Abstract
Severe congenital neutropenia (CN) is a heterogeneous disorder of myelopoiesis which follows an autosomal dominant or autosomal recessive pattern of inheritance. Genetic analyses indicate mutations in the ELA2 gene in most patients. We have identified LEF-1 as a decisive transcription factor in granulopoiesis controlling proliferation and granulocytic differentiation by direct activation of its target gene, C/EBPα. In patients with CN, the expression of LEF-1 and C/EBPα was abrogated in myeloid progenitors leading to maturation arrest of granulopoiesis. In the present study we demonstrated that ELA2 mRNA expression in myeloid progenitors and plasma protein levels of neutrophil elastase (NE) were markedly reduced in patients with CN harboring mutations in either ELA2 or HAX-1 genes. The ELA2 gene promoter is positively regulated by the direct binding of LEF-1 or C/EBPα, documenting the role of LEF1 in the diminished ELA2 expression. We found that transduction of hematopoietic cells with LEF-1 cDNA resulted in the up-regulation of ELA2/NE synthesis, whereas inhibition of LEF-1 by shRNA led to a marked reduction in the levels of ELA2/NE. LEF-1 rescue of CD34+ cells isolated from 2 patients with CN resulted in granulocytic differentiation of the cells which was in line with increased levels of functionally active ELA2/NE.
Introduction
Severe congenital neutropenia (CN) is a hematologic disorder characterized by maturation arrest of granulopoiesis in bone marrow (BM) at the promyelocyte/myelocyte stage, absence of mature granulocytes in peripheral blood, and severe recurrent bacterial infections.1,2 CN is a multigene syndrome caused by inherited mutations in several genes in subgroups of patients. Autosomal dominant mutations in the elastase 2 (ELA2) gene, encoding the neutrophil elastase (NE) protein, have been found in approximately 60% of patients with CN.3 Novel autosomal recessive mutations in the HAX1 gene, encoding the mitochondrial protein HAX1, have been identified in approximately 10% to 15% of patients analyzed at the Hannover Medical School.4 A causal connection between these gene mutations and defective granulopoiesis in CN is still unclear. Arrested promyelocytes from patients with CN showed impaired proliferation and differentiation in response to granulocyte colony-stimulating factor (G-CSF) in vitro.5–7 Only daily administered pharmacologic doses of G-CSF (1-100 μg/kg body weight per day) lead to sufficient numbers of granulocytes in the peripheral blood of patients with CN. BM morphology and response to G-CSF are similar in both subtypes of CN patient with mutations in the ELA2 gene or the HAX-1 gene. This finding suggests that similar factors or signaling pathways downstream of ELA2 and HAX1 mutations are involved in the pathologic process, causing the maturation arrest of myelopoiesis in CN.
We have identified lymphoid enhancer-binding factor 1 (LEF-1) as a decisive transcription factor in granulopoiesis controlling proliferation, proper lineage commitment, and granulocytic differentiation by C/EBPα.8 Myeloid progenitors from patients with CN showed a severe down-regulation or even a lack of LEF-1 and its target genes, including C/EBPα, independent of the inheritance of ELA2 or HAX1 mutations. We postulated that the absence of LEF-1 plays a critical role in the defective maturation program of myeloid progenitors in CN. Moreover, it has been shown that LEF-1 and C/EBPα regulate the expression of ELA2 in BM cells by binding to the ELA2 promoter.9 However, the specificity of this regulatory process for particular populations of BM cells, especially for myeloid progenitors, is not clear.
NE is a protease stored in primary granules of neutrophilic granulocytes that are formed during the promyelocytic phase of granulocyte differentiation.10 NE is released after neutrophil activation and can cleave multiple substrates, including cytokines and chemokines (G-CSF and SDF1α)11–13 as well as cell-surface proteins (G-CSFR, VCAM, c-kit, and CXCR4).12,14–17 G-CSF administration induces an increase in the level of NE in BM myeloid cells and the subsequent mobilization of BM stem cells to the peripheral blood.16 By cleavage of the chemokine receptor CXCR4 and its ligand SDF-1α, NE negatively regulates SDF1α/CXCR4 signaling, which plays a crucial role in the retention of hematopoietic cells within the BM and their mobilization to the peripheral blood.14–21 The degree of mobilization positively correlates with the down-regulation of the surface expression of CXCR4 by mobilized cells. The process of G-CSF–dependent stem cell mobilization is disturbed in patients with CN.
In the present study, we compared the expression levels of ELA2 and NE in myeloid cells and plasma from patients with CN carrying either ELA2 or HAX1 mutations with the expression of LEF1. We also investigated the LEF-1–dependent regulation of ELA2/NE in myeloid cells from patients with CN and in the myeloid cell line, U937. Our studies showed that down-regulation of ELA2/NE causes elevated surface expression of CXCR4 by BM cells from patients with CN.
Methods
Patients and controls
Twelve patients with CN harboring either ELA2 (n = 8) or HAX1 (n = 4) mutations; 4 patients with cyclic neutropenia (CyN), and 3 healthy volunteers participated in this study. All patients with neutropenia were treated long-term (> 1 year) with G-CSF in a dose between 2 and 7.5 μg/kg per day at the time of study. None of the patients have developed acute myeloid leukemia or myelodysplastic syndrome so far. Three healthy volunteers received G-CSF in a dose of 5 μg/kg per day for 2 days before BM was taken. BM samples were collected in association with the annual follow-up recommended by the Severe Chronic Neutropenia International Registry. Approval for this study was obtained from the Hannover Medical School's Institutional Review Board, and informed consent was obtained in accordance with the Declaration of Helsinki.
Purification and separation of CD34+ and CD33+ progenitor cells
BM aspirates were obtained from the posterior iliac crest by standard techniques. BM mononuclear cells were isolated by Ficoll-Hypaque gradient centrifugation (Amersham Bioscience). For shRNA experiments, G-CSF–primed CD34+ cells were harvested by leukapheresis from healthy volunteers. CD33+ and CD34+ cells were positively selected with the use of sequential immunomagnetic labeling and sorting according to the manufacturer's protocol (Miltenyi Biotec Inc). Cells were counted, and viability was assessed by trypan blue dye exclusion. Purity of sorted cells was greater than 96% as tested by FACS analysis and by hematoxylin/eosin staining of cytospin preparations.
Laser-assisted cell picking
Laser-assisted cell picking from BM slides was performed with the use of the PALM Laser-MicroBeam System that enables the contact-free isolation of single cells. The BM slides were fixed and stained with hematoxylin/eosin. The microdissected cells (100 cells per sample) were catapulted into the lid of a 0.5-mL reaction tube with the use of the laser pressure catapulting technique of the instrument. RNA was isolated with TRIzol reagent according to the manufacturer's protocol (Invitrogen) with slight modifications: 10 ng/mL tRNA (Sigma-Aldrich) and 50 ng/mL linear polyacrylamide (LPA; Sigma-Aldrich) were added to the TRIzol reagent to increase the RNA yield. cDNA synthesis was performed with random hexamer priming and MuLV reverse transcriptase (Fermentas).
qRT-PCR
For quantitative real-time reverse transcription PCR (qRT-PCR), we isolated RNA with the use of the QIAGEN RNeasy Mini Kit with the manufacturer's protocol, amplified cDNA with random hexamer primer (Fermentas), and measured mRNA expression with the SYBR green qPCR kit (QIAGEN). Target gene mRNA expression was normalized to β-actin and was represented as arbitrary units (AUs). Primer sequences are available on request.
Western blot analysis
We used the following antibodies: goat polyclonal anti-ELA2 (Santa Cruz Biotechnology Inc), rabbit polyclonal anti-ELA2 (Calbiochem), mouse monoclonal anti–LEF-1 (REMB1; Calbiochem), mouse monoclonal anti–β-actin (Santa Cruz Biotechnology Inc), and secondary anti–mouse or anti–rabbit HRP-conjugated antibody from Santa Cruz Biotechnology Inc. We obtained whole-cell lysates either through lysis of a defined number of cells in lysis buffer or through direct disruption in Laemmli loading buffer followed by brief sonication. We separated proteins by 10% SDS polyacrylamide gel electrophoresis and probed the blots either 1 hour at 24°C or overnight at 4°C.
Analysis of the NE protein by ELISA
The concentrations of NE in plasma and in culture supernatants were assayed in duplicate in appropriately diluted samples with an NE-specific enzyme-linked immunoabsorbent assay (ELISA) kit (Hycult Biotechnology). The detection limit of the assay was 0.4 ng/mL (NE).
FACS analysis of CXCR4 surface expression on BM CD34+ cells
The following antibodies were used: primary unconjugated rat anti-CXCR4 (a gift of Prof R. Förster, Institute of Immunology, Hannover Medical School, Hannover, Germany), secondary APC-conjugated anti–rat antibody. Isotype control mAbs with irrelevant specificities were obtained from Immunotech. Expression of CXCR4 was measured with a FACScan flow cytometer (Becton Dickinson).
Chemotaxis assay
Chemotaxis assay was used to detect CXCR4/SDF1α-dependent chemotactic activity of BM CD34+ cells in vitro. CD34+ cells from 4 patients with CN treated with G-CSF and 2 healthy volunteers treated with G-CSF were suspended at 5 × 106 cells/mL of RPMI 1640 medium and 0.5% BSA. CD34+ cell suspension (100 μL) was placed into the insert of a Transwell chemotaxis chamber, and the bottom well was filled with 600 μL RPMI/0.5% BSA (negative control) or the same medium supplemented with 10 ng/mL SDF1α. Inserts were transferred to the lower chambers and incubated at 37°C and 6% CO2 for 6 hours. When indicated, BM cells were preincubated with 100 μg/mL purified human NE. After the incubation, 50 μL of 70 mM EDTA solution was added into the lower chambers to release adherent cells from the lower surface of the membrane and from the bottom of the well. Plates were further incubated for 30 minutes at 4°C, inserts were removed, and the transmigrated cells were vigorously suspended and counted with a FACSCalibur for 1 minute at 60 μL/min with gating on forward scatter and side scatter. Migration of CD34+ cells from the insert to the bottom well was calculated as the percentage of total cells loaded into the upper chamber.
Transduction and G-CSF stimulation of CD34+ cells from 2 patients with CN
LEF-1 cDNA synthesis and construction of LEF-1–GFP lentiviral vectors, anti–LEF-1 shRNA synthesis and construction of shRNA-RFP containing lentiviral vectors, as well as preparation of recombinant lentiviral supernatants and lentiviral transduction of the myeloid cell lines and primary CD34+ cells, has been described previously.8 For the G-CSF stimulation experiment, sorted GFP+ (for LEF-1 lv) or RFP+ (for anti–LEF-1 shRNA) CD34+ cells were cultured for 4 days in 24-well tissue-culture plates (Costar; 2 × 105 cells/well) in X-vivo medium supplemented with 1% heat-inactivated autologous human serum with or without the addition of 10 ng/mL rhG-CSF. For LEF-1 dose-dependent experiments, GFP+ cells were sorted by FACSCanto flow cytometer dependent on low, medium, or high intensity of GFP. Alternatively, transduced and sorted U937 cells were cultured in RPMI/10% FCS medium.
Determination of ELA2 mRNA stability
Serum-deprived THP1 and NB4 myeloid cell lines were treated with or without G-CSF (10 ng/mL) for 24 hours. Measurements of ELA2 mRNA stability were performed by inhibiting transcription with actinomycin D (5 μg/mL; catalog no. A4262, Sigma-Aldrich) for the indicated time points. Amount of ELA2 mRNA was quantified with qRT-PCR.
Statistical analysis
Statistical analysis was performed with the SPSS Version 9.0 statistical package (SPSS Inc). To analyze differences in mean values between groups, a 2-sided unpaired Student t test was used.
Results
Abrogated levels of ELA2 mRNA in CD33+ myeloid progenitors and of NE in plasma of patients with CN harboring either ELA2 or HAX1 mutations
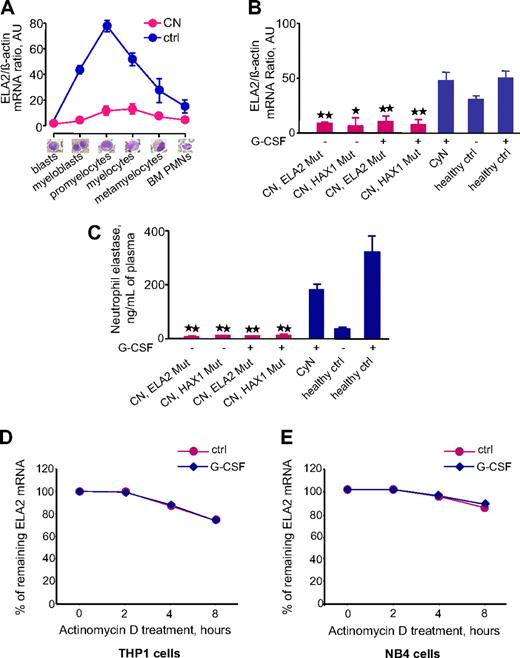
To evaluate the ELA2 mRNA expression profile during granulocyte differentiation of hematopoietic cells, we performed laser-assisted single-cell isolation of defined populations of hematopoietic cells in BM smears from patients with CN and healthy volunteers and measured ELA2 mRNA levels in defined cells. We found the highest ELA2 mRNA levels in promyelocytes from healthy volunteers but very low levels of ELA2 in these cells from patients with CN (Figure 1A). Analysis of ELA2 mRNA expression in CD33+ myeloid progenitors showed G-CSF–dependent up-regulation of ELA2 mRNA in healthy volunteers. By comparison, the CD33+ cells from patients with CN showed markedly lower ELA2 mRNA levels that were not up-regulated by G-CSF (Figure 1B). In contrast, ELA2 mRNA expression in CD33+ cells from patients with CyN was comparable with that of healthy volunteers (Figure 1B). Moreover, both groups of patients with CN, carrying ELA2 or HAX1 mutations, had comparable low levels of ELA2 mRNA (Figure 1B). Consistent with the low level of ELA2 mRNA, we found markedly diminished amounts of NE protein in the plasma from both groups of patients with CN, compared with G-CSF–treated healthy volunteers and patients with CyN (Figure 1C). Furthermore, neutrophils from patients with CN failed to release the NE protein after in vitro stimulation with G-CSF (data not shown).
ELA2 mRNA expression in myeloid cells and NE protein levels in plasma are severely down-regulated in patients with CN. (A) ELA2 mRNA expression in BM cells at different stages of granulopoiesis. Different cell populations were isolated from BM smears from 3 healthy volunteers (blue line) and 4 patients with CN (red line). ELA2 mRNA expression was measured by qRT-PCR normalized to β-actin and is presented as arbitrary units (AUs); data represent means ± SDs of triplicates. (B) ELA2 mRNA expression in CD33+ cells from studied groups: 8 patients with CN harboring ELA2 mutations (CN, ELA2 Mut), 4 patients with CN with HAX1 mutations (CN, HAX1 Mut), 4 patients with CyN, 5 healthy volunteers without G-CSF treatment (ctrl), and 3 healthy volunteers treated with G-CSF (ctrl). ELA2 mRNA expression is normalized to β-actin (and is presented as AUs) l data represent means ± SDs measured in triplicates (*P < .05; **P < .01). (C) NE plasma levels were measured in different groups of patients and healthy volunteers indicated above, using NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate; **P < .01. (D-E) Analysis of the ELA2 mRNA stability in THP1 (D) and NB4 (E) myeloid cell lines after inhibition of mRNA transcription by actinomycin D in the absence (red, ctrl) or presence (blue, G-CSF) of G-CSF. The data represent the percentage of remaining ELA2 mRNA after actinomycin D treatment for the indicated time points, compared with initial ELA2 mRNA amounts. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate.
ELA2 mRNA expression in myeloid cells and NE protein levels in plasma are severely down-regulated in patients with CN. (A) ELA2 mRNA expression in BM cells at different stages of granulopoiesis. Different cell populations were isolated from BM smears from 3 healthy volunteers (blue line) and 4 patients with CN (red line). ELA2 mRNA expression was measured by qRT-PCR normalized to β-actin and is presented as arbitrary units (AUs); data represent means ± SDs of triplicates. (B) ELA2 mRNA expression in CD33+ cells from studied groups: 8 patients with CN harboring ELA2 mutations (CN, ELA2 Mut), 4 patients with CN with HAX1 mutations (CN, HAX1 Mut), 4 patients with CyN, 5 healthy volunteers without G-CSF treatment (ctrl), and 3 healthy volunteers treated with G-CSF (ctrl). ELA2 mRNA expression is normalized to β-actin (and is presented as AUs) l data represent means ± SDs measured in triplicates (*P < .05; **P < .01). (C) NE plasma levels were measured in different groups of patients and healthy volunteers indicated above, using NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate; **P < .01. (D-E) Analysis of the ELA2 mRNA stability in THP1 (D) and NB4 (E) myeloid cell lines after inhibition of mRNA transcription by actinomycin D in the absence (red, ctrl) or presence (blue, G-CSF) of G-CSF. The data represent the percentage of remaining ELA2 mRNA after actinomycin D treatment for the indicated time points, compared with initial ELA2 mRNA amounts. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate.
To evaluate the effects of G-CSF on ELA2 mRNA stability, we inhibited mRNA synthesis in the THP1 and NB4 myeloid cell lines with the use of actinomycin D and compared the amount of remaining mRNA after 2, 4, and 8 hours in G-CSF–stimulated and unstimulated groups of cells. We observed that ELA2 mRNA declined only up to 74.1% in THP1 cells and only up to 84.7% in NB4 cells (Figure 1D-E). We further showed that G-CSF treatment does not alter the ELA2 mRNA stability in both cell lines (Figure 1D-E).
Together, our results show that the levels of ELA2 mRNA and NE protein are dramatically down-regulated in patients with CN, irrespective of gene mutation status.
LEF-1 rescue of CD34+ cells of patients with CN with ELA2 or HAX1 mutations results in elevated ELA2 mRNA and NE protein synthesis
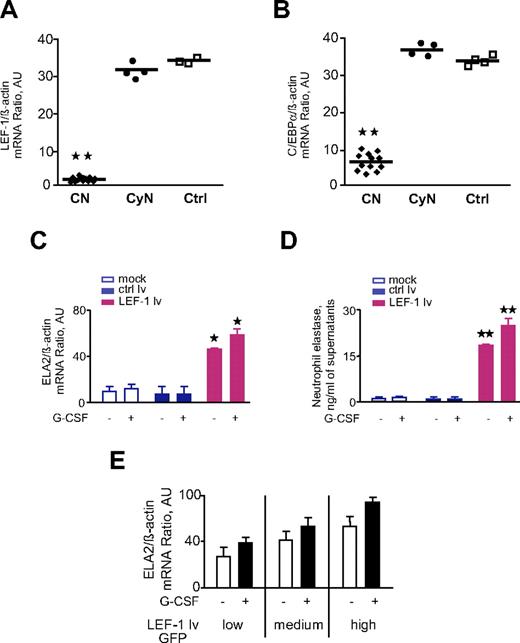
Previously, we have shown that the LEF-1 transcription factor is crucial for neutrophil granulocytopoiesis.8 We found that C/EBPα is a target gene of LEF-18 and that mRNA expression of both LEF-1 and C/EBPα is markedly reduced in patients with CN (Figure 2A-B).8 LEF-1 and C/EBPα regulate ELA2 by binding to the ELA2 gene promoter.9 Because LEF-1 and ELA2 are expressed at maximum levels in promyelocytes, we hypothesized that the lack of ELA2 expression in CN may be because of the absence of LEF-1. To test this hypothesis, we transduced CD34+ cells from 2 patients with CN with LEF-1–expressing lentiviral vector and measured ELA2 mRNA and NE protein expression. We found up-regulation of ELA2 mRNA and elevated synthesis of NE protein in LEF-1–rescued cells of patients with CN, which was even higher after in vitro stimulation of these cells with G-CSF (Figure 2C-D). LEF-1–dependent up-regulation of ELA2 mRNA expression occurs in a dose-dependent manner (Figure 2E). Moreover, previously we have shown that restoration of LEF-1 expression led to normalization of the in vitro granulocytic differentiation of hematopoietic progenitors from patients with CN.8 The findings of the present study confirmed that ELA2/NE is involved in this process.
LEF-1 rescue of CD34+ cells from 2 patients with CN restores diminished ELA2/NE synthesis. (A-B) mRNA expression of LEF-1 (A) and C/EBPα (B) in BM CD33+ cells of studied groups: CN, severe congenital neutropenia; CyN, cyclic neutropenia; Ctrl, control; all treated with G-CSF. Data represent means ± SDs and were measured in triplicate; **P < .01 compared with Ctrl. (C-E) CD34+ cells from 2 patients with CN were transduced with LEF-1 lv GFP or ctrl lv GFP and subsequently incubated without or with G-CSF for 4 days. (C) ELA2 mRNA expression was measured by qRT-PCR normalized to β-actin and is presented as AUs; data represent means ± SDs of 2 experiments measured in triplicates (*P < .05 to ctrl lv samples). (D) NE protein secretion into culture supernatants was assessed with the use of NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (**P < .01 to ctrl lv samples). (E) CD34+ cells from 2 patients with CN were transduced with LEF-1 lv GFP; on day 2 GFP+ cells were sorted depending on the intensity of GFP expression (low, medium, high) and subsequently incubated without or with G-CSF for 4 days. ELA2 mRNA expression was measured by qRT-PCR normalized to β-actin and is presented as AUs; data represent means ± SDs of 2 independent experiments each measured in triplicates.
LEF-1 rescue of CD34+ cells from 2 patients with CN restores diminished ELA2/NE synthesis. (A-B) mRNA expression of LEF-1 (A) and C/EBPα (B) in BM CD33+ cells of studied groups: CN, severe congenital neutropenia; CyN, cyclic neutropenia; Ctrl, control; all treated with G-CSF. Data represent means ± SDs and were measured in triplicate; **P < .01 compared with Ctrl. (C-E) CD34+ cells from 2 patients with CN were transduced with LEF-1 lv GFP or ctrl lv GFP and subsequently incubated without or with G-CSF for 4 days. (C) ELA2 mRNA expression was measured by qRT-PCR normalized to β-actin and is presented as AUs; data represent means ± SDs of 2 experiments measured in triplicates (*P < .05 to ctrl lv samples). (D) NE protein secretion into culture supernatants was assessed with the use of NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (**P < .01 to ctrl lv samples). (E) CD34+ cells from 2 patients with CN were transduced with LEF-1 lv GFP; on day 2 GFP+ cells were sorted depending on the intensity of GFP expression (low, medium, high) and subsequently incubated without or with G-CSF for 4 days. ELA2 mRNA expression was measured by qRT-PCR normalized to β-actin and is presented as AUs; data represent means ± SDs of 2 independent experiments each measured in triplicates.
Hematopoietic progenitor cells of patients with CN showed elevated CXCR4 surface expression and enhanced CXCR4-dependent chemotaxis
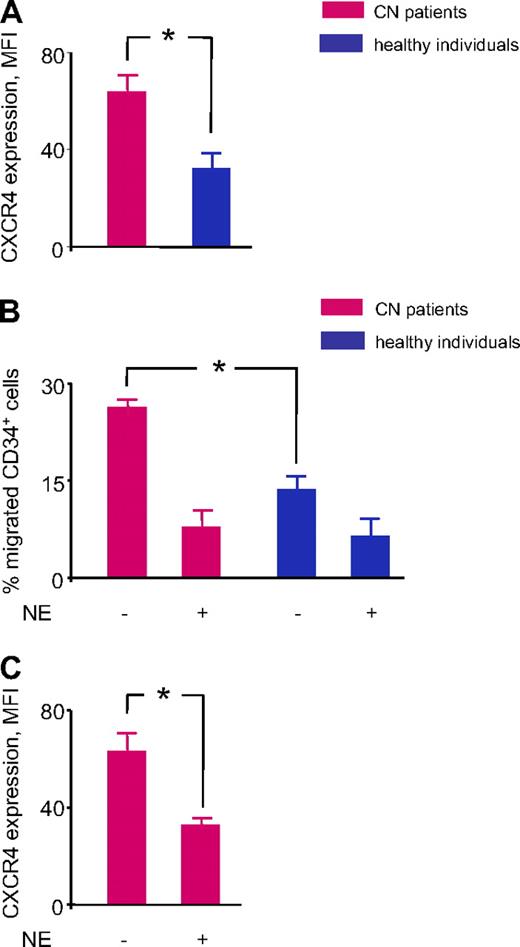
NE is elevated in the BM of healthy persons treated with G-CSF. One of the known functions of NE is that it cleaves CXCR4 expressed on the surface of CD34+ cells.20 Because the synthesis of NE is markedly down-regulated in patients with CN, we measured as a functional readout the surface expression of CXCR4 in CD34+ BM cells from patients with CN treated with G-CSF and compared the levels of expression with CD34+ cells from healthy volunteers treated with G-CSF. As expected, CXCR4 surface expression was up-regulated in CD34+ cells from patients with CN because of the lack of functional NE protein (Figure 3A). Next, we analyzed the functional activity of up-regulated CXCR4 receptors. We compared the ability of CD34+ cells from patients with CN and healthy volunteers to migrate toward the CXCR4 ligand, SDF-1α, by using an in vitro chemotaxis assay. We found that the percentage of migrated CD34+ cells was markedly higher in the CN group, which is consistent with the up-regulated levels of CXCR4 and indicates that the CXCR4 receptors are functionally active (Figure 3B). Because preincubation with functionally active NE induces a marked reduction in the migratory ability of CD34+ cells from patients with CN, which is consistent with the reduced expression of CXCR4 (Figure 3C), we conclude that this process is NE specific. On the basis of these findings, we conclude that the abolished G-CSF–dependent synthesis of NE in patients with CN results in the abnormally high levels of CXCR4 in BM cells.
Elevated CXCR4 expression and increased CXCR4-mediated chemotaxis toward SDF1α in BM CD34+ cells of patients with CN. (A) We measured CXCR4 surface expression in BM CD34+ cells from 4 patients with CN treated with G-CSF and 2 healthy volunteers treated with G-CSF with the use of rat anti–human CXCR4 antibody. Data presented as mean fluorescence intensity (MFI) of CXCR4 staining. Data represent means ± SDs of triplicates (*P < .05). (B) We analyzed chemotactic activity of BM CD34+ cells isolated from 4 patients with CN treated with G-CSF and 2 healthy volunteers treated with G-CSF with the use of Transwell migration assays, as described in “Methods.” The cells were additionally treated or not with 100 μg/mL of purified human NE. Results are expressed as the percentage of CD34+ cells loaded into the upper chamber that had migrated to the bottom well toward 10 ng/mL of SDF1α. Data represent means ± SDs of triplicates (*P < .05). (C) We assessed CXCR4 surface expression on BM CD34+ cells from 4 patients with CN treated with G-CSF which were incubated without or with NE and migrated in the low compartment of a Transwell chamber toward 10 ng/mL of SDF1α. Data presented as mean fluorescence intensity (MFI) of CXCR4 staining. Data represent means ± SDs of triplicates (*P < .05).
Elevated CXCR4 expression and increased CXCR4-mediated chemotaxis toward SDF1α in BM CD34+ cells of patients with CN. (A) We measured CXCR4 surface expression in BM CD34+ cells from 4 patients with CN treated with G-CSF and 2 healthy volunteers treated with G-CSF with the use of rat anti–human CXCR4 antibody. Data presented as mean fluorescence intensity (MFI) of CXCR4 staining. Data represent means ± SDs of triplicates (*P < .05). (B) We analyzed chemotactic activity of BM CD34+ cells isolated from 4 patients with CN treated with G-CSF and 2 healthy volunteers treated with G-CSF with the use of Transwell migration assays, as described in “Methods.” The cells were additionally treated or not with 100 μg/mL of purified human NE. Results are expressed as the percentage of CD34+ cells loaded into the upper chamber that had migrated to the bottom well toward 10 ng/mL of SDF1α. Data represent means ± SDs of triplicates (*P < .05). (C) We assessed CXCR4 surface expression on BM CD34+ cells from 4 patients with CN treated with G-CSF which were incubated without or with NE and migrated in the low compartment of a Transwell chamber toward 10 ng/mL of SDF1α. Data presented as mean fluorescence intensity (MFI) of CXCR4 staining. Data represent means ± SDs of triplicates (*P < .05).
LEF-1 controls the levels of ELA2 mRNA and synthesis of NE in the myeloid cell line U937 and in CD34+ hematopoietic cells in a dose-dependent manner
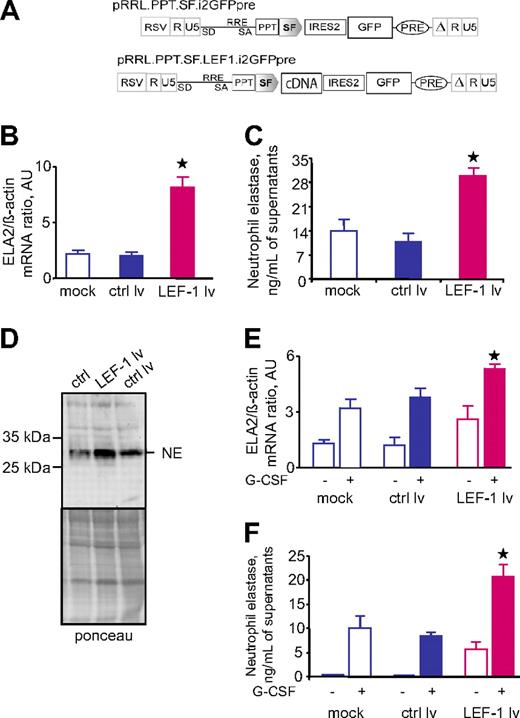
We evaluated whether the effect of LEF-1 on ELA2 and NE is only specific for hematopoietic cells from patients with CN or whether it is a general mechanism in myelopoiesis. We transduced the myeloid cell line U937 with a lentiviral expression vector containing LEF-1 cDNA and GFP as a marker (Figure 4A). We found that overexpression of LEF-1 in the U937 cell line led to the marked up-regulation of ELA2 mRNA, which is consistent with the elevated levels of intracellular NE and NE secreted into the culture medium, compared with untransduced cells or cells transduced with the control lentiviral construct containing GFP but not LEF-1 cDNA (Figure 4B-D). Again, LEF-1 activated ELA2 and NE in a dose-dependent manner (data not shown). Similar results were observed after transduction of CD34+ hematopoietic cells with the LEF-1–GFP construct (Figure 4E-F; data not shown).
Transduction of hematopoietic cells with lentiviral construct contained LEF-1 cDNA led to up-regulation of ELA2/NE. The myeloid cell line U937 was transduced with lentiviral construct with LEF-1 cDNA and green fluorescence protein (GFP) (LEF-1 lv) or only GFP (ctrl lv; A). On day 4 of culture, GFP+ cells were sorted. (B) ELA2 mRNA expression in indicated groups, as measured by qRT-PCR normalized to β-actin, is presented as arbitrary units (AUs); data represent means ± SDs of triplicates (*P < .05, to ctrl lv samples). (C) NE protein secretion into culture supernatants was assessed with the use of NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (*P < .05, to ctrl lv samples). (D) Representative Western blot images showing NE protein expression in total lysates from U937 cells transduced with LEF-1 lv, ctrl lv, or from untransduced U937 cells. Ponceau staining is depicted as a control of the amounts of loaded proteins. (E-F) CD34+ cells from 2 healthy volunteers were transduced with LEF-1 lv GFP or ctrl lv GFP and subsequently incubated without or with G-CSF for 4 days; ELA2 mRNA expression (E) was measured by qRT-PCR normalized to β-actin and is presented as arbitrary units (AUs); data represent means (± SDs) of 2 experiments each measured in triplicates (*P < .05 to G-CSF stimulated ctrl lv samples). NE protein secretion into culture supernatants (F) was assessed with the use of NE-specific ELISA; data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (*P < .05 to G-CSF–stimulated ctrl lv samples).
Transduction of hematopoietic cells with lentiviral construct contained LEF-1 cDNA led to up-regulation of ELA2/NE. The myeloid cell line U937 was transduced with lentiviral construct with LEF-1 cDNA and green fluorescence protein (GFP) (LEF-1 lv) or only GFP (ctrl lv; A). On day 4 of culture, GFP+ cells were sorted. (B) ELA2 mRNA expression in indicated groups, as measured by qRT-PCR normalized to β-actin, is presented as arbitrary units (AUs); data represent means ± SDs of triplicates (*P < .05, to ctrl lv samples). (C) NE protein secretion into culture supernatants was assessed with the use of NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (*P < .05, to ctrl lv samples). (D) Representative Western blot images showing NE protein expression in total lysates from U937 cells transduced with LEF-1 lv, ctrl lv, or from untransduced U937 cells. Ponceau staining is depicted as a control of the amounts of loaded proteins. (E-F) CD34+ cells from 2 healthy volunteers were transduced with LEF-1 lv GFP or ctrl lv GFP and subsequently incubated without or with G-CSF for 4 days; ELA2 mRNA expression (E) was measured by qRT-PCR normalized to β-actin and is presented as arbitrary units (AUs); data represent means (± SDs) of 2 experiments each measured in triplicates (*P < .05 to G-CSF stimulated ctrl lv samples). NE protein secretion into culture supernatants (F) was assessed with the use of NE-specific ELISA; data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (*P < .05 to G-CSF–stimulated ctrl lv samples).
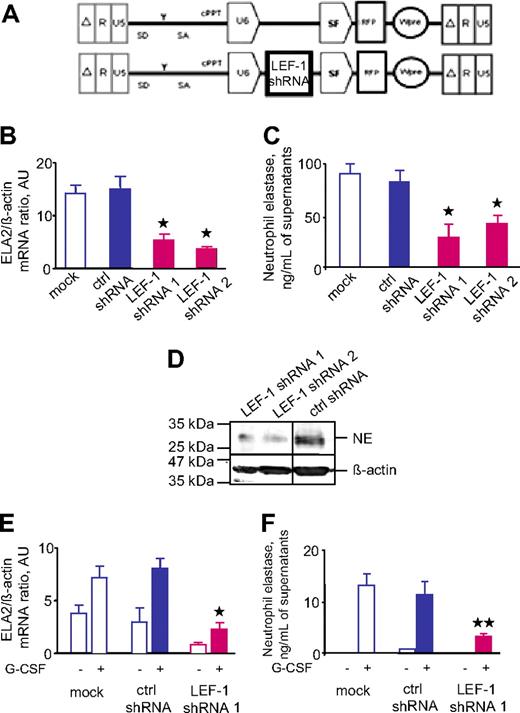
We also evaluated the expression of ELA2 and NE in hematopoietic cells deficient in LEF-1. We inhibited the expression of LEF-1 by transducing the myeloid cell line U937 with a lentiviral vector that expressed LEF-1–specific shRNA and RFP (Figure 5A). We used 2 different LEF-1–specific shRNAs and 1 nonspecific shRNA as a control. Analysis of U937 cells transduced with LEF-1–specific shRNAs showed marked down-regulation of ELA2 mRNA expression, NE protein secretion, and the intracellular NE protein level, compared with control cells (Figure 5B-D). We found similar effects of LEF-1 inhibition in CD34+ hematopoietic cells (Figure 5E-F). Taken together, these data suggest that a LEF-1–dependent mechanism is involved in the regulation of ELA2 and NE in hematopoietic cells.
Transduction of hematopoietic cells with LEF-1 shRNA resulted in down-regulation of ELA2/NE. The myeloid cell line U937 was transduced with lentiviral constructs with red fluorescence protein (RFP) and 2 different LEF-1–specific shRNAs (LEF-1 shRNA 1; LEF-1 shRNA 2) or with irrelevant shRNA (ctrl shRNA; A). On day 4 of culture, we sorted and analyzed RFP+ cells. (B) ELA2 mRNA expression in indicated groups, as measured by qRT-PCR normalized to β-actin and is presented as arbitrary units (AUs); data represent means ± SDs of triplicates (*P < .05 to ctrl shRNA samples). (C) NE protein secretion into culture supernatants was assessed with the use of NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (*P < .05 to ctrl shRNA samples). (D) Representative Western blot images from the same gel and same experiment showing NE protein expression in total lysates from U937 cells transduced with shRNA constructs, as indicated above. β-Actin staining was used as a control of the amounts of loaded proteins. Vertical lines have been inserted to indicate a repositioned gel lane. (E-F) CD34+ cells from 2 healthy volunteers were transduced with lentiviral constructs with RFP and LEF-1–specific shRNA (LEF-1 shRNA 1) or with irrelevant shRNA (ctrl shRNA); ELA2 mRNA expression (E) was measured by qRT-PCR normalized to β-actin and is presented as AUs. Data represent means ± SDs of 2 experiments measured in triplicates (*P < .05 to G-CSF–stimulated ctrl shRNA samples). NE protein secretion into culture supernatants (F) was assessed with the use of NE-specific ELISA; data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (**P < .01 to G-CSF–stimulated ctrl shRNA samples).
Transduction of hematopoietic cells with LEF-1 shRNA resulted in down-regulation of ELA2/NE. The myeloid cell line U937 was transduced with lentiviral constructs with red fluorescence protein (RFP) and 2 different LEF-1–specific shRNAs (LEF-1 shRNA 1; LEF-1 shRNA 2) or with irrelevant shRNA (ctrl shRNA; A). On day 4 of culture, we sorted and analyzed RFP+ cells. (B) ELA2 mRNA expression in indicated groups, as measured by qRT-PCR normalized to β-actin and is presented as arbitrary units (AUs); data represent means ± SDs of triplicates (*P < .05 to ctrl shRNA samples). (C) NE protein secretion into culture supernatants was assessed with the use of NE-specific ELISA. Data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (*P < .05 to ctrl shRNA samples). (D) Representative Western blot images from the same gel and same experiment showing NE protein expression in total lysates from U937 cells transduced with shRNA constructs, as indicated above. β-Actin staining was used as a control of the amounts of loaded proteins. Vertical lines have been inserted to indicate a repositioned gel lane. (E-F) CD34+ cells from 2 healthy volunteers were transduced with lentiviral constructs with RFP and LEF-1–specific shRNA (LEF-1 shRNA 1) or with irrelevant shRNA (ctrl shRNA); ELA2 mRNA expression (E) was measured by qRT-PCR normalized to β-actin and is presented as AUs. Data represent means ± SDs of 2 experiments measured in triplicates (*P < .05 to G-CSF–stimulated ctrl shRNA samples). NE protein secretion into culture supernatants (F) was assessed with the use of NE-specific ELISA; data represent means ± SDs and are derived from 2 independent experiments each measured in triplicate (**P < .01 to G-CSF–stimulated ctrl shRNA samples).
Discussion
In the present study, we found diminished expression of ELA2 mRNA and NE protein in myeloid cells and plasma of patients with CN. Previous studies have reported reduced expression of ELA2 mRNA in the BM of patients with CN.2,3 However, the expression status of ELA2 in the myeloid subpopulation of BM cells was unknown, and the mechanism underlying the reduced expression of ELA2 in patients with CN was not clear. It was also unknown whether ELA2 mutations influenced the expression of ELA2 mRNA or the NE protein directly or both or whether the reduced expression of ELA2 in patients with CN was a result of abnormal ELA2 gene regulation by other factors. Autosomal dominant mutations in the ELA2 gene in patients with CN were initially described in 1999.3 To date, more than 20 different mutations have been identified in the ELA2 gene.22 In some cases, the same ELA2 mutations have been observed in the 2 different syndromes, CN and CyN.3,22,23 ELA2 levels in patients with CyN, in which ELA2 is mutated in most patients, are comparable with the ELA2 levels in healthy persons. Therefore, other factors in addition to ELA2 mutations are responsible for the down-regulation of ELA2 transcription and NE expression in CN. This notion is supported by our data showing that ELA2 expression and NE protein levels in patients harboring HAX1 mutations are similarly abrogated. Moreover, several additional disputed observations suggest that the downstream effects of ELA2 mutations are not the only cause of CN: (1) there is no clear genotype-phenotype correlation, and more than 20 different ELA2 mutations confer widely disparate effects on NE enzymatic activity; (2) analysis of mutated recombinant NE proteins has shown no evident changes in protein stability or substrate specificity and no consistent effects on glycosylation, and proteolytic activity retained some mutants compared with the wild-type protein; and (3) gene targeting of ELA2 has failed to reproduce neutropenic phenotype in mice. Nevertheless, some studies have proposed that mutant ELA2 triggers accelerated apoptosis of granulocyte precursors.24,25 It was shown that ELA2 mutations lead to cytoplasmatic accumulation of the altered protein, disturbance of intracellular trafficking, activation of the unfolded protein response (UPR), and induction of endoplasmic reticulum (ER) stress-triggered apoptosis.24,25 However, it is not clear why ELA2 mutations do not cause UPR in patients with CyN and how HAX1 mutations would induce UPR and ER stress-induced apoptosis. Many unanswered questions remain about ER stress-induced apoptosis. Which molecules, associated with UPR (eg, ATF6, IRE1, PERK) are altered and which caspases are associated with the cell death. Continuous research is still necessary to outline the complexity of UPR. However, this was not the aim of this study.
Previously, we found that the transcription factor LEF-1 plays a decisive role in granulopoiesis by direct activation of the granulocyte-specific transcription factor C/EBPα.8 Like ELA2 mRNA expression, LEF-1 is expressed at the highest levels in BM promyelocytes.8 Both LEF-1 and C/EBPα activate the ELA2 gene by direct binding to the ELA2 promoter.9,26–28 Furthermore, myeloid progenitor cells of patients with CN showed a severe down-regulation or even a lack of LEF-1, C/EBPα, and ELA2/NE expression in both groups, carrying either ELA2 or HAX1 mutations.8 Wang et al28 showed that C/EBPα and G-CSFR signals synergistically induce ELA2 expression. In addition, evidence is clear that G-CSF–dependent granulocytic differentiation is severely affected in patients with CN: (1) serum of these patients contains normal or increased levels of biologically active G-CSF, and G-CSF receptors (G-CSFRs) are expressed by CN myeloid cells at normal levels29,30 ; (2) patients with CN responded only to the regular subcutaneous administration of pharmacologic doses of recombinant human G-CSF (100-1000 times greater than physiologic levels)1,2 ; and (3) in vitro growth of granulocyte colonies in CFU assays is defective with only a few neutrophil colonies formed, despite maximal stimulation with G-CSF.5–7 In U937 cells we have shown that ELA2 expression and NE secretion directly depend on the intracellular levels of LEF-1, inhibition of LEF-1 led to reduced ELA2/NE production, and LEF-1 overexpression markedly enhanced the ELA2/NE levels. In hematopoietic progenitor cells from patients with CN, the in vitro reconstitution of LEF-1 expression by lentivirus-based transduction of LEF-1–GFP cDNA corrected the defective ELA2/NE levels and resulted in the differentiation of these progenitor cells into mature granulocytes. These results suggest that low ELA2/NE levels are involved in the complex multistep pathologic processes that ultimately lead to the maturation arrest of myeloid progenitors from patients with CN downstream of defective LEF-1. The importance of LEF-1 in the regulation of ELA2 is supported by the fact that the levels of ELA2 and NE are unaffected in patients with CyN, in whom the LEF-1 levels were comparable with healthy volunteers.8 However, we cannot exclude the persistence of the other LEF-1–independent mechanisms of ELA2 regulation. As we have shown in Figure 1D and E, one of the possible mechanisms, namely enhancement of ELA2 mRNA stability by G-CSF treatment, is not involved in the control of ELA2 mRNA levels.
The existence of the LEF-1/C/EBPα–independent mechanisms of the regulation of granulopoiesis in patients with CN is supported by the fact that treatment of these patients with G-CSF stimulates granulocytic differentiation even if LEF-1 and C/EBPα are significantly down-regulated. Patients with CN must receive daily pharmacologic doses of G-CSF1,2 to be able to induce additional factors or signaling mechanisms that partially compensate for the deregulated “classical” signaling systems downstream of G-CSFR. Recently, we identified nicotinamide phosphoribosyl transferase (NAMPT), also known as pre–B-cell colony–enhancing factor, as an essential enzyme mediating G-CSF–triggered granulopoiesis in healthy persons and in persons with CN.31 The enzyme NAMPT converts nicotinamide to nicotinamide mononucleotide, which is converted into nicotinamide adenine dinucleotide (NAD+) by nicotinamide mononucleotide adenylyltransferase in the mammalian biosynthetic pathway.9 NAD+ regulates the transcription and function of sirtuins (SIRTs), which are lysine deacetylases for C/EBPα and C/EBPβ.31 Intriguingly, in line with elevated NAMPT levels, amounts of NAD+ and SIRT1 protein in myeloid cells, as well as plasma NAD+ levels, were increased by G-CSF treatment of persons with CN.31 Because in the myeloid cells of patients with CN C/EBPα is severely down-regulated,8 it is not available for binding to SIRT1. However, we found that in contrast to C/EBPα, normal or even elevated amounts of C/EBPβ were expressed in these cells of persons with CN. C/EBPα and C/EBPβ have different functions in granulopoiesis. C/EBPα is responsible for steady state granulopoiesis, whereas C/EBPβ is crucial for cytokine-induced “emergency” granulopoiesis.32 Therefore, we hypothesize that high NAMPT, NAD+, and SIRT1 levels activate a C/EBPβ-dependent emergency pathway of neutrophil granulopoiesis in patients with CN, whereas the steady state regulation by LEF-1 and C/EBPα does not function.8
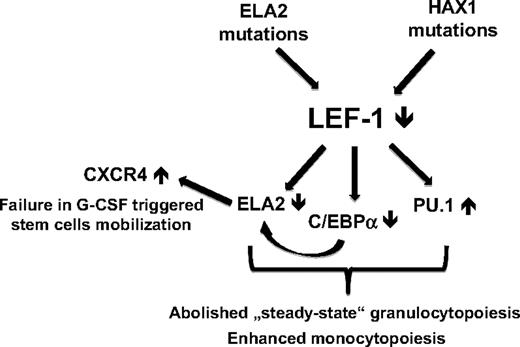
PU.1 is another extensively studied myeloid-specific transcription factor.33,34 The relative levels of PU.1 and C/EBPα in granulocytic-macrophage progenitors have been suggested to regulate monocyte versus neutrophil cell fate choice. Although these factors are known to positively regulate each other in early hematopoietic progenitors, they probably have opposite functions in myelopoiesis. The relative levels of PU.1 and C/EBPα in granulocytic-macrophage progenitors have been suggested to regulate monocyte versus neutrophil cell fate choice.35 Thus, during granulocytic differentiation C/EBPα is up-regulated and PU.1 is repressed (but still expressed at the low levels), and monocytic differentiation occurs if PU.1 is activated and C/EBPα is inhibited, although the underlying molecular mechanism remains obscure. In patients with CN, the myelopoietic maturation program is sharply shifted toward monocytopoiesis (increased levels of monocytes from 2 to 4 times over normal and no granulocytes in peripheral blood), which may be because of the misbalanced C/EBPα/PU.1 expression ratio in their myeloid cells. Indeed, in addition to severely abrogated C/EBPα, PU.1 is slightly up-regulated in myeloid cells from patients with CN.36 Rosenbauer et al37 identified a key upstream regulatory element of PU.1 gene, which contained LEF-1/TCF binding sites. They have shown that LEF-1/TCFs function as transcriptional inhibitors of PU.1 gene expression.37 On the basis of these findings, we conclude that in LEF-1–deficient myeloid cells from patients with CN a disturbed C/EBPα/PU.1 ratio with a strong shift toward PU.1 may play a decisive role in the improper regulation of myelopoiesis with defective granulocytopoiesis and elevated monocytic differentiation.36 This theory was supported by our data showing increased GM-CSF–dependent differentiation of LEF-1–deficient CD34+ cells toward monocytes/macrophages in vitro.8 The aforementioned data suggest the involvement of LEF-1 in the complex regulatory network of the myeloid-specific signaling pathways, which are severely deregulated in patients with CN (Figure 6). However, further experiments are needed to confirm and to understand the ultimate effects of the interaction between these transcription factors on the myeloid lineages decision in CN patients.
The mechanism of dysregulated myeloid differentiation of hematopoietic progenitors in patients with CN. The relative levels of PU.1 and C/EBPα in granulocytic-macrophage progenitors have been suggested to regulate monocyte versus neutrophil cell fate choice. LEF-1 transcription factor could be responsible for the “fine-tuning” of C/EBPα versus PU.1 levels in myeloid cells to induce granulocytic differentiation. LEF-1 transcription factor is absent in myeloid progenitor cells from patients with CN irrespective to mutation status. Therefore, the myelopoietic maturation program in these patients is sharply shifted toward monocytopoiesis. A lack of LEF-1 leads to a severely abrogated C/EBPα and ELA2 expression as well as to elevated PU.1 levels. This misbalanced expression of C/EBPα, ELA2, and PU.1 in hematopoietic cells from patients with CN causes severely diminished granulopoiesis with enhanced monocytic differentiation. In addition, abrogated ELA2 and NE levels cause pathologically high CXCR4 expression on hematopoietic cells of patients with CN, which could cause a defective G-CSF–triggered stem cell mobilization.
The mechanism of dysregulated myeloid differentiation of hematopoietic progenitors in patients with CN. The relative levels of PU.1 and C/EBPα in granulocytic-macrophage progenitors have been suggested to regulate monocyte versus neutrophil cell fate choice. LEF-1 transcription factor could be responsible for the “fine-tuning” of C/EBPα versus PU.1 levels in myeloid cells to induce granulocytic differentiation. LEF-1 transcription factor is absent in myeloid progenitor cells from patients with CN irrespective to mutation status. Therefore, the myelopoietic maturation program in these patients is sharply shifted toward monocytopoiesis. A lack of LEF-1 leads to a severely abrogated C/EBPα and ELA2 expression as well as to elevated PU.1 levels. This misbalanced expression of C/EBPα, ELA2, and PU.1 in hematopoietic cells from patients with CN causes severely diminished granulopoiesis with enhanced monocytic differentiation. In addition, abrogated ELA2 and NE levels cause pathologically high CXCR4 expression on hematopoietic cells of patients with CN, which could cause a defective G-CSF–triggered stem cell mobilization.
The ELA2 gene is specifically transcribed in promyelocytes in the BM, but the NE protein persists in cells through terminal differentiation into neutrophilic granulocytes.10 NE is released after neutrophil activation and cleaves multiple substrates, including cytokines and chemokines (G-CSF and SDF1α)11–13 as well as cell-surface proteins (G-CSFR, VCAM, and c-kit).12,14–16 It was reported that NE cleaves CXCR4 expressed on the surface of CD34+ cells.20 Levesque et al20 have shown that the administration of G-CSF leads to inactivation of the SDF1α/CXCR4 chemotactic pathway due to proteolytic cleavage by NE, thereby facilitating the egress of CD34+ cells from BM into the blood. The proteolytic cleavage of either SDF1α or CXCR4 by NE leads to complete inactivation of the SDF1α/CXCR4 pathway.17–20 We therefore investigated whether expression of CXCR4 in patients with CN is normal or even elevated as a functional consequence of the missing NE activity. Indeed, we have shown that abolished synthesis of the NE protein in CN was associated with abnormally high CXCR4 expression by CD34+ cells. Intriguingly, our attempts to mobilize CD34+ cells in a patient with CN (as a backup treatment before allogenic BM transplantation) were unsuccessful, despite a temporary 10-fold increase in the daily administered G-CSF dose (K.W., unpublished data, December 2000). The unsuccessful mobilization of CD34+ cells in the patient with CN may be due to the elevated expression of CXCR4 that results from the lack of NE production. On the basis of these data, CXCR4 antagonists could be used for stem cell mobilization in patients with CN alternatively to G-CSF.38–40 The potential compensation of defective NE expression and missing protein function by other neutrophil proteases, such as proteinase 3, cathepsin G, or MMP9, is not possible because, in patients with CN, these proteases are down-regulated to the same degree as NE (Kawaguchi41 and our data42 ).
In summary, we have shown that the expression of ELA2 mRNA and the production of functional NE protein were markedly down-regulated in all patients with CN, independent of the presence of ELA2 or HAX1 mutations. We confirmed that the down-regulation of ELA2 mRNA expression and functional NE protein production was due to the absence of expression of the LEF-1 and C/EBPα transcription factors that directly activate ELA2 gene expression (Figure 6).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank R. Förster for providing anti-CXCR4 antibody, and Ch. Baum and A. Schambach for help with lentiviral constructs. We thank all colleagues associated with the Data Collection Center of the Severe Chronic Neutropenia International Registry at the Medizinische Hochschule Hannover, Hannover, Germany (Cornelia Zeidler and Gusal Pracht) for their continued patient care and for providing patient material. We also thank the patients and their families for cooperation.
This work was supported by the Grant of Hannover Medical School (HiLF) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation).
Authorship
Contribution: J.S. and K.W. designed the experiments, supervised experimentation, analyzed the data, and wrote the manuscript; and J.S., J.P.F., L.D., and B.K.T. performed the experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current address for L.D. is Department of Pediatrics, First Affiliated Hospital of Guang Xi Medical University, Nan Ning, China.
Correspondence: Karl Welte, Department of Molecular Hematopoiesis, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: welte.karl.h@mh-hannover.de; or Julia Skokowa, Department of Molecular Hematopoiesis, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: skokowa.julia@mh-hannover.de.
References
Author notes
*J.S., J.P.F, and L.D. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal