In acute graft-versus-host disease (GVHD), naive donor CD4+ T cells recognize alloantigens on host antigen-presenting cells and differentiate into T helper (Th) subsets (Th1, Th2, and Th17 cells), but the role of Th subsets in GVHD pathogenesis is incompletely characterized. Here we report that, in an MHC-mismatched model of C57BL/6 donor to BALB/c recipient, WT donor CD4+ T cells predominantly differentiated into Th1 cells and preferentially mediated GVHD tissue damage in gut and liver. However, absence of interferon-γ (IFN-γ) in CD4+ T cells resulted in augmented Th2 and Th17 differentiation and exacerbated tissue damage in lung and skin; absence of both IL-4 and IFN-γ resulted in augmented Th17 differentiation and preferential, although not exclusive, tissue damage in skin; and absence of both IFN-γ and IL-17 led to further augmentation of Th2 differentiation and idiopathic pneumonia. The tissue-specific GVHD mediated by Th1, Th2, and Th17 cells was in part associated with their tissue-specific migration mediated by differential expression of chemokine receptors. Furthermore, lack of tissue expression of the IFN-γ–inducible B7-H1 played a critical role in augmenting the Th2-mediated idiopathic pneumonia. These results indicate donor CD4+ T cells can reciprocally differentiate into Th1, Th2, and Th17 cells that mediate organ-specific GVHD.
Introduction
Graft-versus-host disease (GVHD) is an exaggerated, undesirable manifestation of a normal inflammatory response, in which naive donor T cells recognize alloantigens on host antigen-presenting cells (APCs).1 The donor CD4+ T-cell interaction with host APCs leads to the activation of the donor T cells and their differentiation into T helper (Th) cells.2 The Th cells then secrete a variety of cytokines to mediate GVHD inflammation.
CD4+ T cells can differentiate into Th1, Th2, and Th17 cells, depending on the cytokine milieu. In the presence of IL-12, CD4+ T cells differentiate into interferon-γ (IFN-γ)–producing Th1 cells, whereas in the presence of IL-4, CD4+ T cells differentiate into IL-4-, IL-5-, and IL-13-producing Th2 cells. Th17 cells produce IL-17A (referred as IL-17), IL-17F, and IL-22.3 Th17 differentiation requires TGF-β and IL-6,4 and IL-23 and IL-21 are critical for their expansion and survival.5,6 It was reported that the differentiation of Th17 cells was potently inhibited by IFN-γ and IL-4.7 Conversely, Th17 cells have also been shown to down-regulate Th1 or Th2 differentiation.8,,–11
However, the role of Th1, Th2, and Th17 cells in acute GVHD pathogenesis is still controversial. Acute GVHD has been proposed to be mediated by Th1 cells,1 but donor T cells deficient in IFN-γ induced exacerbated acute GVHD.12,13 Th2 cells were reported to suppress acute GVHD,14 but Th2-biased STAT4−/− donor cells induced lethal GVHD.15 Th17 cells were reported to be a potent inflammatory mediator in some autoimmune diseases,16,17 but we recently showed that absence of Th17 cells led to exacerbated acute GVHD.8 However, Th17 cells were also shown to augment GVHD in some circumstances,18,19 and in vitro-generated Th17 cells were shown to mediate lung and skin GVHD.20
In addition, Burman and colleagues proposed that the severe lung tissue damage mediated by IFN-γ−/− donor T cells was associated with the lack of an IFN-γ–inducible protective mechanism possessed by host lung tissue,21 but the protective molecule has not yet been identified. PD1/B7-H1 axis plays an important role in inducing T-cell anergy and apoptosis.22 PD1 is expressed by activated T cells22 ; B7-H1 is constitutively expressed by APCs such as dendritic cells, but its expression on mesenchymal tissue cells is induced by IFN-γ.23 Taken together, we hypothesize that donor naive alloreactive T cells reciprocally differentiate into Th1, Th2, and Th17 cells, and each Th subset contributes to specific GVHD target tissue damage. In addition, lack of host tissue expression of IFN-γ–inducible coinhibitory B7-H1 contributes to tissue damage in recipients transplanted with IFN-γ−/− donor T cells. The current studies tested these hypotheses.
Methods
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from NCI Laboratories. IFN-γ−/− C57BL/6 and IL-4Rα−/− BALB/c were purchased from The Jackson Laboratory and Taconic Farms, respectively. IL-17−/− C57BL/6 and B7-H1−/− BALB/c were established as previously described.24,25 IFN-γ−/−IL-17−/− C57BL/6 mice were generated by crossing IFN-γ−/− mice with IL-17−/− mice.26 As a quality control, CD4+ T cells from recipients given IFN-γ−/−IL-17−/− donor cells were shown to produce neither IFN-γ nor IL-17 (see supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Mice were maintained in a pathogen-free room at City of Hope (COH) Research Animal Facilities. Mice at age of 8 to 12 weeks were used. All animal protocols were approved by the COH Research Animal Care Committee.
Induction and assessment of GVHD as well as histopathology and scoring
The procedures for induction of acute GVHD were described in previous publications27 and the supplemental Methods.
Monoclonal antibodies and flow cytometric analysis as well as in vivo neutralizing of cytokines
Statistical analysis
Body weight and survival in different groups were compared using the log-rank test (GraphPad Prism Version 5.0; GraphPad Software). Comparison of 2 means was analyzed using the unpaired 2-tailed Student t test. Comparison of frequencies was analyzed by the χ2 test.
Results
IFN-γ−/− donor CD4+ T cells induced preferential tissue damage in lung and skin, which was associated with augmented Th2 and Th17 differentiation
The Th1, Th2, and Th17 differentiation of IFN-γ−/− donor T cells in hematopoietic cell transplantation (HCT) recipients and their pathogenic role has not been studied. To address this question, sorted CD4+ T cells (106) and T cell–depleted bone marrow cells (TCD-BM, 5 × 106) from wild-type (WT) or IFN-γ−/− C57BL/6 donors were transplanted into TBI-conditioned BALB/c recipients. We found that whereas the majority (> 85%) of the recipients given WT donor CD4+ T cells survived for more than 30 days, the majority (> 90%) of the recipients given IFN-γ−/− donor CD4+ T cells died within 30 days after HCT (P < .01; Figure 1A), although the recipients given WT or IFN-γ−/− donor TCD-BM cells all survived for more than 50 days (data not shown).
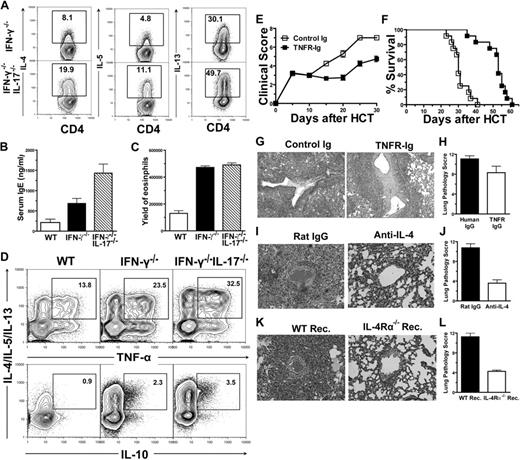
IFN-γ−/− donor CD4+ T cells induced preferential tissue damage in lung and skin in association with augmented differentiation of Th17 and Th2 cells. (A-C) Percent survival, percentage of mice with diarrhea, and body-weight change after HCT. There were 12 mice in each group, combined from 3 replicate experiments. (D-E) Hematoxylin and eosin (H&E) staining of colon, liver, lung, and skin sections of recipients 20 to 25 days after HCT and mean ± SE of histopathology scores (n = 12). (F) Mean ± SE of T-bet, Gata-3, and RORγt expression in sorted splenic CD4+ T cells (purity > 90%) from recipients given IFN-γ−/− donor cells 5 days after HCT (n = 4). Relative gene expression levels were normalized within each sample to GAPDH and were presented relative to the expression of WT control. Data are representative of 2 replicate experiments. (G) Intracellular cytokine profiles of splenic CD4+ T cells 7 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. A representative of 4 replicate experiments is shown. (H) Seven days after HCT, sorted H-2b+CD4+ T cells from the spleen of recipients given WT or IFN-γ−/− donor cells were restimulated with plate-bound anti-CD3/CD28. Mean ± SE of supernatant cytokines 24 hours after culture. These were 4 samples in each group, combined from 2 replicate experiments.
IFN-γ−/− donor CD4+ T cells induced preferential tissue damage in lung and skin in association with augmented differentiation of Th17 and Th2 cells. (A-C) Percent survival, percentage of mice with diarrhea, and body-weight change after HCT. There were 12 mice in each group, combined from 3 replicate experiments. (D-E) Hematoxylin and eosin (H&E) staining of colon, liver, lung, and skin sections of recipients 20 to 25 days after HCT and mean ± SE of histopathology scores (n = 12). (F) Mean ± SE of T-bet, Gata-3, and RORγt expression in sorted splenic CD4+ T cells (purity > 90%) from recipients given IFN-γ−/− donor cells 5 days after HCT (n = 4). Relative gene expression levels were normalized within each sample to GAPDH and were presented relative to the expression of WT control. Data are representative of 2 replicate experiments. (G) Intracellular cytokine profiles of splenic CD4+ T cells 7 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. A representative of 4 replicate experiments is shown. (H) Seven days after HCT, sorted H-2b+CD4+ T cells from the spleen of recipients given WT or IFN-γ−/− donor cells were restimulated with plate-bound anti-CD3/CD28. Mean ± SE of supernatant cytokines 24 hours after culture. These were 4 samples in each group, combined from 2 replicate experiments.
Interestingly, the severity of the clinical signs of GVHD was not consistent with the mortality of the recipients given IFN-γ−/− donor CD4+ T cells. Although the mortality of recipients given IFN-γ−/− donor CD4+ T cells was markedly higher than that of recipients given WT CD4+ T cells (90% vs 20%), by 30 days after HCT, the former showed significantly less frequent and less severe diarrhea 20 to 50 days after HCT, compared with the latter (P < .01; Figure 1B), and no difference was observed in bodyweight changes between the 2 groups (Figure 1C). These results indicate that it is unlikely the increased mortality in recipients given IFN-γ−/−CD4+ donor T cells resulted from gut damage. It was of interest that the recipients given IFN-γ−/− donor cells showed labored breathing before falling into moribundity (data not shown). Next, we compared the histopathology of the colon, liver, lung, and skin of the 2 groups of recipients. We found that, compared with recipients given WT donor CD4+ T cells, recipients given IFN-γ−/− CD4+ T cells had significantly less damage in the colon and liver but greater damage in lung and skin (P < .01; Figure 1D-E). These results indicate that IFN-γ−/− donor CD4+ T cells preferentially damage lung and skin tissues of recipients and that the earlier mortality of those recipients is associated with severe lung damage.
To explore the mechanisms of the preferential lung and skin damage caused by IFN-γ−/− donor CD4+ T cells, we used intracellular staining and in vitro cytokine production assays to compare the cytokine profile of donor CD4+ T cells from recipients given WT or IFN-γ−/− donor CD4+ T cells, and observed that, compared with WT donor CD4+ T cells, the IFN-γ−/− donor CD4+ T cells from the spleen of the recipients produced 5 times more IL-17 (Figure 1G-H). The IFN-γ−/− donor T cells also produced approximately 3 times more IL-4, IL-5, and IL-13 (Figure 1G-H). The differential cytokine patterns were consistent with lineage analysis of transcription factors in donor CD4+ T cells from GVHD recipients. Whereas CD4+ T cells from recipients given IFN-γ−/− CD4+ T cells markedly increased expression of Gata3 and RORγT, they dramatically decreased expression of T-bet (P < .01; Figure 1F). Furthermore, this in vivo differentiation pattern was also recapitulated by an in vitro culture of donor CD4+ T cells stimulated with host dendritic cells (DCs; see supplemental Figure 2). These results indicate that deficiency of IFN-γ in donor CD4+ T cells result in augmented differentiation of Th2 and Th17 cells, which preferentially cause the damage in skin and lung tissues.
Absence of IL-17 did not ameliorate GVHD idiopathic pneumonia but did ameliorate tissue damage in gut and skin of recipients given IFN-γ−/−IL17−/− donor CD4+ T cells
It was also recently reported that IFN-γ−/− donor CD4+ T cells showed augmented Th17 differentiation in another MHC-mismatched model and that Th17 cells were indicated to mediate the GVHD idiopathic pneumonia by mild reduction in pathology after neutralizing IL-17.28 To further address the role of Th17 cells in GVHD pathogenesis, we compared the ability of IFN-γ−/−IL-17−/− and IFN-γ−/− donor CD4+ T cells to induce GVHD. We found that, compared with the recipients given IFN-γ−/− donor CD4+ T cells, the recipients given IFN-γ−/−IL-17−/− donor CD4+ T cells survived significantly longer (P < .01; Figure 2A), although recipients still died within 35 days after HCT (Figure 2A). In addition, we observed that the recipients given IFN-γ−/−IL-17−/− donor CD4+ T cells had a significant reduction in diarrhea 5 to 10 days after HCT (P < .01; Figure 2B), although their body weight was only slightly increased (Figure 2C). A comparison of the histopathology of the 2 groups revealed a significant reduction in tissue damage in gut and skin of the recipients given IFN-γ−/−IL-17−/− donor CD4+ T cells (P < .01; Figure 2D-E) but no reduction, and even a slight increase, in lung damage of these recipients (Figure 2D-E), nor was a significant change in liver damage observed (Figure 2D-E). These results indicate that a deficiency in IL-17 does not ameliorate GVHD idiopathic pneumonia mediated by IFN-γ−/− donor CD4+ T cells and that Th17 cells do not play a critical role in GVHD idiopathic pneumonia. These results also indicate that IL-17 play an important role in GVHD skin damage.
IFN-γ−/−IL-17−/− donor CD4+ T cells induced little tissue damage in gut, liver, and skin, but severe damage in lung. (A-C) Percent survival, percentage of mice with diarrhea, and body-weight change after HCT. There were 12 mice in each group, combined from 3 replicate experiments. (D-E) H&E staining of colon, liver, lung, and skin tissue sections of recipients 20 to 25 days after HCT and mean ± SE of histopathology scores (n = 12).
IFN-γ−/−IL-17−/− donor CD4+ T cells induced little tissue damage in gut, liver, and skin, but severe damage in lung. (A-C) Percent survival, percentage of mice with diarrhea, and body-weight change after HCT. There were 12 mice in each group, combined from 3 replicate experiments. (D-E) H&E staining of colon, liver, lung, and skin tissue sections of recipients 20 to 25 days after HCT and mean ± SE of histopathology scores (n = 12).
IFN-γ−/−IL-17−/− donor CD4+ T cells predominantly differentiated into Th2 and mediated GVHD idiopathic pneumonia
Next, we tested whether Th2 differentiation was augmented in recipients of IFN-γ−/−IL17−/− donor CD4+ T cells and whether Th2 cells mediated GVHD idiopathic pneumonia in those recipients. Accordingly, donor CD4+ T cells and TCD-BM cells from IFN-γ−/− or IFN-γ−/−IL17−/− donors were transplanted into recipients as described above. Seven days after HCT, we found that, compared with the recipients given IFN-γ−/− donor CD4+ T cells, there was a 2- to 3-fold increase in the percentage of IL-4-, IL-5-, and IL-13–producing CD4+ T cells among spleen donor CD4+ T cells of the recipients given IFN-γ−/−IL-17−/− donor CD4+ T cells (Figure 3A). This in vivo pattern was recapitulated by in vitro culture (see supplemental Figure 3). These results indicate that in the absence of IFN-γ and IL-17, Th2 differentiation is further augmented. The recipients given IFN-γ−/−IL-17−/− donor CD4+ T cells also showed significant amelioration of tissue damage in the colon, liver, and skin, but a slight exacerbation of damage in the lung, compared with that of recipients given INF-γ−/− donor cells (Figure 2). Therefore, Th2 cells are likely to play a major role in mediating GVHD idiopathic pneumonia.
IFN-γ−/−IL-17−/− donor CD4+ T cells predominantly differentiated into Th2 and induced lung damage. (A) Intracellular cytokine profiles of splenic CD4+ T cells 7 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. A representative of 3 replicated experiments is shown. (B) Mean ± SE of serum IgE (n = 8) combined from 2 replicate experiments. (C) Mean ± SE of yield of eosinophil (CCR3+Gr-1+) in lungs 10 days after HCT (n = 4). (D) Intracellular cytokine profiles of CD4+ T cells among lung mononuclear cells 10 days after HCT. Gated CD4+ T cells were shown as IL-4/IL-5/IL-13 versus TNF-α or IL-10. Percentage of TNF-α+IL-4/IL-5/IL-13+ or IL-10+IL-4/IL-5/IL-13+ cells were calculated among total CD4+ T cells. A representative of 3 replicate experiments is shown. (E-F) Clinical scores and percent survival of recipients treated with TNFR-IgG or control IgG are shown. There were 12 mice in each group, combined from 3 replicate experiments. (G-H) H&E staining of lung tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 6). (I-J) BALB/c recipients given IFN-γ−/−IL-17−/− donor cells were treated with anti–IL-4 or rat IgG. H&E staining of lung tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 6). (K-L) WT or IL-4Rα−/− BALB/c recipients were transplanted with IFN-γ−/−IL-17−/− donor cells. H&E staining of lung tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 6).
IFN-γ−/−IL-17−/− donor CD4+ T cells predominantly differentiated into Th2 and induced lung damage. (A) Intracellular cytokine profiles of splenic CD4+ T cells 7 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. A representative of 3 replicated experiments is shown. (B) Mean ± SE of serum IgE (n = 8) combined from 2 replicate experiments. (C) Mean ± SE of yield of eosinophil (CCR3+Gr-1+) in lungs 10 days after HCT (n = 4). (D) Intracellular cytokine profiles of CD4+ T cells among lung mononuclear cells 10 days after HCT. Gated CD4+ T cells were shown as IL-4/IL-5/IL-13 versus TNF-α or IL-10. Percentage of TNF-α+IL-4/IL-5/IL-13+ or IL-10+IL-4/IL-5/IL-13+ cells were calculated among total CD4+ T cells. A representative of 3 replicate experiments is shown. (E-F) Clinical scores and percent survival of recipients treated with TNFR-IgG or control IgG are shown. There were 12 mice in each group, combined from 3 replicate experiments. (G-H) H&E staining of lung tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 6). (I-J) BALB/c recipients given IFN-γ−/−IL-17−/− donor cells were treated with anti–IL-4 or rat IgG. H&E staining of lung tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 6). (K-L) WT or IL-4Rα−/− BALB/c recipients were transplanted with IFN-γ−/−IL-17−/− donor cells. H&E staining of lung tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 6).
Consistently, we observed that serum levels of IgE in recipients given IFN-γ−/−IL17−/− donor cells was increased 2- and 5-fold, compared with recipients given WT or IFN-γ−/− donor cells, respectively (P < .01; Figure 3B). In addition, the yield of CCR3+Gr-1+ eosinophils among lung-infiltrating cells of the recipients given IFN-γ−/−IL-17−/− donor cells was 3-fold higher than that of recipients given WT donor cells (P < .01; Figure 3C), although there was no significant increase in that of recipients given IFN-γ−/− donor cells (Figure 3C). These results further indicate that the lung-tissue damage in the recipients given IFN-γ−/−IL-17−/− donor CD4+ T cells is mediated through Th2 cells.
Th2 cells were reported to include anti-inflammatory IL-10–producing and proinflammatory TNF-α–producing subsets.29 We observed that, among the Th2 cells from the idiopathic pneumonia tissues, the percentage of tumor necrosis factor α (TNF-α)–producing Th2 cells was around 10-fold higher than the percentage of the IL-10–producing cells (Figure 3D); and the percentage of the TNF-α–producing Th2 cells in the lung tissue of recipients given IFN-γ−/−IL-17−/− donor cells was approximately 3- and 1.5-fold higher than that of the recipients given WT or IFN-γ−/− donor cells, respectively (Figure 3D). These results suggest that the proinflammatory TNF-α–producing Th2 cells may mediate the idiopathic pneumonia.
Next, we tested the role of TNF-α in the pathogenesis of idiopathic pneumonia. Recipients given IFN-γ−/−IL17−/− donor cells were injected intraperitoneally with TNF-αR Ig or control Ig every other day after HCT until the recipients became moribund. Treatment with TNF-αR Ig significantly reduced the systemic clinical signs of GVHD and significantly prolonged the survival of the recipients (P < .01; Figure 3E-F) but only slightly reduced lung tissue damage (Figure 3G-H). These results indicate that, although TNF-α plays an important pathogenic role in systemic GVHD, it plays a minimal role in tissue-specific idiopathic pneumonia.
To further investigate the role of Th2 cells in mediating GVHD idiopathic pneumonia in the recipients given IFN-γ−/−IL-17−/− donor CD4+ T cells, these recipients were treated with anti–IL-4 days 0, 1, 2, 3, 5, and 7 after HCT. We found that anti–IL-4 treatment blocked Th2 differentiation (see supplemental Figure 4) and ameliorated lung idiopathic pneumonia (P < .01; Figure 3I-J). In addition, inactivation of host IL-4 and IL-13 signaling through the use of IL-4Rα−/− recipients also significantly ameliorated idiopathic pneumonia (P < .01; Figure 3K-L), although there was no significant change in Th2 differentiation (data not shown). Furthermore, we found that anti–IL-4 treatment of recipients given IFN-γ−/− donor CD4+ T cells suppressed Th2 but augmented Th17 differentiation (Figure 4A). The treatment also ameliorated lung idiopathic pneumonia but augmented skin damage (Figure 4B-C). These results indicate that Th2 cells mediate GVHD idiopathic pneumonia through either IL-4 or IL-13. It was reported by others that IL-13 mediated allergic pneumonia via IL-13.30
Absence of IL-4 and IFN-γ led to augmented Th17 differentiation and exacerbated damage in skin but not in lung. BALB/c recipients given IFN-γ−/− donor cells were treated with rat-IgG or anti–IL-4. (A) Intracellular cytokine profiles of splenic CD4+ T cells 13 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. Representative of 4 replicate experiments is shown. (B-C) H&E staining of lung- and skin-tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 8).
Absence of IL-4 and IFN-γ led to augmented Th17 differentiation and exacerbated damage in skin but not in lung. BALB/c recipients given IFN-γ−/− donor cells were treated with rat-IgG or anti–IL-4. (A) Intracellular cytokine profiles of splenic CD4+ T cells 13 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. Representative of 4 replicate experiments is shown. (B-C) H&E staining of lung- and skin-tissue sections of recipients 15 days after HCT and mean ± SE of histopathology scores (n = 8).
Tissue-specific GVHD caused by Th1, Th2, and/or Th17 cells was in part due to tissue-specific T-cell migration mediated by chemokine receptors and chemokines
To explore the mechanisms of tissue-specific GVHD, we first compared the yield of donor CD4+ T cells and Th subsets in GVHD target tissues of recipients given WT, IFN-γ−/−, orIFN-γ−/−IL-17−/− donor CD4+ T cells. As mentioned above, WT donor CD4+ T cells preferentially differentiated into Th1 cells, and IFN-γ−/− or IFN-γ−/−IL-17−/− donor T cells preferentially differentiated into Th2/Th17 or Th2 cells (Figures 1 and 3). We found that Th1 cells preferentially, although not exclusively, migrated to liver and gut tissues, and that Th2 and Th17 cells preferentially migrated to lung and skin tissues (Figure 5A and Table 1). Although the yield of total donor CD4+ T cells in the spleen was no different in recipients given WT, IFN-γ−/−, or IFN-γ−/−IL-17−/− donor CD4+ T cells, the yield of total donor CD4+ T cells in the gut or liver of recipients given WT T cells was 10- or 3-fold higher than the recipients given IFN-γ−/− or IFN-γ−/−IL-17−/− donor T cells (P < .01). In contrast, the yield of total donor CD4+ T cells in the lung or skin of the recipients given WT cells was 10- or 3-fold lower than in the recipients given IFN-γ−/− or IFN-γ−/−IL-17−/− donor cells (P < .01; Figure 5A). Furthermore, the yield of Th1 cells in the gut or liver of recipients given WT cells was 15- or 3-fold higher than Th2 cells (P < .01), but the difference was diminished in lung or skin tissues (Table 1).
Tissue-specific GVHD caused by Th1, Th2, and Th17 cells was associated with tissue-specific T-cell migration. (A) Yield of H-2b+CD4+ T cells in spleen or tissues of recipients given WT or IFN-γ−/− donor cells 13 days after HCT. Mean ± SE is shown (n = 8), combined from 3 replicated experiments. (B-C) Five days after HCT, splenocytes from recipients were stained for H-2b, CD4, and chemokine receptors. Gated H-2b+CD4+ T cells are shown in CD4 versus chemokine receptors. Representative of 4 replicate experiments is shown. (D-E) Mean ± SE of chemokine expression levels by GVHD target tissues 5 days after HCT (n = 4). Relative gene expression levels were normalized within each sample to housekeeping gene GAPDH. Data are a representative of 2 replicated experiments.
Tissue-specific GVHD caused by Th1, Th2, and Th17 cells was associated with tissue-specific T-cell migration. (A) Yield of H-2b+CD4+ T cells in spleen or tissues of recipients given WT or IFN-γ−/− donor cells 13 days after HCT. Mean ± SE is shown (n = 8), combined from 3 replicated experiments. (B-C) Five days after HCT, splenocytes from recipients were stained for H-2b, CD4, and chemokine receptors. Gated H-2b+CD4+ T cells are shown in CD4 versus chemokine receptors. Representative of 4 replicate experiments is shown. (D-E) Mean ± SE of chemokine expression levels by GVHD target tissues 5 days after HCT (n = 4). Relative gene expression levels were normalized within each sample to housekeeping gene GAPDH. Data are a representative of 2 replicated experiments.
Yield of CD4+ T-cell subsets in GVHD target organs 13 days after HCT (mean ± SE 10−3; n = 4)
| . | Gut . | Liver . | Lung . | Skin . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Th1 . | Th2 . | Th17 . | Th1 . | Th2 . | Th17 . | Th1 . | Th2 . | Th17 . | Th1 . | Th2 . | Th17 . | |
| WT | 30.6 ± 3.5 | 2.7 ± 0.9 | 1.1 ± 0.1 | 498.0 ± 85.1 | 160.5 ± 39.5 | 9.3 ± 1.8 | 64.2 ± 13.9 | 41.7 ± 10.6 | 1.9 ± 0.5 | 80.0 ± 17.5 | 67.5 ± 12.1 | 2.4 ± 0.7 |
| IFN-γ−/− | NA | 1.6 ± 0.2 | 0.7 ± 0.2 | NA | 149.5 ± 13.4 | 35.9 ± 2.4 | NA | 410.5 ± 29.5 | 116.8 ± 10.5 | NA | 365.5 ± 32.1 | 45.1 ± 12.1 |
| IFN-γ−/− IL-17−/− | NA | 1.5 ± 0.6 | NA | NA | 189.5 ± 13.4 | NA | NA | 684.3 ± 102.6 | NA | NA | 280.5 ± 21.0 | NA |
| . | Gut . | Liver . | Lung . | Skin . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Th1 . | Th2 . | Th17 . | Th1 . | Th2 . | Th17 . | Th1 . | Th2 . | Th17 . | Th1 . | Th2 . | Th17 . | |
| WT | 30.6 ± 3.5 | 2.7 ± 0.9 | 1.1 ± 0.1 | 498.0 ± 85.1 | 160.5 ± 39.5 | 9.3 ± 1.8 | 64.2 ± 13.9 | 41.7 ± 10.6 | 1.9 ± 0.5 | 80.0 ± 17.5 | 67.5 ± 12.1 | 2.4 ± 0.7 |
| IFN-γ−/− | NA | 1.6 ± 0.2 | 0.7 ± 0.2 | NA | 149.5 ± 13.4 | 35.9 ± 2.4 | NA | 410.5 ± 29.5 | 116.8 ± 10.5 | NA | 365.5 ± 32.1 | 45.1 ± 12.1 |
| IFN-γ−/− IL-17−/− | NA | 1.5 ± 0.6 | NA | NA | 189.5 ± 13.4 | NA | NA | 684.3 ± 102.6 | NA | NA | 280.5 ± 21.0 | NA |
GVHD indicates graft-versus-host disease; HCT, hematopoietic cell transplantation; and NA, not applicable.
Interestingly, the yield of Th1 cells in gut, liver, lung, and skin of recipients given WT T cells were all more than 30-fold higher than Th17 cells (P < .01; Table 1). In addition, augmentation of Th2 and Th17 differentiation resulted from the absence of IFN-γ led to a 5- to 10-fold increase in the yield of Th2 and a more than 20-fold increase of Th17 cells in lung and skin tissues (P < .01) but no significant changes in the gut or liver. Further augmentation of Th2 differentiation resulted from absence of both IFN-γ and IL-17 led to further increase of Th2 cell yield in the lung (P < .01) but not in the skin (Table 1). These results indicate that, although Th1, Th2, and Th17 cells can migrate to all GVHD target tissues, Th1 cells preferentially migrate to gut and liver tissues; Th2 and Th17 cells preferentially migrate to skin and lung tissues, especially with augmented Th2 or Th17 differentiation.
Next, we compared donor T expression of chemokine receptors and GVHD target tissue release of chemokines, because chemokine receptors and their ligands have been proposed to play a critical role in donor T-cell migration into GVHD target tissues.31,–33 We found that donor CD4+ T cells from recipients given WT donor CD4+ T cells expressed much higher levels of the gut-homing receptors α4β7 and CCR934 as well as the liver-homing receptors CCR5 and CXCR6,35,,–38 compared with donor CD4+ T cells from recipients given IFN-γ−/− or IFN-γ−/−IL-17−/− donor T cells. In contrast, donor CD4+ T cells from former recipients expressed much lower levels of lung- and skin-homing receptors CCR3, CCR4, and CCR6,39,40 compared with the latter recipients (P < .01; Figure 5C). Because we had demonstrated that WT donor T cells predominantly differentiate into Th1 cells, and IFN-γ−/− donor T cells predominantly differentiate into Th2 and Th17 cells (Figure1), taken together, these results indicate that Th1 cells express high levels of the gut-homing receptor α4β7 and CCR9 as well as the liver-homing receptor CCR5 and CXCR6; in contrast, Th2 and Th17 cells expressed high levels of lung- and skin-homing CCR3, CCR4, and CCR6.
On the other hand, we found that, in recipients given WT donor CD4+ T cells, mRNA expression levels of CCL25 (CCR9 ligand) in allogeneic recipient gut tissues was more than 10-fold higher than in syngeneic recipient gut tissues (data not shown). It was also more than 10-fold higher than liver, lung, and skin tissues of the same allogeneic recipients (P < .01; Figure 5D). Although expression of CCL3-5 (CCR5 ligands) and CXCL16 (CXCR6 ligand) in allogeneic recipient liver tissues was more than 10-fold higher than in syngeneic recipients, there was no significant difference among liver, gut, lung, and skin tissues of the same allogeneic recipients (data not shown). However, it was shown by others that liver vascular epithelial cells expressed much higher levels of CXCL16 protein as measured by immunohistochemistry methods.38
We also found that, in allogeneic recipients given WT donor CD4+ T cells, expression of CCL11 (CCR3 ligand) by lung tissues was more than 10-fold higher than gut, liver, or skin tissues (P < .01; Figure 5E); expression of CCL17/CCL22 (CCR4 ligands) by lung tissues was 3-fold higher than skin tissues and 10-fold higher than gut and liver tissues (P < .01; Figure 5F-G); expression of CCL20 by skin tissues was approximately 2-fold higher than lung and gut tissues and 5-fold higher than liver tissues (P < .01; Figure 5H). In addition, transplantation of IFN-γ−/− or IFN-γ−/−IL-17−/− donor CD4+ T cells instead of WT CD4+ T cells did not significantly change the gut, liver, lung, and skin tissue expression of ligands of CCR9, CCR5, CXCR6, CCR3, and CCR4 (data not shown). However, transplantation of IFN-γ−/− donor CD4+ T cells instead of WT or IFN-γ−/−IL-17−/− donor CD4+ T cells augmented the skin expression of CCL20 by 10-fold and lung expression of CCL20 by 2-fold (P < .01; Figure 5I).
Taken together, these results indicate that the preferential migration of Th1 cells to gut and liver tissues is mediated by Th-cell expression of α4β7/CCR9 or CCR5/CXCR6 and gut and liver tissue expression of their ligands, and that Th2 and Th17 cells to lung and skin tissues is mediated by Th2- or Th17-cell expression of high levels of CCR3, CCR4, and CCR6 as well as lung and skin tissue expression of high levels of the chemokine receptor ligands.
Lack of host tissue expression of IFN-γ–inducible B7-H1 contributed to Th2-mediated idiopathic pneumonia
Next, we assessed whether the lack of expression of B7-H1 in lung tissue contributed to the idiopathic pneumonia mediated by Th2 donor cells. Because we have shown that Th2 cells play a critical role in mediating severe idiopathic pneumonia in both recipients given IFN-γ−/− donor cells and recipients given IFN-γ−/−IL17−/− donor cells (Figures 3 and 4), we focused on studying the role of B7-H1 in idiopathic pneumonia induced by IFN-γ−/− donor cells.
Because IFN-γR−/− donor CD4+ T cells secrete IFN-γ but IFN-γ−/− donor CD4+ T cells do not, we first compared the Th2 differentiation, severity of idiopathic pneumonia, and the host lung tissue expression of B7-H1 in the recipients given IFN-γR−/− or IFN-γ−/− donor CD4+ T cells. We observed that, compared with WT recipients, the percentage of IL-4–, IL-5–, and IL-13–producing Th2 cells in the recipients given IFN-γR−/− or IFN-γ−/− donor cells was markedly greater (Figure 6A). Therefore, similar to an IFN-γ deficiency in donor T cells, inactivating IFN-γ receptor signaling also leads to augmented Th2 differentiation. Furthermore, we observed that, compared with recipients given IFN-γ−/− donor cells, recipients given IFN-γR−/− donor cells showed a significant reduction in clinical GVHD, as determined by prolonged survival and increased body weight (P < .01; Figure 6B-C) and marked reduction in severity of idiopathic pneumonia (P < .01; Figure 6D and see supplemental Figure 5A). Their clinical GVHD and lung tissue damage was similar to that of recipients given WT donor cells (Figure 6B-D).
Lack of host lung tissue expression of IFN-γ–inducible B7-H1 contributed to Th2-mediated idiopathic pneumonia. (A) Intracellular cytokine profiles of splenic CD4+ T cells 10 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. Representative of 4 replicate experiments is shown. (B-C) Percent survival and body-weight change of recipients after HCT. There were 12 mice in each group, combined from 3 replicate experiments. (D) Mean ± SE of histopathology scores (n = 6). (E) Ten days after HCT, cells from lungs of recipients were stained for CD45, CD11c, and B7-H1 or isotype control. Gated CD11c+ or CD45− cells were shown in histogram for B7-H1 or isotype control. Representative of 3 replicate experiments is shown. (F) Immunohistochemical staining of B7-H1 in lung tissues harvested 6 days after HCT. One representative picture of 3 replicate experiments is shown. (G-H) IFN-γ−/− or IFN-γR−/− donor cells were transplanted into B7-H1−/−, or B7-H1−/− chimera recipients. Percent survival curves are shown. There were 6 to 8 mice in each group, combined from 2 replicate experiments. (I) IFN-γR−/− donor cells were transplanted into WT, B7-H1−/−, or B7-H1−/− chimera recipients. Mean ± SE of histopathology scores is shown (n = 6). (J) IFN-γ−/− donor cells were transplanted into WT or B7-H1−/− recipients with or without injection of recombinant IFN-γ. Mean ± SE of histopathology scores is shown (n = 6).
Lack of host lung tissue expression of IFN-γ–inducible B7-H1 contributed to Th2-mediated idiopathic pneumonia. (A) Intracellular cytokine profiles of splenic CD4+ T cells 10 days after HCT. Gated CD4+ T cells are shown in CD4 versus cytokines. Representative of 4 replicate experiments is shown. (B-C) Percent survival and body-weight change of recipients after HCT. There were 12 mice in each group, combined from 3 replicate experiments. (D) Mean ± SE of histopathology scores (n = 6). (E) Ten days after HCT, cells from lungs of recipients were stained for CD45, CD11c, and B7-H1 or isotype control. Gated CD11c+ or CD45− cells were shown in histogram for B7-H1 or isotype control. Representative of 3 replicate experiments is shown. (F) Immunohistochemical staining of B7-H1 in lung tissues harvested 6 days after HCT. One representative picture of 3 replicate experiments is shown. (G-H) IFN-γ−/− or IFN-γR−/− donor cells were transplanted into B7-H1−/−, or B7-H1−/− chimera recipients. Percent survival curves are shown. There were 6 to 8 mice in each group, combined from 2 replicate experiments. (I) IFN-γR−/− donor cells were transplanted into WT, B7-H1−/−, or B7-H1−/− chimera recipients. Mean ± SE of histopathology scores is shown (n = 6). (J) IFN-γ−/− donor cells were transplanted into WT or B7-H1−/− recipients with or without injection of recombinant IFN-γ. Mean ± SE of histopathology scores is shown (n = 6).
In addition, we observed that the CD45− host lung mesenchymal cells of the recipients given WT or IFN-γR−/− donor cells but not the recipients given IFN-γ−/− donor cells up-regulated expression of B7-H1, although donor CD11c+ DC expression of B7-H1 was up-regulated in all types of recipients (Figure 6E). Consistently, although lung-tissue expression of recipients given IFN-γ−/− donor cells showed little expression of B7-H1, as determined by immunohistochemical staining of B7-H1, the lung tissues of recipients given IFN-γR−/− or WT donor cells markedly up-regulated B7-H1 (Figure 6F). These results indicate that expression of the IFN-γ–inducible coinhibitory molecule B7-H1 by host lung mesenchymal cells may play an important role in preventing GVHD idiopathic pneumonia.
To further test the role of B7-H1 in preventing the induction of idiopathic pneumonia, CD4+ T cells and TCD-BM cells from IFN-γ−/− or IFN-γR−/− donors were transplanted into B7-H1−/− mice as well as chimeric B7-H1−/− mice reconstituted with WT bone marrow cells, WT-BM to B7-H1 chimera that expressed B7-H1 on hematopoietic cells such as DC cells (see supplemental Figure 6). We found that, although IFN-γR−/− donor CD4+ T cells induced much weaker clinical GVHD and idiopathic pneumonia in WT recipients, compared with IFN-γ−/− donor CD4+ T cells (Figure 6B-D), they induced severe clinical GVHD in both B7-H1−/− mice and B7-H1−/− chimeras. All the recipients died within 2 weeks after HCT, and there was no difference in survival time between the recipients given IFN-γ−/− or IFN-γR−/− donor cells (Figure 6G-H). Furthermore, the IFN-γR−/− donor cells induced much more severe idiopathic pneumonia in B7-H1−/− recipients as well as in WT-BM to B7-H1−/− chimeras, compared with WT recipients (P < .01; Figure 6I and see supplemental Figure 5B). In addition, we found that, although giving recombinant IFN-γ to WT recipients given IFN-γ−/− donor cells ameliorated idiopathic pneumonia, giving recombinant IFN-γ to B7-H1−/− recipients given IFN-γ−/− donor cells did not ameliorate idiopathic pneumonia (P < .01; Figure 6J and see supplemental Figure 5C). Taken together, these results demonstrate that IFN-γ–induced tissue expression of B7-H1 plays an important role in prevention of idiopathic pneumonia and that lack of lung tissue expression of B7-H1 in recipients given IFN-γ−/− donor cells plays an important role in augmenting induction of idiopathic pneumonia by donor Th2 cells.
Discussion
We observed that, in general, Th1 cells play a critical role in mediating acute GVHD pathogenesis, and Th2 and Th17 cells can mediate acute GVHD under special circumstances (ie, absence of IFN-γ), in which donor T cells can differentiate into Th2 and Th17 cells to mediate acute GVHD. In turn, Th2 or Th17 cells can down-regulate Th1 differentiation and Th1-mediated GVHD, and absence of either Th2 or Th17 cells can lead to augmentation of Th1 differentiation and GVHD.8,41 These findings can provide insightful explanation into many previous contradictory reports. For example, it was reported that in vitro–biased Th2 cells ameliorated GVHD mediated by Th1 cells14,42 but Stat4−/− donor cells that were originally thought to preferentially differentiated into Th2 cells still caused lethal GVHD.15 Based on our new observation, Stat4−/− donor cells may differentiate into both Th2 and Th17 cells, and they may be responsible for tissue-specific GVHD. In addition, previous reports showed that blockade of IFN-γ or IL-4 either augmented or ameliorated GVHD.12,13,41,43,44 These contradictory reports may result from the reciprocal differentiation of Th1, Th2, and Th17 cells after blockade of different cytokines.
We observed that the preferential tissue damage mediated by Th1, Th2, and Th17 cells was in part associated with tissue-specific infiltration of the Th subsets, which was mediated by preferential expression of tissue-homing chemokine receptors by different Th subsets and preferential expression of chemokines by different GVHD target tissues. However, differential tissue response to inflammatory cytokines from T cells also plays an important role in tissue damage. First, although Th2 cells infiltrated lung and skin tissues, Th2 cells caused severe tissue damage in the lung but not in the skin. It is reported that the Th2 cytokine IL-13 enhances lung mucus cell secretion and bronchial muscle cell spasm to cause blockage of the respiratory tract and subsequent pulmonary failure.30 We also observed that blockade of IL-13 and IL-4 signaling pathway ameliorated GVHD idiopathic pneumonia.
Second, although Th17 cells infiltrated skin and lung tissues, our studies showed that Th17 cells played a critical role in skin-tissue damage but not in lung-idiopathic pneumonia. We found that augmented Th17 differentiation in recipients given IFN-γ−/− donor T cells led to a marked increase in Th17 infiltration of the skin tissues and a severe epidermal hyperplasia; in contrast, the skin T-cell infiltration and skin-tissue damage were markedly reduced in recipients given IFN-γ−/−IL17−/− donor T cells. Thus, Th17 cells play an important role in mediating skin GVHD. It was reported that Th17 cytokines IL-17 can activate STAT3 in keratinocytes and cause epidermal hyperplasia.45 We also observed activation of STAT3 in skin keratinocytes in IFN-γ−/− donor cell recipients but not in IFN-γ−/−IL17−/− donor cell recipients (see supplemental Figure 7).
On the other hand, we did not find an important role for Th17 cells in GVHD idiopathic pneumonia, because, although both Th2 and Th17 cells infiltrated lung tissues in recipients given IFN-γ−/− donor T cells, augmentation of Th17 differentiation by blocking Th2 differentiation ameliorated, rather than exacerbated, idiopathic pneumonia. In addition, IFN-γ−/−IL17−/− donor T cells still induced severe idiopathic pneumonia. Thus, Th17 cells did not play a critical role in mediating idiopathic pneumonia. We should point out that this conclusion is contradictory to a report by Mauermann et al.28 Their conclusion was based on data that anti–IL-17 treatment of 4 recipients mildly ameliorated the lung infiltration, of which the statistic significance was not mentioned. In addition, no survival result was shown. Therefore, it is conceivable that Th17 cells may play a minor role in mediating idiopathic pneumonia and anti–IL-17 treatment can modestly reduce the infiltration of Th17 cells in lung tissues, because we observed that augmented Th17 differentiation significantly increased lung tissue expression of CCL20 and increased Th17 infiltration.
We should also point out that the tissue-specific GVHD mediated by Th1, Th2, and Th17 is preferential but not exclusive, because the difference in chemokine receptor expression by Th subsets and the difference in chemokine expression by GVHD target tissues is quantitative, so that each T-cell subset can migrate to all GVHD target tissues, although there is a preference for specific tissues. Therefore, under a biased condition, a Th subset can induce tissue damage in nonpreferential tissues.
We observed that lack of tissue expression of the IFN-γ– inducible B7-H1 played an important role in augmenting the idiopathic pneumonia mediated by Th2 cells. Augmentation of Th2 differentiation alone was not sufficient to induce severe idiopathic pneumonia, because Th2 differentiation in recipients given IFN-γR−/− donor cells was significantly stronger than in recipients given IFN-γ−/− donor cells, but GVHD idiopathic pneumonia was markedly weaker in the former recipients. This difference was due to the expression of B7-H1 in the lung mesenchymal tissue of recipients given IFN-γR−/− donor cells. In addition, although no difference in Th2-cell differentiation was observed between WT and B7-H1−/− recipients, the yield of Th2 cells in lung was markedly increased in B7-H1−/− recipients compared with WT recipients (see supplemental Figure 8). This indicates that lung tissue expression of B7-H1 may tolerize the lung infiltrating T cells. This is consistent with reports that tissue expression of B7-H1 tolerize infiltrating T cells in the lung and other peripheral organs.46,47 This finding provides a likely answer to questions brought up by Burman and colleagues regarding an IFN-γ–induced unknown protective mechanism possessed by the host tissues.21 Furthermore, we observed that all GVHD target tissues could up-regulate expression of B7-H1 in an IFN-γ–dependent manner and that tissue expression of B7-H1 also played an important role in ameliorating Th1-mediated GVHD tissue damage in gut and liver (Yi T, Zeng D, unpublished observation).
We are aware that amelioration of donor CD8+ T-mediated GVHD by IFN-γ was shown via augmenting activation-induced cell death.48 We speculate that IFN-γ induced up-regulation of B7-H1 on host-tissue cells, and B7-H1 interaction with PD1 on donor CD8+ T cells may contribute to the augmentation of activation-induced cell death. We are also aware that, although IFN-γ−/− donor CD4+ T cells induced less severe liver damage in the current study, it was reported that IFN-γ−/− donor CD8+ T cells induced more severe liver damage.48 This discrepancy may be due to the differential sensitivity of CD4+ and CD8+ T cells to the tolerant effect mediated by PD1/B7-H1 interaction, because we previously reported that the apoptosis of activated CD8+ but not CD4+ T cells was significantly reduced in the liver of B7-H1−/− mice.25
In summary, we propose that WT naive alloreactive CD4+ T cells predominantly differentiate into Th1 cells, while a portion of them also differentiate into Th2 or Th17 cells after interaction with host APCs. Th1 cells can down-regulate Th2 and Th17 cells or vice versa. In the absence of IL-17 or IL-4 (eg, genetic deficiency or severe neutralization), Th1 differentiation is augmented, and tissue damage in the gut and liver is preferentially exacerbated. In contrast, in the absence of IFN-γ, both Th2 and Th17 differentiation is augmented, and tissue damage in lung and skin is exacerbated. Absence of both IFN-γ and IL-17 leads to further augmentation of Th2 differentiation and exacerbated lung damage (idiopathic pneumonia). Lack of both Th1 and Th2 cells further augments Th17 differentiation and exacerbates skin damage. Therefore, in addition to the well-established balance between regulatory and effector T cells,4,49,50 our studies indicate that the balance among Th1, Th2, and Th17 effector subsets also plays an important role in regulating T-cell immune response and that neutralizing either Th1, Th2, or Th17 cytokines may lead to biased Th1, Th2, or Th17 differentiation and cause organ-specific tissue damage.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Keely Walker for critical reading of the manuscript, Lucy Brown and her staff at COH Flow Cytometry Facility, Sofia Loera and her staff at COH Anatomic Pathology Laboratory for their excellent technical assistance, and Dr Richard Ermel and his staff at COH Research Animal Facility for providing excellent animal care.
D.H. is supported by Eugene and Ruth Summer Student Academy, COH Beckman Research Institute. This work was supported by National Institutes of Health grant R01 AI066008.
National Institutes of Health
Authorship
Contribution: T.Y. designed and performed research, analyzed data, and wrote the manuscript; Y.C., L.W., G.D., D.H., D. Zhao, H.J., J.Y., and I.T. performed research, D.T.U., L.C., and Y.I. provided critical mouse strains; F K. and S.F. reviewed manuscript; and D. Zeng designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Defu Zeng, The Beckman Research Institute, Gonda Bldg, R2017, COH National Medical Center, 1500 East Duarte Rd, Duarte, CA 91010; e-mail: dzeng@coh.org.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal