Neutropenia is a recognized adverse event in patients treated with the humanized anti-CD52 monoclonal antibody alemtuzumab. However, as it is widely believed that neutrophils do not express CD52, the etiology of alemtuzumab-associated neutropenia is unclear. We have found that neutrophils express both mRNA coding for CD52 and the protein itself on the cell surface. We confirmed cell-surface expression using 3 different anti-CD52 antibodies, and note that neutrophils express lower levels of CD52 than lymphocytes and eosinophils. Further, incubation of alemtuzumab with neutrophils results in dose-dependent, complement-mediated lysis in the presence of both heterologous and autologous complement. These data offer an explanation for the etiology of alemtuzumab-associated neutropenia. In a climate of increased use of alemtuzumab in leukemia and other disease states, as well as in transplantation, these data highlight the need for increased vigilance of emerging neutropenia in patients treated with alemtuzumab.
Introduction
Neutropenia is a recognized adverse event in patients treated with alemtuzumab, a humanized monoclonal CD52-specific antibody.1 A highly lytic antibody, alemtuzumab mediates cytotoxicity by antibody-dependent cell-mediated cytotoxicity and potent activation of human complement.2 It is approved for use in chronic lymphocytic leukemia (CLL)3 but is also used in non-Hodgkin lymphoma,4 T-cell malignancies,4 rheumatoid arthritis,5 vasculitis,6 scleroderma,7 eosinophilia,8 and prevention of graft-versus-host-disease and graft rejection in bone marrow,9 stem cell,10 and solid organ transplantation.11,–13
Alemtuzumab administration is sometimes associated with a cytokine-release syndrome, which can include pyrexia, headaches, nausea, urticaria, and rigors.2 Myelotoxicity may result in anemia, thrombocytopenia, and neutropenia.14 In particular, postalemtuzumab neutropenia occurs in both fludarabine-refractory1 and treatment-naive14 CLL. The etiology of postalemtuzumab neutropenia, and its associated morbidity and mortality, is poorly understood.15
The obvious mechanism for postalemtuzumab neutropenia would be through neutrophil CD52 expression. However, it is widely believed that neutrophils do not express CD52,11 unlike T and B lymphocytes, natural killer (NK) cells, monocytes, dendritic cells, and male reproductive tract cells.2 Within the granulocyte population, it has been reported that eosinophils, but not neutrophils, express CD52.16 We revisited this question and have shown that neutrophils contain CD52 mRNA, express surface CD52, and are susceptible to complement-mediated lysis in the presence of alemtuzumab. The level of CD52 on neutrophils is lower than on eosinophils and T and B lymphocytes, which could be why it has been difficult to detect.
Methods
Cell isolation
Blood was separated into peripheral blood mononuclear cells (PBMCs) and granulocytes using density-gradient centrifugation over Polymorphprep (Axis-Shield). RPMI-1640 with 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine (Invitrogen), and either 2% or 10% human male AB serum (Biowest) was used for all washes and incubations. Eosinophils were negatively selected from the granulocytes using CD16-conjugated magnetic beads (Miltenyi Biotec). Permission to use human blood samples was granted by the institutional ethics review board of Imperial College London and donor informed consent was obtained in accordance with the Declaration of Helsinki.
Flow cytometry
Cells were stained for 30 minutes at 4°C with saturating concentrations of a specific monoclonal antibody or an isotype-matched control (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), acquired on a FACSCalibur flow cytometer, and analyzed using CellQuest software (BD Biosciences).
Reverse-transcription–polymerase chain reaction
Total RNA was isolated from cells using an RNeasy mini kit (QIAGEN). A total volume of 15 μL containing 5 μg RNA, 0.5 μg Oligo-dT primer (Invitrogen), and water was incubated at 65°C for 10 minutes and cooled. To this, the following was added: 1 μL dNTP mix (each 10 mM; Promega), 200 U Moloney murine leukemia virus reverse transcriptase, 7 μL 5× buffer, 5 μM DTT (Invitrogen), and 1 μL RNase ribonuclease inhibitor (Promega), and the volume made up to 35 μL with water. To perform first-strand cDNA synthesis, this 35-μL mixture was incubated at 37°C for 90 minutes. cDNA was amplified in a 25-μL reaction mixture containing 0.625 U Taq DNA polymerase, reaction buffer (Eppendorf), 0.2 mM each dNTP, 0.2 μM each primer, and water. The polymerase chain reaction was incubated in a Primus 96 Plus thermocycler (MWG Biotech) with initial denaturation (95°C, 4 minutes), 35 cycles of denaturation, annealing and extension (95°C, 30 seconds; 55°C, 30 seconds; 68°C, 1 minute), and final extension (72°C, 10 minutes).
Complement-dependent cytotoxicity
Alemtuzumab-induced complement-dependent cytotoxicity was measured using a standard technique: 2 × 103 cells were incubated in Terasaki trays with 1 to 300 μg/mL alemtuzumab for 30 minutes. Standard rabbit complement (5 μL; Cedarlane Laboratories) or autologous serum (5 μL) was added and incubated for 60 minutes. Cells were stained with 5 μL vital dye mix (propidium iodide, acridine orange, ink) and read under fluorescence microscopy. The anti–HLA class I mAb (W6/32; Sigma-Aldrich) was the positive control; the negative control was male AB serum (Biowest).
Results and discussion
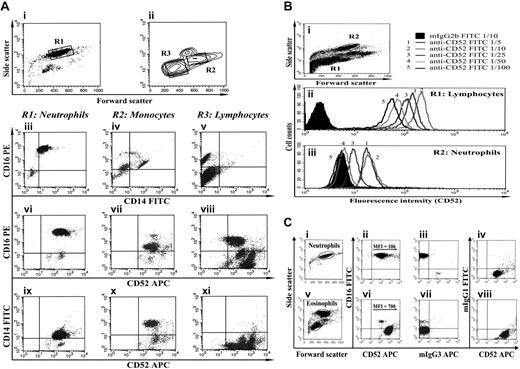
In all 13 individuals studied, we have found that neutrophils contain CD52 mRNA (supplemental Figure 3) and express surface CD52 (Figure 1A), albeit at lower levels than lymphocytes (Figure 1B) or eosinophils (Figure 1C). On the basis of relative mean fluorescent intensities, we estimate that neutrophils have 22% the CD52 of lymphocytes. Anti-CD52 titration demonstrated that, at lower mAb concentrations, at which lymphocytes still appear positive (Figure 1Bii), neutrophils appear negative (Figure 1Biii). We speculate that neutrophil CD52 may have hitherto remained undetected due to the use of antibody concentrations that were nonsaturating at such low-level expression.
Neutrophils express CD52. (A) Purified neutrophils (i) and mononuclear cells (ii) were stained with anti-CD16, anti-CD14, and anti-CD52 mAbs. Surface expression of each of these markers in each of the 3 populations (R1 in panel i and R2 and R3 in panel ii) is shown in panels iii through xi. Neutrophils were identified on the basis of size and granularity (i), and by the expression of a CD16highCD14low phenotype (iii). In this way, they were differentiated from monocytes (CD16lowCD14high, iv) and NK cells (CD16low CD14neg, v). Neutrophils express CD52 (vi,ix). T and B lymphocytes express very high levels of CD52 (viii, bottom right quadrant), whereas monocytes (vii,x) and NK cells (viii, top right quadrant) express lower levels. (B) Unseparated leukocytes were stained with various dilutions of an anti-CD52 mAb (clone HI186). Gating on lymphocytes and neutrophils (R1 and R2 in panel i, respectively), the flow cytograms show that CD52 is expressed at lower levels on neutrophils (iii) than on lymphocytes (ii). Similar results were obtained for the anti-CD52 mAb clone YTH34.5 (data not shown). Open histograms represent staining with an anti-CD52 mAb; shaded histograms represent staining with an isotype-matched control mAb of irrelevant specificity. (C) Using CD16 to differentiate neutrophils (CD16high, ii) from eosinophils (CD16neg/low, vi), we observed that both granulocyte populations express CD52, with significantly lower expression by neutrophils (ii). Open histograms represent staining with an anti-CD52 mAb; shaded histograms represent staining with an isotype-matched control mAb of irrelevant specificity.
Neutrophils express CD52. (A) Purified neutrophils (i) and mononuclear cells (ii) were stained with anti-CD16, anti-CD14, and anti-CD52 mAbs. Surface expression of each of these markers in each of the 3 populations (R1 in panel i and R2 and R3 in panel ii) is shown in panels iii through xi. Neutrophils were identified on the basis of size and granularity (i), and by the expression of a CD16highCD14low phenotype (iii). In this way, they were differentiated from monocytes (CD16lowCD14high, iv) and NK cells (CD16low CD14neg, v). Neutrophils express CD52 (vi,ix). T and B lymphocytes express very high levels of CD52 (viii, bottom right quadrant), whereas monocytes (vii,x) and NK cells (viii, top right quadrant) express lower levels. (B) Unseparated leukocytes were stained with various dilutions of an anti-CD52 mAb (clone HI186). Gating on lymphocytes and neutrophils (R1 and R2 in panel i, respectively), the flow cytograms show that CD52 is expressed at lower levels on neutrophils (iii) than on lymphocytes (ii). Similar results were obtained for the anti-CD52 mAb clone YTH34.5 (data not shown). Open histograms represent staining with an anti-CD52 mAb; shaded histograms represent staining with an isotype-matched control mAb of irrelevant specificity. (C) Using CD16 to differentiate neutrophils (CD16high, ii) from eosinophils (CD16neg/low, vi), we observed that both granulocyte populations express CD52, with significantly lower expression by neutrophils (ii). Open histograms represent staining with an anti-CD52 mAb; shaded histograms represent staining with an isotype-matched control mAb of irrelevant specificity.
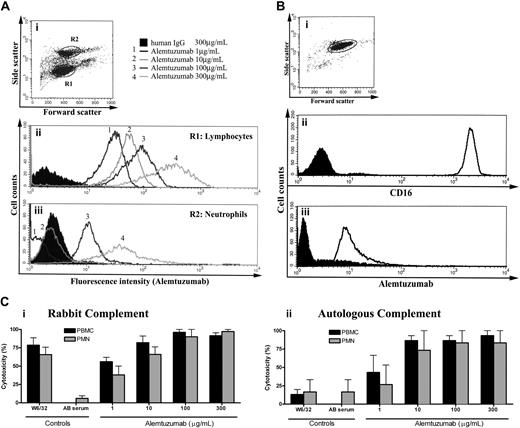
We confirmed specificity of the cell-surface protein using 3 different anti-CD52 antibodies, recognizing at least 2 different epitopes17 (supplemental Figure 2). Alemtuzumab binds neutrophil CD52 (Figure 2A-B) and induces dose-dependent complement-mediated lysis in the presence of either heterologous or autologous complement (Figure 2Ci-ii, respectively), and the concentration of alemtuzumab observed therapeutically18 certainly exceeds that needed to cause both complement-dependent and antibody-dependent cell-mediated cytotoxicity in vitro.18,19
Alemtuzumab binds neutrophil CD52 and induces complement-mediated lysis. (A) Unseparated leukocytes were stained with various dilutions of alemtuzumab. Gating on lymphocytes (R1 in panel i) and neutrophils (R2 in panel i), the flow cytograms show that alemtuzumab binds neutrophil CD52 but requires higher concentrations for detection (iii; 3-4, 100-300 μg/mL), compared with lymphocyte binding (ii; 1-4, 1-300 μg/mL). (B) Gating on a purified neutrophil population (i) stained with CD16 (ii) and alemtuzumab (iii) confirms the binding of alemtuzumab to neutrophils. (C) Neutrophils and mononuclear cells were lysed in a dose-dependent manner in the presence of alemtuzumab with either purified rabbit complement (i) or autologous serum (ii) as the complement source. The anti–HLA class I antibody W6/32 induced cell death in the presence of rabbit complement (i), but not autologous serum (ii), due to the inhibition of autologous complement activation by complement regulators (such as decay accelerating factor and CD59) expressed on PBMCs and neutrophils. In the autologous serum condition, alemtuzumab itself served as the positive control antibody, as its ability to activate human complement is not inhibited by complement regulators. Open histograms represent staining with alemtuzumab; shaded histograms represent staining with an isotype-matched control mAb.
Alemtuzumab binds neutrophil CD52 and induces complement-mediated lysis. (A) Unseparated leukocytes were stained with various dilutions of alemtuzumab. Gating on lymphocytes (R1 in panel i) and neutrophils (R2 in panel i), the flow cytograms show that alemtuzumab binds neutrophil CD52 but requires higher concentrations for detection (iii; 3-4, 100-300 μg/mL), compared with lymphocyte binding (ii; 1-4, 1-300 μg/mL). (B) Gating on a purified neutrophil population (i) stained with CD16 (ii) and alemtuzumab (iii) confirms the binding of alemtuzumab to neutrophils. (C) Neutrophils and mononuclear cells were lysed in a dose-dependent manner in the presence of alemtuzumab with either purified rabbit complement (i) or autologous serum (ii) as the complement source. The anti–HLA class I antibody W6/32 induced cell death in the presence of rabbit complement (i), but not autologous serum (ii), due to the inhibition of autologous complement activation by complement regulators (such as decay accelerating factor and CD59) expressed on PBMCs and neutrophils. In the autologous serum condition, alemtuzumab itself served as the positive control antibody, as its ability to activate human complement is not inhibited by complement regulators. Open histograms represent staining with alemtuzumab; shaded histograms represent staining with an isotype-matched control mAb.
Alemtuzumab's ability to activate autologous complement, despite the presence of cell-surface complement regulators, represents a potential mechanism by which alemtuzumab may be therapeutically effective. Incidentally, we did not observe the down-regulation of CD16 that is characteristic of neutrophil activation (data not shown), making it unlikely that the adverse effects of alemtuzumab could be attributed to it activating neutrophils. In a multicenter trial of alemtuzumab in CLL (n = 149),14 77% of patients developed neutropenia20 and 9.5% required granulocyte colony-stimulating factor (G-CSF).14 Our data provide a potential mechanism for neutropenia in alemtuzumab-treated patients.
In the only trial to use alemtuzumab with concurrent, prophylactic G-CSF, 64% (9/14) of patients developed grade 3 to 4 neutropenia.15 Four developed late-onset neutropenia (> week 10), which was unresponsive to increased doses of G-CSF but reversed within 3 to 5 weeks of withdrawing alemtuzumab. It is unclear whether the unresponsiveness to G-CSF was due to alemtuzumab-mediated consumption of mature neutrophils, or alemtuzumab-mediated interference with bone marrow neutrophil development and release. Gilleece and Dexter reported that alemtuzumab treatment does not affect myeloid progenitor cells,21 but others reported that Campath-1 (alemtuzumab's rat precursor; Genzyme) reduces granulocyte-macrophage colony-forming cells.22 In addition, it is possible that alemtuzumab-mediated depletion of CD52+ neutrophils favors selection of CD52− neutrophil clones, a process known to occur in T cells,23 and for other neutrophil surface proteins.24
Neutropenia after alemtuzumab therapy in solid organ transplant recipients is not widely reported, perhaps due to the frequent concomitant use of (neutrophilia-inducing) steroids. Where neutropenia is reported, use of other bone marrow–suppressing agents often renders etiologic conclusions impossible. Considering the current trend toward steroid-free immunosuppression in solid organ transplantation, it is possible that alemtuzumab-associated neutropenia may be unmasked. In the largest series of live donor renal transplant recipients undergoing alemtuzumab and steroid induction followed by tacrolimus monotherapy (n = 205), 15% experienced 40 episodes of neutropenia requiring G-CSF.25
Of course, patients being treated with alemtuzumab are at risk of neutropenia from causes other than the alemtuzumab: the underlying bone marrow disease itself, as a complication of immunosuppression, such as Epstein-Barr virus infection, or as an adverse effect of other drugs used.
Our data are of relevance to eosinophil studies that have used CD52 as a marker to positively select eosinophils from a granulocyte population during purification.16 Failure to double-stain the resulting population for CD16 may result in not detecting contaminating (CD52+) neutrophils. In addition, if activated during purification, these neutrophils may express only low levels of CD16, and be mistaken for (CD16−) eosinophils.
These data offer an explanation for the etiology of alemtuzumab-associated neutropenia. Our data do not suggest avoiding or discontinuing alemtuzumab, nor do published guidelines.1 Alemtuzumab is an important and effective treatment and its adverse events are generally predictable, transient, and manageable.1 These data simply highlight the need for vigilance for neutropenia after alemtuzumab, especially in patients undergoing medium- to long-term treatment and in solid organ transplantation regimens avoiding steroids.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Matt Butler for useful scientific and technical discussions and Drs Mark Little, Efrem Eren, and Paul Brookes for review of the paper.
We are grateful to the National Institutes for Health Research (NIHR) Biomedical Research Center funding scheme for supporting this work.
This work was funded by a British Transplantation Society PhD Research Fellowship and a grant from the Charitable Funds of Hammersmith and Queen Charlotte's Hospital to L.R.A.
Authorship
Contribution: L.R.A. designed, performed, and analyzed the research and wrote the paper; A.-S.M. contributed to the design and analysis of the molecular work and donated reagents; and A.N.W. contributed to the design and analysis of the research and to the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lyn Ambrose, Immunology Unit, Rm 236, Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, Keppel St, London WC1E 7HT, United Kingdom; e-mail: lynambrose@googlemail.com.