphoma.
EBV latent life cycle. Virus enters though mucosal routes (shown is the buccal cavity), then infects normal naive B cells circulating through mucosal sites. Virus expresses type 3 latency, which drives B-cell proliferation and expands the infected memory pool. B-cell differentiation into the memory compartments occurs in germinal centers driven by type 2 latency proteins. Infected memory B cells exiting the germinal center down-regulate viral proteins and are invisible to the immune response. EBNA1 is expressed during homeostatic proliferation to maintain the latent viral episome. Virus replication is induced at mucosal sites, and virus is released into the saliva. PTLD indicates posttransplantation lymphoproliferative disease; HD, Hodgkin disease; NPC, nasopharyngeal cancer, and BL, Burkitt lymphoma.
Most infected circulating memory B cells express no viral antigens (type 0 latency), allowing them to remain invisible to the host immune system.5 EBNA1, which acts on a latent origin of replication, is responsible for coordinating replication of the latent episome in concert with replication of the host cells and is therefore expressed in all types of latency associated with cell division.6 Type 1 latency is associated with expression of only EBNA-1 and is seen in circulating B cells when they proliferate and in Burkitt lymphoma. In type 2 latency, LMP1 and LMP2 are also expressed in addition to EBNA1, and this pattern of expression is seen in germinal center B cells in healthy tonsils. The most immunogenic form of latency is type 3, in which all nuclear proteins (EBNAs -1, -2, -3A, -3B, -3C, and -LP) and 2 membrane proteins (LMP1 and LMP2) are expressed together with 2 small RNAs (EBERs). Type 3 latency occurs in B cells immortalized in vitro by EBV into permanently growing lymphoblastoid cell lines. Type 3 latency B cells are rarely detected in healthy seropositive persons, but their occurrence can be inferred from the high frequency of T cells specific for the EBNA3 proteins that persist long-term, and it is probable that periodic reactivation to type III latency is rapidly controlled by the EBV-specific T-cell response. Patients who are severely immunocompromised, however, so that that EBV-specific T cells are severely diminished, may develop B-cell proliferations that express the type 3 latency pattern of antigens. After SOT or HSCT, these disorders have been labeled posttransplantation lymphoproliferative disease (PTLD).7
Biology of PTLD
Because the immune deficiency of patients after HSCT or SOT may disrupt the normal balance between latently infected B-cell proliferation and the EBV-specific T-cell response, the increased number of latently infected B cells8 may develop into PTLD, which typically presents with lymphadenopathy or discrete nodules but may be localized to one specific site or involve the allograft after SOT. PTLD may also present as more diffuse disease that is more difficult to diagnose and may be misinterpreted as a fulminant sepsis syndrome.9
Histologically, PTLD includes a heterogeneous group of lymphoproliferative disorders ranging from reactive, polyclonal hyperplasia to aggressive non-Hodgkin lymphomas. A revised classification was published in 2008 by the World Health Organization and recommends classifying PTLD into 4 categories: (1) early lesions, (2) polymorphic PTLD, (3) monomorphic PTLD, and (4) classic Hodgkin lymphoma-type PTLD10 (Table 1). All types are associated with EBV. Despite this and earlier efforts to standardize the pathologic classification of PTLD, neither histology nor clonality consistently predicts outcome.11
PTLD histologic classification (WHO classification 2008)
| Category . | Clonal status . | EBV status . | Oncogene/tumor suppressor gene changes . |
|---|---|---|---|
| ″Early″ lesions | |||
| Plasmacytic hyperplasia, infectious mononucleosis-like lesion | Polyclonal | Always EBV+ | None |
| Polymorphic PTLD | Monoclonal | Always EBV+ | None |
| Monomorphic | |||
| B-cell lymphomas (DLCL, Burkitt, plasma cell myeloma, Plasmacytoma-like lesions | Monoclonal | Frequently EBV+ | |
| T-cell lymphoma (peripheral T-cell lymphoma, hepatosplenic) | Rarely EBV+ | ||
| Classic Hodgkin lymphoma-like PTLD | Monoclonal | Frequently EBV+ | None |
| Category . | Clonal status . | EBV status . | Oncogene/tumor suppressor gene changes . |
|---|---|---|---|
| ″Early″ lesions | |||
| Plasmacytic hyperplasia, infectious mononucleosis-like lesion | Polyclonal | Always EBV+ | None |
| Polymorphic PTLD | Monoclonal | Always EBV+ | None |
| Monomorphic | |||
| B-cell lymphomas (DLCL, Burkitt, plasma cell myeloma, Plasmacytoma-like lesions | Monoclonal | Frequently EBV+ | |
| T-cell lymphoma (peripheral T-cell lymphoma, hepatosplenic) | Rarely EBV+ | ||
| Classic Hodgkin lymphoma-like PTLD | Monoclonal | Frequently EBV+ | None |
Data from Swerdlow et al.10
PTLD after HSCT is predominantly derived from donor B cells and typically occurs within the first 6 months after transplantation, before reconstitution of the EBV-specific cytotoxic T lymphocyte (CTL) response. It can, however, occur later in the most severely immunocompromised patients. Risk factors include the degree of mismatch between donor and recipient, manipulation of the graft to deplete T cells, and the degree and duration of immunosuppression used to prevent and treat graft-versus-host disease (GVHD).9 Although the proliferating B cells are almost always of donor origin, recent reports have described a high incidence of PTLD in pediatric patients who received reduced intensity conditioning regimens that included ATG or Campath, and may be a consequence of persisting recipient-derived B cells.12,13
In recipients of SOTs, the severe impairment of T-cell function as result of the immunosuppression required to prevent allograft rejection also places these patients at risk for the development of PTLD. In SOT recipients, PTLD is predominantly of recipient origin. Risk factors for developing this complication include the degree of immunosuppression and development of primary infection after transplantation, so a higher incidence is seen in lung and small bowel transplant recipients as well as in EBV-seronegative pediatric patients receiving a transplant from an EBV-seropositive donor.14 Most PTLD occurs in the first year after SOT, and cases occurring later may be EBV-negative and have cytogenetic abnormalities.
Diagnosis
As PTLD may evolve progressively from a polyclonal disorder to a more aggressive monoclonal variant, early diagnosis is important so that treatment can be promptly instituted. There has therefore been much interest in developing predictive assays to identify patients with early disease. Measurement of EBV load by quantitative polymerase chain reaction amplification assays can be a sensitive aid to diagnosis, but it is unfortunately not always specific for disease onset. Furthermore, different assays use whole blood, serum, or peripheral blood mononuclear cells (PBMCs) and require differing interpretation. When PBMCs are assayed, an elevated EBV DNA may reflect both EBV in normal B cells (a population that may be expanded in immunosuppressed patients) and EBV in transformed cells. Assays of EBV in serum reflect virus shedding, which occurs intermittently in normal seropositive persons from epithelium and also from lytically transformed B cells as well as virus released from necrotic transformed cells. Assays measuring whole blood will measure EBV from all these sources. In general, assays using PBMCs are the most sensitive; but in all assays, elevated loads may not always reflect PTLD.
Initial studies in recipients of HSCT that were selectively T cell–depleted to prevent GVHD suggested that an elevated EBV-DNA load was highly predictive of EBV-PTLD.15,16 Follow-up studies, however, which included a broader range of HSCT recipients, showed that only 50% of patients with elevated EBV-DNA subsequently developed PTLD.17 Nevertheless, recent evidence-based guidelines from the European Conference in Infections in Leukemia recommend weekly screening of EBV-DNA for at least 3 months in high-risk allogeneic HSCT recipients.18 Similarly, whereas up to 50% of SOT recipients may have an elevated EBV-DNA load after transplantation, only a much smaller subset will develop PTLD.19 In both sets of patients, serial monitoring is therefore important to distinguish patients with a stable-elevated EBV-DNA load from those with increasing EBV-DNA, which may indicate developing PTLD. Combined monitoring of EBV-DNA and EBV-specific CTL responses appears to better predict individual patients at risk for PTLD development.20-23 CTL response can be assessed by major histocompatibility complex class I peptide-multimer complexes, which enumerate EBV-specific CTL, or functionally by measuring interferon-γ secretion in response to antigenic stimulation. Unfortunately, neither assay is available outside the research setting. Changes in EBV-DNA load therefore need to be considered along with clinical symptoms, such as fever and lymphadenopathy, and imaging studies, before deciding on the need for intervention. The context and risk profile are also important in deciding when to intervene, and I would have a lower threshold for intervention in a patient early after HSCT for immune deficiency than a stable patient several months after kidney transplantation. Similarly, early intervention or even prophylactic administration may be warranted where there is a very high risk of developing PTLD, such as a patient with severe GVHD who has received potent T cell–depleting or –suppressing antibodies.24
Treatment options
Treatment options for EBV-PTLD include manipulating the balance between outgrowing EBV-infected B cells and the EBV CTL response, or targeting the B cells with monoclonal antibodies or chemotherapy.
Restoring immune response to EBV
EBV-associated tumors express viral derived antigens and are excellent antigen-presenting cells, expressing high levels of immune system costimulatory molecules. Therefore, one therapeutic option is to manipulate the immune system to target and eradicate these malignancies. In HSCT recipients in particular, treatment strategies aim to tilt the balance toward EBV immune responses either by depleting the B-cell population (including EBV-infected B cells) or by augmenting the cellular immune response to EBV.
Reducing immunosuppression.
Reducing immunosuppression to restore immune responses to EBV is usually not a useful approach for treating LPD early after HSCT because the patients are profoundly immunosuppressed and the regenerating immune system usually cannot recover fast enough to eradicate the malignant cells. When patients are later after transplantation with partial immune recovery, reducing immunosuppression alone may be successful,25 and it has also been used as a prophylactic strategy in recipients with increasing EBV DNA levels but no evidence of lymphoma.26 In SOTs, where it is a previously normal immune system that has been immunosuppressed, reduction of antirejection drugs may be more useful, and reductions in immunosuppression in response to a rising EBV viral load have indeed reduced the incidence of PTLD in pediatric liver transplant recipients.19 Studies of reducing immunosuppression in SOT recipients with overt PTLD, however, have yielded variable results, probably because the process has been empiric with multiple regimens and endpoints used. Because reduction of immunosuppressive therapy carries the risk of graft rejection, graft function must be monitored closely, especially in heart, lung, and liver allograft recipients, for whom no effective alternatives are available if rejection occurs. Nevertheless, single-center studies report response rates of up to 75% in patients treated with this modality alone or in combination with surgery.27,28 Factors predicting the failure of reduction of immunosuppression as a single treatment modality include elevated lactate dehydrogenase, organ dysfunction, and multiorgan involvement.27 The Southwest Oncology Group and the Eastern Cooperative Oncology Group have reported a prospective clinical trial to evaluate reduction of immunosuppression, which mandated a 50% reduction in immunosuppressive agents for 2 weeks followed by a further 50% reduction for 1 week if not in complete remission. In this study, only 1 of 16 patients attained a partial response, a much lower response rate than reported in single-center studies.29 This strategy is still reasonable in SOT recipients with limited disease, but the patient should be monitored closely so he or she can be transitioned to alternate therapies if needed.
Unmanipulated donor T cells.
Because most HSCT cell donors are EBV seropositive, an EBV-specific T-cell response can often be provided simply by infusing unmanipulated donor lymphocytes. Although this approach has shown clinical efficacy in HSCT patients with established PTLD with response rates of more than 70%, it carries a significant risk of inducing severe or fatal GVHD, as the frequency of alloreactive T cells in the cell product is more than a log higher than the frequency of virus-reactive T cells.30,31 One investigational approach to circumvent this problem is to transduce T cells with a suicide gene, such as the thymidine kinase gene, which can be activated by infusion of ganciclovir should the recipient develop GVHD. This approach has proved effective in early phase studies and is being evaluated in a phase 3 licensing trial in Europe.32
EBV-specific T cells.
The problem of alloreactivity can also be overcome by infusing EBV-specific CTLs generated using EBV-transformed lymphoblastoid B-cell lines, which will effectively present the viral antigens expressed on the tumor cells as antigen-presenting cells. We have given donor-derived EBV-specific CTL to more than 100 patients after HSCT and found them to be highly effective as prophylaxis in high-risk patients with a history of PTLD or patients receiving selective T-cell depletion.33,34 They have also proved effective as treatment for overt PTLD in more than 80% of patients,35 a finding confirmed by other investigators, who have also shown that EBV-CTLs are active in transplant recipients with rituximab-resistant PTLD.36-39 In the SOT recipient, there are additional challenges because patients remain on long-term immunosuppression. Several studies have nonetheless shown that it is possible to generate autologous EBV CTLs from these patients, which will restore short-term EBV-specific immunity that controls disease progression. Long-term persistence, however, has not been apparent in patients who continue to receive immunosuppression.40-42 In addition, both these approaches are currently confined to experimental protocols, and additional drawbacks are the time (2-3 months) and facilities required for CTL production.
One solution to make this strategy more accessible to a larger number of patients is to bypass the development of separate lines for each affected patient by developing a bank of partially human leukocyte antigen (HLA)–matched, allogeneic lines, which can be readily and rapidly available. Investigators in Great Britain reported a phase 2 clinical trial using partially HLA-matched allogeneic CTL for PTLD therapy in a cohort of HSCT and SOT recipients who had failed to respond to conventional PTLD therapy, obtaining a 64% (21 of 33) and 52% (17 of 33) response rate at 5 weeks and 6 months, respectively.43 Patients with closer HLA-matching donors showed better responses at 6 months.
Targeting B cells
Antibody therapy.
As most cases of PTLD arise in donor- or recipient-derived B cells, one strategy for prevention and treatment is to eliminate EBV-infected B cells. Antibody therapy targets B cell–specific surface antigens present on the EBV-transformed malignant cells. The most widely used antibody is rituximab, a chimeric murine/human monoclonal anti-CD20 antibody. Rituximab has been used as prophylaxis and treatment for PTLD after HSCT, with initial response rates between 55% and 100%,44-47 a range that probably reflects differences in the treated patient populations. Rituximab also has activity in PTLD after SOT, in which response rates of 44% to 100% have been reported in several small studies.48-51 The results of these studies are summarized in Table 2. Fewer late-phase studies have been reported, but a recent phase 2 clinical trial using rituximab to treat PTLD reported a response rate of 44% at day 80. This trial included patients whose only previous therapy was reduction of immunosuppression but excluded patients with central nervous system (CNS) PTLD.52
Rituximab response rates
| Type of transplantation . | Response rate . | Reference . |
|---|---|---|
| Cord | 5/9 | Brunstein et al45 |
| T cell–depleted HSCT | 3/3 | van Esser et al46 |
| Allogeneic HSCT | 3/3 | Kuehnle et al44 |
| Autologous CD34 selected HSCT | 4/5 | Powell et al47 |
| Solid organ transplantation | 6/6 | Savoldo et al48 |
| Solid organ transplantation | 7/8 | Ganne et al49 |
| Solid organ transplantation | 6/11 CR; 1/11 PR | Blaes et al50 |
| Solid organ transplantation | 9/17/ CR; 1/17 PR | Oertel et al51 |
| Solid organ transplantation | 19/43 | Choquet et al52 |
| Type of transplantation . | Response rate . | Reference . |
|---|---|---|
| Cord | 5/9 | Brunstein et al45 |
| T cell–depleted HSCT | 3/3 | van Esser et al46 |
| Allogeneic HSCT | 3/3 | Kuehnle et al44 |
| Autologous CD34 selected HSCT | 4/5 | Powell et al47 |
| Solid organ transplantation | 6/6 | Savoldo et al48 |
| Solid organ transplantation | 7/8 | Ganne et al49 |
| Solid organ transplantation | 6/11 CR; 1/11 PR | Blaes et al50 |
| Solid organ transplantation | 9/17/ CR; 1/17 PR | Oertel et al51 |
| Solid organ transplantation | 19/43 | Choquet et al52 |
Initial response rates to rituximab in several small trials in patients with PTLD after transplantation.
CR indicates complete response; and PR, partial response.
Because CD20 expression is not confined to the malignant cells, normal B cells are also destroyed. This can be a significant concern in patients who are already immunosuppressed, and fatal viral infections have been reported after rituximab therapy.53 As rituximab can deplete B cells for more than 6 months in these already immunosuppressed patients, it should be used as preemptive therapy for PTLD only where there is a strong probability of subsequent lymphoma. An additional concern is that, when used as therapy, it does not restore the cellular immune response to EBV, which is a crucial requirement if EBV-mediated B-cell proliferation is to be controlled long-term.48 This may not be a major problem in most HSCT recipients, in whom recovery of a donor-derived immune response should provide long-term protection, but it is a concern in SOT patients who remain on long-term immunosuppression. Indeed, a long-term follow-up study of SOT patients treated with rituximab showed that 57% had progressive disease at 12 months,54 whereas another study showed PTLD recurrence in 50%.48 A final concern is that only one antigen is targeted and antigen-loss tumor cell variants may be selected.
Antiviral agents.
Antiviral treatment for lytic EBV infection currently makes use of nucleoside analogs, which target the virus-specific enzyme, thymidine kinase (TK) expressed in lytically infected cells. The lack of viral TK expression in EBV-positive tumors during viral latency, however, makes antiviral therapy alone ineffective as an antineoplastic therapy. Antiviral agents may have a role in prophylaxis in EBV-seronegative SOT recipients as they can block EBV production in donor B cells and subsequent infection of recipient B cells.55 Moreover, long-term prophylaxis with antiviral agents or intravenous immunoglobulin may decrease the incidence of PTLD by limiting intercellular virus transmission; several centers use prophylactic acyclovir or intravenous immunoglobulin in the first 6 months after allograft, during which time the immunosuppression is most intense.56 Once PTLD is established, however, antiviral agents will have no effect on the growth of type II latency cells that are already transformed. One possible means of exploiting the susceptibility of lytic cycle cells to acyclovir or ganciclovir is to induce reexpression of the viral-associated TK or the lytic cycle of EBV infection, using chemotherapy57 or arginine butyrate.58 A trial of arginine butyrate and ganciclovir included 6 patients with PTLD and encouragingly reported 2 complete and 3 partial responses.58
Radiation therapy and surgery.
When PTLD is confined to one site, radiation and/or surgery can effectively control local disease. Indeed, in one report on the outcome of PTLD occurring late after SOT, all patients treated with surgery or radiotherapy for limited disease and reduction of immunosuppression obtained sustained complete responses.59 Surgery and radiation also have a role in managing local complications of PTLD, such as compression of vital organ structures.
Chemotherapy.
Chemotherapy with regimens used in lymphoma therapy, such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), remains a therapeutic option for patients who fail to respond to immune manipulation or rituximab. One concern is that PTLD patients may be more susceptible to chemotherapy toxicity (particularly HSCT recipients who have already received intensive chemotherapy), and they are also at increased risk of infection because of their preexisting immune suppression. Although high toxicity rates were seen in initial reports, more recent reports have shown better outcomes. A recent retrospective analysis of standard CHOP in 26 adults with PTLD demonstrated an overall response rate of 65% and median overall and progression-free survivals of 13.9 and 42 months, respectively, after a median follow-up of 8.8 years.60 PTLD generally remains chemotherapy-sensitive after progression or failure to respond to rituximab used as first-line therapy, and CHOP salvage therapy can achieve an overall response rate up to 70% in these patients.61 By comparison, EBV-unrelated, PTLD after SOT has a lower response rate but may respond to high-dose chemotherapy followed by HSC rescue.62 To reduce toxicity from chemotherapy, a study evaluated lower-dose chemotherapy with cyclophosphamide and prednisone in 36 pediatric patients who had failed frontline therapy and reported an excellent overall response rate of 83%.63 This lower-dose regimen therefore appears effective as salvage therapy for children with PTLD.
PTLD involving the CNS can present a particular challenge as multiple lesions can be present and the prognosis is poor.64,65 In the largest reported series, the best outcomes were seen with radiation for isolated CNS disease.65 Responses have also been reported with reduction in immunosuppression and rituximab, although this agent crosses the blood-brain barrier poorly.66 Chemotherapy with high-dose methotrexate has also induced responses in a report of administration to 4 children with CNS PTLD after liver transplantation.67
Hydroxyurea is another less toxic option that can eradicate episomal DNA elements, which may be required for the continued growth of EBV-associated lymphomas. There are several case reports showing the activity of this agent in EBV-associated B-cell lymphoproliferations, particularly with CNS involvement, but these results have not yet been confirmed in prospective trials.68,69 Rapamycin also has activity against EBV-transformed B cells in vitro, and there are anecdotal reports of using the agent as a substitute immunosuppressive agent that can treat PTLD.70
Model treatment schema
Perhaps one of the most contentious issues with PTLD is not how but when to treat. My general philosophy is to reserve preemptive treatment or treatment based on EBV DNA alone for very-high-risk situations, such as a patient with a diagnosis of X-linked lymphoproliferative disease or Wiskott-Aldrich syndrome.71 Treatment for rejection or GVHD with an intensive anti-T-cell antibody that does not also target B cells would also place a patient in a high-risk category, in which preemptive treatment of a rising EBV DNA in the absence of other symptoms or signs could be considered.24
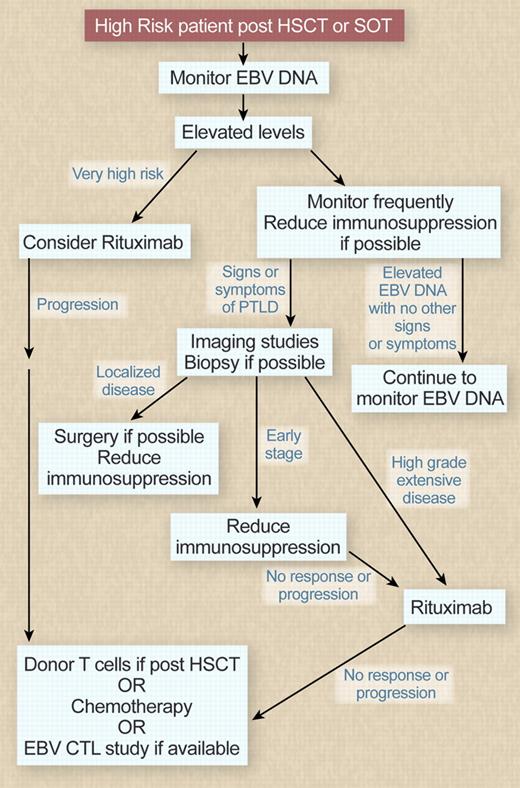
In other settings, my goal would be to diagnose PTLD at an early stage so it could be treated promptly using the diagnostic tests reviewed in Table 3. I would therefore monitor EBV DNA in high-risk recipients at 1 to 2 week intervals. In patients who develop elevated levels in the absence of symptoms, modulation of immunosuppressive therapy (if feasible) is a reasonable strategy. I would have a low threshold for imaging studies in patients with elevated EBV DNA who also had clinical symptoms, such as fever, that were consistent with the diagnosis. If imaging or physical examination shows enlarged lymph nodes or other evidence of disease, it is helpful to biopsy sites of involvement to confirm the diagnosis and exclude other infections that may have a similar imaging appearance. Biopsy also allows confirmation of CD20 expression, allowing rituximab to be used. In patients with later PTLD after SOT, cytogenetic studies are also helpful in identifying lymphomas that will be less susceptible to immune manipulation and should be treated like lymphomas arising in immunocompetent persons. A monitoring and treatment algorithm is outlined in Figure 2. In patients with localized disease, surgery alone or with reduction of immunosuppression may provide sufficient control of disease. In SOT patients with early disease, a trial of reduction in immunosuppression alone is reasonable, with the addition of rituximab in patients who fail to respond or progress. SOT patients with higher-grade disease and HSCT recipients unless they have some evidence of recovery of an EBV-specific T-cell response should proceed to rituximab.
Diagnosis of PTLD
| Test . | Information gained . | Limitations . |
|---|---|---|
| EBV viral load | Elevated level supports diagnosis | High sensitivity; specificity varies with clinical scenario |
| Imaging | Enlarged lymph nodes or nodules support diagnosis | Differential causes for lymphadenopathy |
| Biopsy affected organ | Confirm EBV positivity by LMP1 immunostaining and EBERs; assess histology (high-grade monoclonal lymphoma vs polyclonal lymphoproliferation); immunophenotyping (CD20 expression); cytogenetics | Invasive procedure depending on organ involved |
| Test . | Information gained . | Limitations . |
|---|---|---|
| EBV viral load | Elevated level supports diagnosis | High sensitivity; specificity varies with clinical scenario |
| Imaging | Enlarged lymph nodes or nodules support diagnosis | Differential causes for lymphadenopathy |
| Biopsy affected organ | Confirm EBV positivity by LMP1 immunostaining and EBERs; assess histology (high-grade monoclonal lymphoma vs polyclonal lymphoproliferation); immunophenotyping (CD20 expression); cytogenetics | Invasive procedure depending on organ involved |
Monitoring and treatment algorithm. Professional illustration by Debra T. Dartez.
Monitoring and treatment algorithm. Professional illustration by Debra T. Dartez.
In patients with more extensive higher-grade disease or patients who progress after initial maneuvers, the options include chemotherapy or T-cell therapies. Donor T cells are only an option in HSCT recipients, whereas EBV-CTLs are investigational and will be restricted to centers participating in clinical trials. Nevertheless, given the morbidity of standard chemotherapy, particularly in HSCT recipients who have often received extensive previous chemotherapy, I think it is reasonable to consider cell therapy at this stage.
In conclusion, one problem in developing a treatment algorithm for treating EBV-associated PTLD is the lack of later-phase trials from which to develop evidence-based guidelines. Hopefully, as newer therapies, such as allogeneic EBV CTLs, and more targeted chemotherapy regimens are evaluated, more definitive trials to treat EBV lymphoproliferations will be designed and completed so that treatment in the future can be more evidence-based.
Acknowledgments
The author thanks Dr Cliona Rooney for her contribution to Figure 1.
This work was supported by the National Institutes of Health (grants PO1 CA94237, P50CA126752) and the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: H.E.H. wrote the manuscript.
Conflict-of-interest disclosure: The author declares no competing financial interests.
Correspondence: Helen E. Heslop, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1620, Houston, TX 77030; e-mail: hheslop@bcm.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal