Depolymerized holothurian glycosaminoglycan (DHG) is a fucosylated chondroitin sulfate with antithrombin-independent antithrombotic properties. Heparin cofactor II (HCII)-dependent and -independent mechanisms for DHG inhibition of plasma thrombin generation were evaluated. When thrombin generation was initiated with 0.2 pM tissue factor (TF), the half maximal effective concentration (EC50) for DHG inhibition was identical in mock- or HCII-depleted plasma, suggesting a serpin-independent mechanism. In the presence of excess TF, the EC50 for DHG was increased 13- to 27-fold, suggesting inhibition was dependent on intrinsic tenase (factor IXa-factor VIIIa) components. In factor VIII–deficient plasma supplemented with 700 pM factor VIII or VIIIa, and factor IX–deficient plasma supplemented with plasma-derived factor IX or 100 pM factor IXa, the EC50 for DHG was similar. Thus, cofactor and zymogen activation did not contribute to DHG inhibition of thrombin generation. Factor IX–deficient plasma supplemented with mutant factor IX(a) proteins demonstrated resistance to DHG inhibition of thrombin generation [factor IX(a) R233A > R170A > WT] that inversely correlated with protease-heparin affinity. These results replicate the effect of these mutations with purified intrinsic tenase components, and establish the factor IXa heparin-binding exosite as the relevant molecular target for inhibition by DHG. Glycosaminoglycan-mediated intrinsic tenase inhibition is a novel antithrombotic mechanism with physiologic and therapeutic applications.
Introduction
Depolymerized holothurian glycosaminoglycan (DHG) is a low molecular weight (average MW 12 500) fucosylated chondroitin sulfate isolated from the sea cucumber Stichopus japonicus and prepared by partial oxidative depolymerization with hydrogen peroxide.1,2 DHG demonstrates antithrombotic efficacy in models of murine thrombin-induced pulmonary thromboembolism, thrombin-induced venous thrombosis in the rat, and canine dialysis during renal failure.3,,–6 DHG does not bind antithrombin with high affinity, and exhibits antithrombin-independent antithrombotic efficacy in vivo.3,7 Compared with equitherapeutic doses of unfractionated or low-molecular-weight heparins (LMWHs), DHG demonstrates significantly reduced effects on tail transection and template bleeding assays in rat and dog models.4,5,8 Thus, DHG has potential as an antithrombotic agent with reduced bleeding risk relative to heparin. In vitro testing has suggested that DHG accelerates thrombin inhibition by heparin cofactor II (HCII), inhibits factorVIII activation by thrombin, and inhibits factor X activation by the intrinsic tenase complex.9,–11 Herein, we investigate the relevant mechanism(s) for the antithrombotic effect of DHG in human plasma.
In vitro and ex vivo modeling of the coagulation cascade indicates that factor X activation by the intrinsic tenase complex (factor IXa-factor VIIIa) is the rate-limiting step for thrombin generation.12,,–15 The heparin-binding exosite on factor IXa is the interactive site for the factor VIIIa A2 domain, contributing to stabilization of cofactor activity and allosteric activation of the protease within the enzyme complex.16,–18 The physiologic importance of this exosite is demonstrated by its critical role in the regulation of thrombin generation in human plasma and saphenous vein thrombosis in the mouse.19 In an experimental system with purified components, the factor IXa heparin-binding exosite is the molecular target for antithrombin-independent inhibition of the intrinsic tenase complex by both LMWH and DHG.11,17
Since in vitro data demonstrates that DHG inhibits the intrinsic tenase complex by interacting with heparin-binding exosite of factor IXa, and this exosite is a critical regulator of plasma thrombin generation and murine venous thrombosis, we hypothesized that DHG regulates thrombin generation via interaction with the factor IXa heparin-binding exosite. The effect of DHG on plasma thrombin generation was evaluated by fluorogenic substrate cleavage and Western blot analysis in HCII- or mock-immunodepleted plasma, factor VIII– or IX–deficient human plasma, and factor IX–deficient plasma reconstituted with recombinant factor IX(a) possessing selected mutations in the heparin-binding exosite. The results demonstrate that DHG inhibits plasma thrombin generation by targeting the heparin-binding exosite of factor IXa. Inhibition of plasma thrombin generation by DHG was independent of effects on factor VIII or IX activation or acceleration of thrombin inhibition by HCII. These data provide proof of principle that glycosaminoglycan-mediated targeting of the factor IXa heparin binding exosite is a novel antithrombotic mechanism.
Methods
Materials
Human pooled plasma, factor IX–deficient, and factor VIII–deficient patient plasmas were purchased from George King. HCII- or mock-immunodepleted human plasma prepared from the same parent pooled plasma was purchased from Affinity Biologicals. Corn trypsin inhibitor (CTI) was obtained from Haematologic Technologies. Human plasma-derived factor IX, IXa, and thrombin were purchased from Enzyme Research. Recombinant human factor VIII (Kogenate FS) was generously provided by Andreas Mueller-Beckhaus of Bayer HealthCare LLC. Phosphatidylserine (PS) and phosphatidylcholine (PC) were purchased from Avanti Lipids. Cholesterol was purchased from Calbiochem. Phosphatidylcholine:phosphatidlylserine:cholesterol (molar ratio 75:25:1) phospholipid vesicles (PC:PS vesicles) were prepared by extrusion through a 100 nm polycarbonate filter.20 Bovine serum albumin (A-9647) was purchased from Sigma-Aldrich. Dimethylsulfoxide (DMSO) was purchased from Mallinckrodt. Lyophilized bovine thrombin-α2-macroglobulin complex was purchased from Thrombinoscope BV. Thromborel S, a human thromboplastin from Dade Behring, was used as the source of relipidated human tissue factor (TF; 200 ng/mL).19 The fluorogenic substrate Z-Gly-Gly-Arg-AMC·HCl was obtained from Bachem. DHG was generously provided by Kazuhisa Minamiguchi of Taiho Pharmaceuticals.
Expression and purification of recombinant factor IX
Stable HEK 293 cell lines expressing human factor IX wild-type (WT) and R233A were constructed as previously described.17,18 A HEK 293 cell lines stably transfected with human factor IX R170A was provided by Darrel Stafford (University of North Carolina, Chapel Hill).21 Recombinant factor IX proteins were purified to homogeneity from conditioned media and quantitated by absorbance at 280 nm. A portion of the factor IX was activated to factor IXa with human factor XIa, and factor IXa catalytic sites were quantitated by titration with antithrombin III, as previously described.18
Fluorogenic assay for detection of plasma thrombin generation
Thrombin generation in human plasma was detected as previously described.19,22 Thrombin activity was determined by 7-amino-4-methycoumarin (AMC) release from the fluorogenic substrate Z-Gly-Gly-Arg-AMC, detected by a 360/40-nm-excitation and 460/40-nm-emission filter set in a Biotek Synergy HT fluorescent plate reader equipped with Gen 5 software (Biotek Instruments). Calibration was performed as previously described.19 The raw data were imported into Technothrombin TGA evaluation software from Technoclone to construct calibration curves for each plasma.
For TF-triggered assays, the initiator solution was composed of 0.12 mg/CTI, 25 μM PC:PS vesicles, 0.6 pM or 12 pM TF, and 0-50 μM DHG in TGA buffer. Before each assay, a fresh fluorogenic substrate and calcium solution (FluCa substrate) containing 2.5 mM Z-Gly-Gly-Arg-AMC and 100 mM CaCl2 was prepared as described.19 Plasma (60 μL) and initiator solution (20 μL) were added to each well and preheated at 37°C for 10 minutes. FluCa substrate (20 μL) preheated to 37°C was then added, mixed at medium intensity for 5 seconds, and readings were obtained at 30-second intervals for 1 hour. Final concentrations (extrapolated to the 60 μL plasma volume) were 0.2 pM (limiting) or 4 pM (excess) TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI. DHG (0-10 μM) concentrations were based on overall assay volume (100 μL). In factor VIII–deficient plasma, thrombin generation was triggered with 0.2 pM TF in the presence of a final plasma concentration of 700 pM factor VIII or thrombin-activated factor VIIIa. Recombinant factor VIII (2.1 nM) was added to the TF initiator solution, or factor VIII was activated with a 1:2 molar ratio of thrombin in 0.15 M NaCl, 5 mM CaCl2, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4, and 0.01% Tween-80 at 37°C for 30 seconds, and immediately added to assay wells followed by FluCa substrate to initiate the reaction. In factor IX–deficient plasma, thrombin generation was triggered with 0.2 pM TF in the presence of 100% plasma levels (90 nM) of plasma-derived or recombinant factor IX, or 100 pM factor IXa in the absence of TF. For factor IXa-triggered assays, TF was omitted from the initiator solution, and protease was added to the assay well just before the FluCa substrate.
Fluorescent signal data were exported to Technothrombin TGA evaluation software, and thrombin generation over time was determined using the appropriate calibration curve for each plasma. Thrombin generation parameters lag time (start until first burst in thrombin formation), peak thrombin concentration, time to thrombin peak, and velocity index (slope between the end of lag time and peak thrombin) were determined using the software. To determine the relative potency of DHG for inhibition of plasma thrombin generation under each experimental condition, inhibitor concentration was plotted versus the relative velocity index (the ratio of the velocity index at each DHG concentration to velocity index in the absence of DHG). Data were fitted to a binding equation to obtain the half maximal effective concentration (EC50) as previously described.18,23
The time course of plasma thrombin generation by Western blot analysis
Thrombin generation was initiated in 1.5-mL eppendorf tubes with 0.2 or 4 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock-depleted plasma with or without 0.5 or 2.5 μM DHG at 37°C. Individual reactions were quenched by addition of 100 μL 2× sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer containing 5 M urea at the indicated time points. Quenched samples were incubated at 37°C for 5 minutes, boiled for 5 minutes, diluted 1:20 in nonreducing SDS-PAGE loading buffer, and subjected to 10% (for 0.2 pM TF condition) or 12.5% (for 4 pM TF condition) SDS-PAGE and then immunoblotted as previously described.19
Results
Effect of TF concentration on thrombin generation in factor IX–deficient plasma
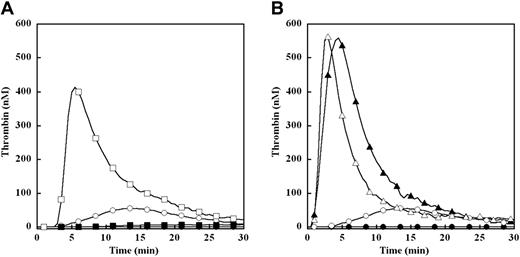
Thrombin generation was triggered by 0.2 or 4 pM TF in factor IX–deficient plasma with or without addition of 100% (90 nM) plasma-derived factor IX (Figure 1). Control reactions in the absence of TF demonstrated that factor IX–deficient plasma alone failed to generate detectable thrombin, while addition of 90 nM factor IX resulted in a modest, delayed thrombin peak (Figure 1A-B). In the presence of 0.2 pM TF, minimal thrombin generation was observed in factor IX–deficient plasma alone, while peak thrombin was approximately 400 nM at 6 minutes when supplemented with 100% plasma-derived factor IX (Figure 1A). In the presence of 4 pM TF, peak thrombin was approximately 550 nM with or without addition of 100% plasma-derived factor IX, with modest shortening of time to peak thrombin when factor IX was present (Figure 1B). Thus, the magnitude of plasma thrombin generation was highly dependent on factor IX in the presence of limiting TF (0.2 pM), and largely independent of factor IX in the presence of excess TF (4 pM).
Effect of limiting (0.2 pM) and excess (4 pM) TF on plasma thrombin generation in factor IX–deficient plasma. Thrombin generation was initiated with 0.2 pM (A) or 4 pM (B) human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor IX–deficient plasma with or without addition of 100% (90 nM) of plasma factor IX (pFIX): no TF or pFIX control (●), 90 nM pFIX control (○), 0.2 pM TF (■), 0.2 pM TF and 90 nM pFIX (□), 4 pM TF (▴), 4 pM TF and 90 nM pFIX (▵). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points.
Effect of limiting (0.2 pM) and excess (4 pM) TF on plasma thrombin generation in factor IX–deficient plasma. Thrombin generation was initiated with 0.2 pM (A) or 4 pM (B) human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor IX–deficient plasma with or without addition of 100% (90 nM) of plasma factor IX (pFIX): no TF or pFIX control (●), 90 nM pFIX control (○), 0.2 pM TF (■), 0.2 pM TF and 90 nM pFIX (□), 4 pM TF (▴), 4 pM TF and 90 nM pFIX (▵). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points.
Effect of DHG on TF triggered thrombin generation in mock- or HCII-immunodepleted plasma
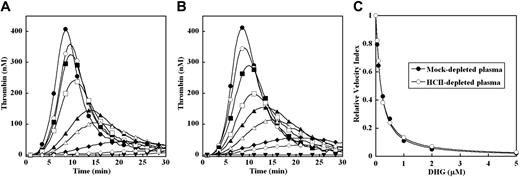
Plasma thrombin generation was triggered by limiting TF (0.2 pM) in the presence of increasing concentrations of DHG (0-5 μM) in either mock- or HCII-immunodepleted plasma (Figure 2A-B). The parent pooled plasma underwent identical immunodepletion protocols to minimize preanalytical plasma variables.24,25 In the absence of TF, no detectable thrombin was generated in either plasma (Figure 2A-B). In mock-depleted plasma, increasing DHG concentration resulted in a dramatic dose-dependent decrease in velocity index (slope) and peak thrombin concentration, with a more gradual prolongation of the lag time and time to peak thrombin generation (Figure 2A). At the highest DHG concentration, thrombin generation approached the level observed in the absence of TF. In HCII-depleted plasma, increasing DHG concentration demonstrated very similar effects on plasma thrombin generation (Figure 2B). The potency of DHG inhibition was estimated by velocity index for plasma thrombin generation at each DHG concentration relative to the absence of glycosaminoglycan (Figure 2C). The mean EC50 for reduction in the velocity index by DHG was identical for both mock-depleted plasma and HCII-depleted plasma (Table 1).
DHG inhibition of thrombin generation triggered by limiting TF in mock- or HCII-depleted pooled plasma. Thrombin generation was initiated with 0.2 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock- (A) or HCII-depleted (B) pooled plasma in the presence of increasing DHG: 0 (●), 0.05 (○), 0.1 (■), 0.25 (□), 0.5 (▴), 1 (▵), 2 (♦), and 5 μM (◇). Control reactions without TF or DHG are presented (▾). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and the data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (C). Representative curves are presented.
DHG inhibition of thrombin generation triggered by limiting TF in mock- or HCII-depleted pooled plasma. Thrombin generation was initiated with 0.2 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock- (A) or HCII-depleted (B) pooled plasma in the presence of increasing DHG: 0 (●), 0.05 (○), 0.1 (■), 0.25 (□), 0.5 (▴), 1 (▵), 2 (♦), and 5 μM (◇). Control reactions without TF or DHG are presented (▾). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and the data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (C). Representative curves are presented.
The EC50 (± SEM) for DHG inhibition of the mean velocity index for thrombin generation (μM) in immunodepleted plasma
| TF, pM . | Mock-depleted plasma . | HCII-depleted plasma . |
|---|---|---|
| 0.2 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| 4 | 2.02 ± 0.09 | 4.31 ± 0.23 |
| TF, pM . | Mock-depleted plasma . | HCII-depleted plasma . |
|---|---|---|
| 0.2 | 0.16 ± 0.01 | 0.16 ± 0.01 |
| 4 | 2.02 ± 0.09 | 4.31 ± 0.23 |
Thrombin generation was initiated with 0.2 or 4 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock- or HCII-depleted plasma in the presence of increasing DHG (0-10 μM). Duplicate wells were averaged to determine individual thrombin generation curves. The relative velocity index was plotted versus DHG concentration, and EC50 values for each replicate (n = 3) were determined as described in “Fluorogenic assay for detection of plasma thrombin generation.” The mean and standard error of the EC50 values were determined from the 3 independent replicates.
Statistical significance analysis: EC50 values for DHG inhibition at 4 pM TF were significantly different from 0.2 pM in both plasma. At 4 pM TF, EC50 value in HCII-depleted plasma was significantly different from mock-depleted plasma (Student t test, P < .002).
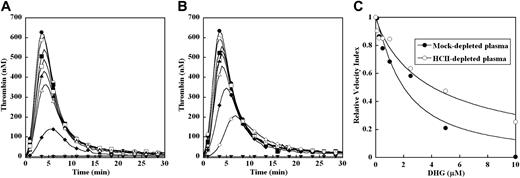
Compared with limiting TF conditions, thrombin generation triggered by excess TF (4 pM) demonstrated enhanced peak thrombin concentration and shortening of time to peak in both mock- and HCII-depleted plasma. Significantly higher concentrations of DHG were required to inhibit thrombin generation in mock-depleted plasma (Figure 3A) under these conditions. This inhibition was primarily reflected in reduction of velocity index and peak thrombin concentration, with no discernable effects on the lag time or time to peak thrombin generation. The mean EC50 for reduction in relative velocity index by DHG inhibition was increased 13-fold (Table 1) for mock-depleted plasma in the presence of excess versus limiting TF concentration, suggesting that components of the intrinsic tenase complex contribute to this inhibition. In HCII-depleted plasma, even higher concentrations were required to inhibit thrombin generation, with incomplete inhibition even at 10 μM DHG (Figure 3B). Similarly, this inhibition was characterized by reduction in velocity index and peak thrombin concentrations, with minimal effects on the time to peak thrombin generation. The mean EC50 for reduction in the velocity index by DHG in HCII-depleted plasma triggered by excess TF was more than 2-fold higher than mock-depleted plasma (Figure 3C) in the presence of excess TF, and 27-fold higher than limiting TF conditions (Table 1).
DHG inhibition of thrombin generation triggered by excess TF in mock- or HCII-depleted pooled plasma. Thrombin generation was initiated with 4 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock- (A) or HCII-depleted plasma (B) in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). Control reactions without TF or DHG are presented (▾). The time course of thrombin generation was measured as described in the “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and the data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (C). Representative curves are presented.
DHG inhibition of thrombin generation triggered by excess TF in mock- or HCII-depleted pooled plasma. Thrombin generation was initiated with 4 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock- (A) or HCII-depleted plasma (B) in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). Control reactions without TF or DHG are presented (▾). The time course of thrombin generation was measured as described in the “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and the data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (C). Representative curves are presented.
Effect of DHG on TF triggered plasma thrombin generation detected by Western blot analysis
To confirm that DHG primarily inhibits plasma thrombin generation instead of accelerating thrombin inhibition, the time course of thrombin activation and inhibition products was monitored by Western blot analysis. Thrombin generation was initiated by 0.2 pM TF in mock-depleted plasma with or without 0.5 μM DHG. The reaction products were analyzed by SDS-PAGE under nonreducing conditions using a primary antibody that simultaneously detected prothrombin, thrombin-related cleavage products, HCII-thrombin complex, and the thrombin-antithrombin complex (TAT; Figure 4A). When thrombin generation was triggered by 0.2 pM TF, mock-depleted plasma demonstrated appearance of free thrombin and the TAT complex at 6 minutes, peak free thrombin at 9 minutes, and progressive accumulation of TAT over time in the absence of DHG. In the presence of 0.5 μM DHG, peak thrombin generation was delayed until approximately 15 minutes, and the intensity of free thrombin and TAT bands was reduced relative to control. Likewise, depletion of the prothrombin/meizothrombin band occurred more slowly and to a lesser extent than in the absence of DHG (Figure 4A). The time course of plasma thrombin generation by Western blot analysis correlated well with results of the fluorogenic substrate assay under these conditions. Finally, a very modest (relative to TAT) thrombin-HCII band was noted only in the presence of DHG.
Western blot analysis of limiting (A) and excess (B) TF triggered thrombin generation in mock-depleted plasma with or without DHG. Thrombin generation was initiated with 0.2 (A) or 4 (B) pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock-depleted plasma in the absence and presence of 0.5 μM or 2.5 μM DHG. Individual reactions were quenched over time with loading buffer containing 5 M urea and analyzed by SDS-PAGE under nonreducing conditions as described in “The time course of plasma thrombin generation by Western blot analysis.” Proteins transferred to Immobilon-P were detected with a polyclonal sheep anti-human thrombin primary antibody, followed by a peroxidase-conjugated affinity purified donkey anti-sheep immunoglobulin G (IgG), and subsequent development of signal with chemiluminescent substrate. Prothrombin indicates prothrombin/meizothrombin band. TAT indicates thrombin-antithrombin complex, and HCII-thrombin indicates heparin cofactor II-thrombin complex.
Western blot analysis of limiting (A) and excess (B) TF triggered thrombin generation in mock-depleted plasma with or without DHG. Thrombin generation was initiated with 0.2 (A) or 4 (B) pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in mock-depleted plasma in the absence and presence of 0.5 μM or 2.5 μM DHG. Individual reactions were quenched over time with loading buffer containing 5 M urea and analyzed by SDS-PAGE under nonreducing conditions as described in “The time course of plasma thrombin generation by Western blot analysis.” Proteins transferred to Immobilon-P were detected with a polyclonal sheep anti-human thrombin primary antibody, followed by a peroxidase-conjugated affinity purified donkey anti-sheep immunoglobulin G (IgG), and subsequent development of signal with chemiluminescent substrate. Prothrombin indicates prothrombin/meizothrombin band. TAT indicates thrombin-antithrombin complex, and HCII-thrombin indicates heparin cofactor II-thrombin complex.
A similar analysis was undertaken for thrombin generation initiated by 4 pM TF in mock-depleted plasma with 0, 0.5, or 2.5 μM DHG present (Figure 4B). Peak free thrombin was observed at 3 minutes under all conditions, but was reduced with increasing DHG. Prothrombin/meizothrombin depletion was rapid and dramatic in the presence of 0 or 0.5 μM DHG, with little or no detectable prothrombin/meizothrombin band from 6 minutes onward. In the presence of 2.5 μM DHG, the prothrombin band decreased with time but persisted through 9 minutes. The TAT complex appeared at 3 minutes under all conditions, and predominated in the absence of DHG. Minimal thrombin-HCII complex was noted in the absence of DHG, but an increasing proportion of thrombin was found in thrombin-HCII relative to TAT complex with increasing DHG concentration. In contrast, HCII-thrombin was barely detectable under limiting TF conditions in the presence of 0.5 μM DHG (Figure 4A). Thus, higher concentrations of DHG were associated with enhanced formation of thrombin-HCII complex when plasma thrombin generation was triggered by excess TF.
Effect of factor VIII or factor IX activation on inhibition of plasma thrombin generation by DHG
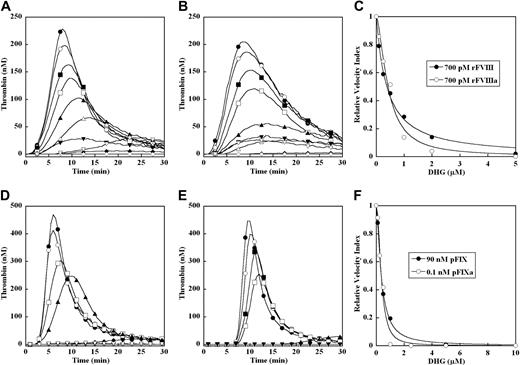
The markedly increased EC50 for inhibition of plasma thrombin generation by DHG in the presence of excess versus limiting TF concentration suggests that components of the intrinsic tenase complex contribute to this inhibition (Figures 1, 2A, and 3A). The potential contribution of factor VIII or factor IX activation to the DHG inhibition mechanism was examined in factor VIII–deficient and factor IX–deficient plasma, respectively. Thrombin generation was triggered by 0.2 pM TF in factor VIII–deficient plasma supplemented with either 700 pM recombinant factor VIII or thrombin-activated factor VIIIa (Figure 5A-B). No significant thrombin response was observed in the absence of factor VIII(a) and TF, while 0.2 pM TF or factor VIII alone generated only very modest increases over baseline thrombin. As expected, supplementing the factor VIII–deficient plasma with 700 pM factor VIII resulted in a marked increase in thrombin generation in the presence of 0.2 pM TF (Figure 5A). To bypass factor VIII activation, 700 pM thrombin-activated factor VIIIa was added to the factor VIII–deficient plasma under identical conditions, which generated a similar thrombin response (Figure 5B). Controls demonstrated no significant effect of “carryover” thrombin from the factor VIII activation mixture (data not shown). In both cases, addition of increasing concentration of DHG (0-5 μM) reduced the velocity index and peak thrombin concentration, and modestly prolonged lag time and time to peak thrombin (Figure 5A-B). The mean EC50 for inhibition of the velocity index for thrombin generation by DHG in factor VIII–deficient plasma was 0.41 (± 0.02) μM in the presence of 700 pM recombinant factor VIII and 0.44 (± 0.05) μM in the presence of 700 pM thrombin-activated factor VIIIa. Representative curves are shown (Figure 5C).
Effect of factor VIII and factor IX activation on inhibition of thrombin generation by DHG. Thrombin generation was initiated with 0.2 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor VIII–deficient plasma supplemented with 700 pM recombinant factor VIII (rFVIII) (A) or 700 pM thrombin-activated factor (rFVIIIa) (B); or factor IX–deficient plasma supplemented with 100% (90 nM) plasma-derived factor IX (D) or 100 pM plasma-derived factor IXa in the absence of TF (E) in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). In panels A and B, control reactions are: no TF or factor VIII(a) (◇), 0.2 pM TF only (▾), and factor VIII(a) only (▿). Control for exogenous thrombin control used in factor VIII activation is not shown. In panels D and E, control reactions are: no TF or factor IX (▾) and 0.2 pM TF only (▿). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition in factor VIII–deficient plasma (C) and in factor IX–deficient plasma (F). Representative curves are presented.
Effect of factor VIII and factor IX activation on inhibition of thrombin generation by DHG. Thrombin generation was initiated with 0.2 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor VIII–deficient plasma supplemented with 700 pM recombinant factor VIII (rFVIII) (A) or 700 pM thrombin-activated factor (rFVIIIa) (B); or factor IX–deficient plasma supplemented with 100% (90 nM) plasma-derived factor IX (D) or 100 pM plasma-derived factor IXa in the absence of TF (E) in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). In panels A and B, control reactions are: no TF or factor VIII(a) (◇), 0.2 pM TF only (▾), and factor VIII(a) only (▿). Control for exogenous thrombin control used in factor VIII activation is not shown. In panels D and E, control reactions are: no TF or factor IX (▾) and 0.2 pM TF only (▿). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition in factor VIII–deficient plasma (C) and in factor IX–deficient plasma (F). Representative curves are presented.
To evaluate the potential role of factor IX activation, thrombin generation was compared in factor IX–deficient plasma supplemented with 0.2 pM TF and 100% levels of plasma-derived factor IX or 100 pM plasma-derived factor IXa in the absence of TF (Figure 5D-E). The magnitude of thrombin generation in factor IX–deficient plasma was similar under both conditions, and no significant levels of thrombin generation were noted in the absence of factor IX or factor IXa. Addition of increasing DHG concentration (0-10 μM) inhibited plasma thrombin generation in a similar fashion in the presence of either 100% plasma-derived factor IX or 100 pM plasma-derived factor IXa, although the lag time and time to peak were slightly prolonged in the latter case (Figure 5E). The mean EC50 plus or minus the standard error of the mean plus or minus SEM for inhibition of the velocity index for thrombin generation by DHG in factor IX–deficient plasma was 0.36 (± 0.01) μM for 100% plasma-derived factor IX and 0.34 (± 0.02) μM for plasma-derived FIXa. Representative curves are shown (Figure 5F).
Effect of mutations in the heparin-binding exosite of recombinant factor IX on the ability of DHG to inhibit TF triggered plasma thrombin generation
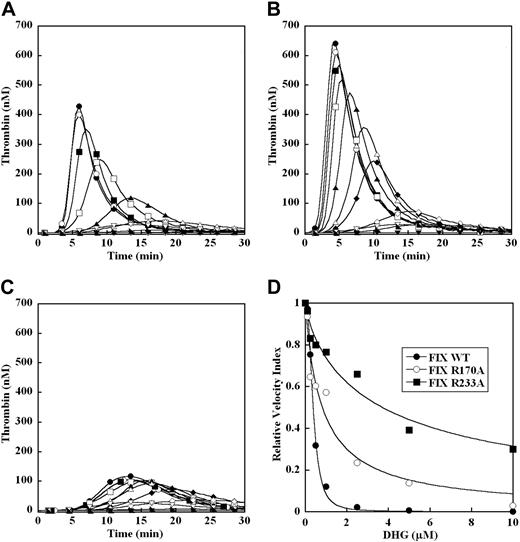
The effect of mutations in the heparin-binding exosite of factor IXa on the ability of DHG to inhibit plasma thrombin generation was assessed with recombinant factor IX WT, R170A, and R233A. The respective proteases derived from these zymogens demonstrate a progressive decrease in heparin affinity (WT > R170A > R233A) and increase in resistance to inhibition by both DHG and LMWH using purified intrinsic tenase components.11,17 The ability of DHG to inhibit thrombin generation triggered by 0.2 pM TF was evaluated in factor IX–deficient plasma supplemented with 100% levels of recombinant factor IX WT, R170A, and R233A (Figure 6A-C). In the absence of DHG, factor IX R170A (Figure 6B) demonstrated increased peak thrombin concentration (1.5-fold) and velocity index (2-fold), while the response for factor IX R233A (Figure 6C) was blunted and delayed relative to wild-type protease, as previously described.19 Minimal thrombin response was noted in the absence of factor IX. For all 3 recombinant factor IX proteins, addition of increasing DHG concentrations to factor IX–deficient plasma resulted in a progressive reduction in peak thrombin, and prolongation of the time to peak thrombin concentration triggered by 0.2 pM TF. In the presence of factor IX WT, plasma thrombin generation was completely inhibited by 5 μM DHG, and the mean EC50 for inhibition of the velocity index was 0.38 (± 0.01) μM (Figure 6D and Table 2), similar to plasma-derived factor IX. In the presence of factor IX R170A, up to 10 μM DHG failed to completely suppress thrombin generation, and the mean EC50 for inhibition of the velocity index was more than 2-fold higher than in the presence of factor IX WT (Figure 6D and Table 2). In the presence of factor IX R233A, the magnitude of baseline thrombin generation was reduced relative to factor IX WT, but demonstrated remarkable resistance to inhibition by DHG (Figure 6C). The mean EC50 for inhibition of the velocity index was more than 9-fold higher than in the presence of factor IX WT (Figure 6D and Table 2).
Effect of recombinant factor IX mutants on DHG inhibition of plasma thrombin generation. Thrombin generation was initiated with 0.2 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor IX–deficient plasma supplemented with 100% (90 nM) recombinant factor IX WT (A), R170A (B), and R233A (C) in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). Control reactions are: no TF or factor IX (▾), 0.2 pM TF only (▿), and 90 nM recombinant factor IX only ( ). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (D). Representative curves are presented.
). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (D). Representative curves are presented.
Effect of recombinant factor IX mutants on DHG inhibition of plasma thrombin generation. Thrombin generation was initiated with 0.2 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor IX–deficient plasma supplemented with 100% (90 nM) recombinant factor IX WT (A), R170A (B), and R233A (C) in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). Control reactions are: no TF or factor IX (▾), 0.2 pM TF only (▿), and 90 nM recombinant factor IX only ( ). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (D). Representative curves are presented.
). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Fluorogenic assay for detection of plasma thrombin generation” to determine the EC50 for inhibition (D). Representative curves are presented.
EC50 (± SEM) for DHG inhibition of the mean velocity index for thrombin generation in factor IX–deficient plasma (μM)
| Protein . | Factor IX(a) WT . | Factor IX(a) R170A . | Factor IX(a) R233A . |
|---|---|---|---|
| Zymogen | 0.38 ± 0.01 | 0.86 ± 0.06 | 3.55 ± 0.47 |
| Protease | 0.38 ± 0.01 | 1.02 ± 0.02 | 2.98 ± 0.64 |
| Protein . | Factor IX(a) WT . | Factor IX(a) R170A . | Factor IX(a) R233A . |
|---|---|---|---|
| Zymogen | 0.38 ± 0.01 | 0.86 ± 0.06 | 3.55 ± 0.47 |
| Protease | 0.38 ± 0.01 | 1.02 ± 0.02 | 2.98 ± 0.64 |
Thrombin generation was initiated with 0.2 pM human TF, 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor IX–deficient plasma supplemented with 100% (90 nM) recombinant factor IX WT, R170A, and R233A in the presence of increasing DHG (0-10 μM). For factor IXa–initiated assays, TF was omitted, and 100 pM recombinant factor IXa was added. Duplicate wells were averaged to determine individual thrombin generation curves. The relative velocity index was plotted versus DHG concentration, and EC50 values for each replicate (n = 3) were determined as described in “Fluorogenic assay for detection of plasma thrombin generation.” The mean and standard error of the EC50 values were determined from the 3 independent replicates.
Statistical significance analysis: EC50 values for each zymogen were not significantly different from their respective protease (Student t test, P > .2). EC50 values for factor IX(a) R170A and R233A were significantly different from factor IX(a) WT (Student t test, P ≤ .01). EC50 values for factor IX(a) R170A were significantly different from factor IX(a) R233A (Student t test, P < .02).
Effect of mutations in the heparin-binding exosite of recombinant factor IXa on the ability of DHG to inhibit factor IXa triggered plasma thrombin generation
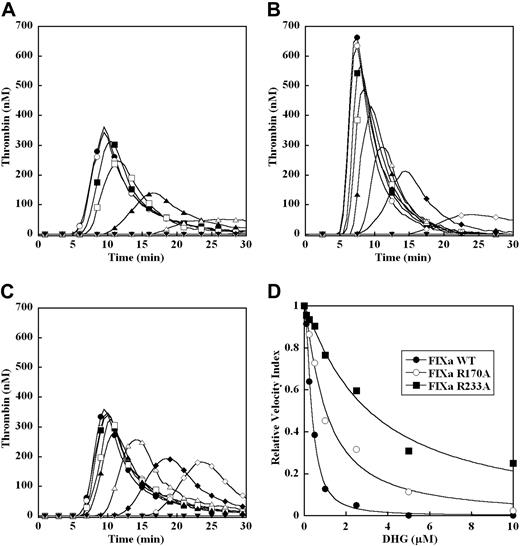
To exclude glycosaminoglycan effects on the activation of factor IX by the TF-factor VIIa complex, the ability of DHG to inhibit thrombin generation was evaluated in factor IX–deficient plasma triggered by 100 pM recombinant factor IXa WT, R170A, and R233A (Figure 7A-C). Under these conditions, factor IXa WT supported a slightly delayed but similar magnitude of thrombin generation to that triggered by 0.2 pM TF in the presence of 100% levels of the zymogen factor IX (Figure 7A). In the absence of factor IXa, no significant thrombin generation was observed. In the absence of DHG, factor IXa R170A demonstrated a 2-fold increase in peak thrombin concentration and up to 2-fold increase in velocity index (Figure 7B), while the response for factor IXa R233A (Figure 7C) was similar to WT protease. Addition of increasing DHG concentrations to factor IX–deficient plasma again resulted in a progressive reduction in peak thrombin, and prolongation of the time to peak thrombin concentration in all cases. In the presence of factor IXa WT, the mean EC50 for inhibition of the velocity index by DHG was essentially identical to that observed for the factor IX WT zymogen (Figure 7D and Table 2). In the presence of factor IXa R170A, the enhanced level of thrombin generation demonstrated increased resistance to DHG, with a mean EC50 for inhibition of the velocity index nearly 3-fold higher than the WT protease (Figure 7D and Table 2). In the presence of factor IXa R233A, marked resistance to inhibition of plasma thrombin generation by DHG was observed, with a mean EC50 for inhibition of the velocity index nearly 8-fold higher than factor IXa WT (Figure 7D and Table 2).
Effect of recombinant factor IXa mutants on DHG inhibition of plasma thrombin generation. Thrombin generation was initiated with 100 pM recombinant factor IXa WT (A), R170A (B), or R233A (C), 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor IX–deficient plasma in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). Control reactions without recombinant factor IXa are presented (▾). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Methods” to determine the EC50 for inhibition (D). Representative curves are presented.
Effect of recombinant factor IXa mutants on DHG inhibition of plasma thrombin generation. Thrombin generation was initiated with 100 pM recombinant factor IXa WT (A), R170A (B), or R233A (C), 8.3 μM PC:PS vesicles, and 40 μg/mL CTI (plasma concentrations) in factor IX–deficient plasma in the presence of increasing DHG: 0 μM (●), 0.1 μM (○), 0.25 μM (■), 0.5 μM (□), 1 μM (▴), 2.5 μM (▵), 5 μM (♦), 10 μM (◇). Control reactions without recombinant factor IXa are presented (▾). The time course of thrombin generation was measured as described in “Fluorogenic assay for detection of plasma thrombin generation.” Thrombin generation curves represent the mean thrombin concentration over the first 30 minutes from replicate determinations (n = 3) and are identified by representative points. The relative velocity index for thrombin generation was plotted versus DHG concentration for each condition, and data were fit as described in “Methods” to determine the EC50 for inhibition (D). Representative curves are presented.
Discussion
DHG is a fucosylated chondroitin sulfate that demonstrates antithrombin-independent antithrombotic activity in animal models of thrombosis, with apparent reduced bleeding risk relative to equitherapeutic heparin doses.3,,–6 Although several in vitro activities have been described, the relevant mechanism for the antithrombotic efficacy of DHG was previously undefined. To determine the relevant mechanism of action, the effect of DHG on TF triggered thrombin generation in human plasma was examined. Plasma thrombin generation has been proposed as a useful phenotypic characterization of thrombotic tendency, and accumulating evidence suggests that increased magnitude of thrombin generation is associated with elevated risk of recurrent or unprovoked venous thrombosis.26,,,,–31 Our results demonstrate that DHG inhibits plasma thrombin generation in a serpin-independent manner, by interacting with the heparin-binding exosite on the factor IXa protease domain.
The magnitude of thrombin generation in factor IX–deficient plasma was dependent on addition of factor IX when triggered by limiting (0.2 pM) TF and independent of factor IX when triggered by excess (4 pM) TF (Figure 1). These results are consistent with previous characterizations of thrombin generation as dependent on the intrinsic tenase complex in the presence of limiting or dilute TF.12,13,32 Furthermore, Western blot analysis demonstrated that substantial prothrombin persisted throughout the thrombin time course triggered by limiting TF, whereas rapid and complete prothrombin depletion occurred with excess TF (Figure 4A-B). Thus, the time course of plasma thrombin generation triggered by excess TF was both independent of the intrinsic tenase (factor IXa-factor VIIIa) complex and influenced by substrate depletion, suggesting limited physiologic relevance. However, comparison of TF concentrations provided insight into the mechanism of DHG action in plasma. DHG inhibited the velocity index (slope) of plasma thrombin generation with 13-fold higher potency in the presence of limiting versus excess TF, suggesting that the intrinsic tenase complex contributed significantly to the mechanism of action for this glycosaminoglycan (Figures 2C and 3C and Table 1). Likewise, Western blot analysis in the presence of limiting TF demonstrated that DHG delayed conversion of prothrombin to free thrombin and TAT complex, confirming that this glycosaminoglycan inhibits plasma thrombin generation primarily by reducing prothrombin activation rather than accelerating inhibition of thrombin by HCII (Figure 4A).
The contribution of HCII to the inhibition of plasma thrombin generation by DHG was likewise compared in the presence of both limiting and excess TF. With limiting TF, the potency of inhibition by DHG was equivalent (identical EC50 values) in both mock- and HCII-depleted plasma (Figure 2C and Table 1), and thrombin-HCII complex was barely detectable by Western blot analysis at a DHG concentration in excess of the EC50 (Figure 4A), indicating that HCII did not significantly contribute to the inhibition mechanism. In the presence of excess TF, HCII depletion resulted in an additional 2-fold decrease in DHG potency relative to mock-depleted plasma (Figure 3A-C and Table 1), and Western blot analysis demonstrated predominance of thrombin-HCII relative to TAT complex at a DHG concentration in excess of the EC50 (Figure 4B). Thus, HCII contributed modestly to inhibition of plasma thrombin generation when triggered by excess TF, but required substantially higher DHG concentrations. In contrast, HCII did not significantly contribute to inhibition of plasma thrombin generation by DHG in the more physiologic limiting TF condition.
The specific mechanism for DHG inhibition of plasma thrombin generation was further defined by evaluating the effect of this glycosaminoglycan on intrinsic tenase complex assembly versus activity. The EC50 for DHG inhibition of the velocity index for plasma thrombin generation was similar in factor VIII–deficient plasma supplemented with factor VIII or factor VIIIa (Figure 5A-C), and factor IX–deficient plasma supplemented with factor IX or factor IXa (Figure 5D-F) demonstrating that the inhibition mechanism does not involve reduced cofactor or protease activation. To evaluate the molecular target for DHG on the intrinsic tenase complex, the dose response for DHG inhibition of thrombin generation was compared in factor IX–deficient plasma supplemented with factor IX(a) WT, R170A, or R233A (Figures 6 and 7). The rank order of the EC50 for DHG in the presence of either zymogen or protease forms [factor IX(a) R233A > R170A > WT] correlated with heparin affinity (lower to higher), and the resistance to inhibition of intrinsic tenase activity by LMWH or DHG (higher to lower) with purified components (Table 2).11,17 The higher DHG concentrations required for inhibition in plasma likely results from extensive protein-glycosaminoglycan binding, and the less dramatic differences between factor IX(a) mutants and WT likely reflects the substantial influence of plasma inhibitors on the more complex end point of thrombin generation. The EC50 values for zymogen and protease forms of the recombinant proteins were not significantly different (Figures 6D and 7D and Table 2), confirming that factor IX activation is not involved in the DHG inhibition mechanism. Further, while the amount of plasma thrombin generation supported by the mutant factor IXa proteases varied significantly based on cofactor affinity and stability,17,19 these properties did not correlate with the relative resistance to DHG inhibition (Figures 6 and 7). Thus, the factor IXa heparin-binding exosite is the relevant molecular target for DHG inhibition of plasma thrombin generation.
The factor IXa heparin-binding exosite plays an essential role in regulation of the coagulation response. Factor X activation by the intrinsic tenase complex is the rate-limiting step for thrombin generation,12,,–15 and mutations in the heparin-binding exosite regulate the rate of plasma thrombin generation and formation of saphenous vein thrombi in response to FeCl3-induced injury in the mouse.19 This enzymatic exosite interacts with the factor VIIIa A2 domain, forming a relatively unstable protein-protein interaction that is critical for cofactor activation of the protease, and is specifically targeted in vitro by glycosaminoglycans such as DHG and heparin.11,17,18 The recognized mechanisms for glycosaminoglycanmediated regulation of the coagulation response in human plasma are predominantly serpin-dependent.33 The unique serpin-independent mechanism for inhibition of plasma thrombin generation by DHG further supports the critical role of the factor IXa heparin-binding exosite in the regulation of ex vivo and in vivo coagulant responses.19 Furthermore, this mechanism may potentially be used by other glycosaminoglycans that bind factor IXa with sufficient affinity. Therapeutic concentrations of unfractionated and low molecular-weight heparin inhibit factor X activation by the intrinsic tenase complex in purified systems, but the contribution of this mechanism to antithrombotic efficacy is unclear due to their concomitant antithrombin-dependent activities in plasma.34,35 The lack of therapeutically relevant serpin-dependent mechanisms for the fucosylated chondroitin sulfate DHG provides proof of principle that the factor IXa heparin binding exosite is a novel antithrombotic target.
The major limiting factor in the application of current antithrombotic agents to human disease remains the relatively narrow therapeutic range and increased risk of bleeding, especially in high-risk populations. In the setting of atrial fibrillation and venous thromboembolic disease, extended use of the antithrombin-dependent pentasaccharide idraparinux has been associated with an increased risk of major hemorrhage relative to vitamin K antagonists.36,37 In contrast, animal models suggest that targeting of the intrinsic tense complex may improve the risk/benefit ratio of antithrombotic therapy. Treatment with active site-blocked factor IXai in canine coronary thrombosis, murine stroke, or baboon cardiopulmonary bypass models is as effective as unfractionated heparin (UFH), and demonstrates a significant reduction in template bleeding or blood loss.38,–40 Similarly, monoclonal antibody versus the factor IXa Gla domain is at least as effective as LMWH in a rat carotid thrombosis model, with markedly reduced activated partial thromboplastin time (APTT) prolongation and blood loss with injury.41,42 These animal models suggest that selective inhibition of factor IX(a), in the presence of intact TF-induced coagulation, may reduce bleeding risk. Likewise, targeting enzymatic exosites is an important strategy that may enhance safety by providing a more graded effect on function, as opposed to the all or none nature of active site inhibition. Thus, targeting the intrinsic tenase complex via the antithrombin-independent, exosite-mediated inhibition mechanism demonstrated for DHG represents a promising approach to antithrombotic therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This research was supported by National Institutes of Health grant no. HL080452 (J.P.S).
We would like to thank Darrel Stafford for providing the factor IX R170A cell line, Kazuhisa Minamiguchi of Taiho Pharmaceuticals (Saitama, Japan) for DHG, Andreas Mueller-Beckhaus of Bayer HealthCare LLC for recombinant factor VIII (Kogenate FS); and Technoclone Ltd for the Technothrombin TGA software for analysis of plasma thrombin generation.
National Institutes of Health
Authorship
Contribution: Y.B. performed the research, analyzed and interpreted data, and wrote the manuscript; and J.P.S. designed the research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John P. Sheehan, Department of Medicine/Hematology, University of Wisconsin, 1300 University Ave, Medical Sciences Center, Rm 4285, Madison, WI 53706; e-mail: jps@medicine.wisc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal