Activating mutations in the receptor tyrosine kinase FLT3 are present in up to approximately 30% of acute myeloid leukemia (AML) patients, implicating FLT3 as a driver of the disease and therefore as a target for therapy. We report the characterization of AC220, a second-generation FLT3 inhibitor, and a comparison of AC220 with the first-generation FLT3 inhibitors CEP-701, MLN-518, PKC-412, sorafenib, and sunitinib. AC220 exhibits low nanomolar potency in biochemical and cellular assays and exceptional kinase selectivity, and in animal models is efficacious at doses as low as 1 mg/kg given orally once daily. The data reveal that the combination of excellent potency, selectivity, and pharmacokinetic properties is unique to AC220, which therefore is the first drug candidate with a profile that matches the characteristics desirable for a clinical FLT3 inhibitor.
Introduction
The presence of genetic changes in cancer cells that lead to dysregulated activation of kinases frequently signals that the activated kinase is a contributing driver of disease,1,,–4 and inhibitors of activated kinases can have a dramatic impact on disease progression in patients with these genetic alterations.5,6 To clearly define the role of the dysregulated kinase, and to determine whether inhibition of the mutant kinase is sufficient to induce a therapeutic benefit, requires drugs capable of selectively, potently, and preferably sustainably inhibiting the activated kinase in patients.
Activating mutations in the FLT3 receptor tyrosine kinase have been identified in up to 30% of acute myeloid leukemia (AML) patients.7,8 The most common class of mutation is internal tandem duplications (ITDs) in the juxtamembrane domain7,9 that lead to constitutive, ligand-independent activation of the kinase.7,10 The prognosis for patients with FLT3-ITD mutations is significantly worse than that for patients with wild-type FLT3 when treated with standard therapy.7,–9,11,,,,–16 The presence of activating FLT3 mutations and the correlation of FLT3 activation with a poor prognosis strongly suggest that FLT3 is a driver of disease in AML, at least in patients with FLT3-ITD mutations. Several small molecule kinase inhibitors with activity against FLT3 have been evaluated in AML patients, including CEP-701 (lestaurtinib), PKC-412 (midostaurin), MLN-518 (tandutinib; previously known as CT-53518), sunitinib (SU-11248), sorafenib (BAY-43-9006), and KW-2449. The compounds inhibit FLT3 in cellular assays and are efficacious in mouse models of FLT3-ITD AML.17,,,,–22 In phase 1 and phase 2 clinical trials, conducted primarily in relapsed or refractory AML patients, responses were consistently observed with each of these drugs,21,23,,,,,,,–31 however, responses generally were relatively limited and not durable.21,23,–25,30 The studies did reveal a relationship between the likelihood of observing a clinical response and the pharmacokinetics/pharmacodynamics of FLT3 inhibition, and highlight the importance of substantial and sustained inhibition of FLT3.19,21,25,26,32 FLT3 inhibitory activity has been reported for several additional compounds, including TKI-258 (dovitinib; formerly known as CHIR-258),33 ABT-869,34 FI-700,35 NVP-AST487,36 and Ki23819.37 The foregoing clinical compounds have FLT3 inhibitory activity; however, they were not expressly developed or optimized as FLT3 inhibitors.38,,,–42 To fully explore the potential of FLT3 inhibition as AML therapy, and to determine whether FLT3 inhibition is sufficient to yield a therapeutic benefit,26 may require a second-generation inhibitor that has been expressly optimized to inhibit FLT3 with very high potency and to be highly selective against other kinases, together with pharmacokinetic properties that afford complete and sustained inhibition of FLT3 in patients' leukemic blast cells.
AC220 is a novel compound expressly optimized as a FLT3 inhibitor for the treatment of AML. We show here that AC220 inhibits FLT3 with low nanomolar potency in cellular assays and is highly selective when screened against the majority of the human protein kinome. We further demonstrate that the combination of high potency and selectivity exhibited by AC220 is unique compared with CEP-701, PKC-412, MLN-518, sunitinib, and sorafenib. AC220 inhibits FLT3 activity in vivo, significantly extends survival in a mouse model of FLT3-ITD AML at doses as low as 1 mg/kg when dosed orally once a day, eradicates tumors in a FLT3-dependent mouse xenograft model at 10 mg/kg, and potently inhibits FLT3 activity in primary patient cells. The results presented here support testing AC220 in clinical trials for the treatment of AML, and these trials are in progress.
Methods
Compounds
MLN-518 was custom synthesized by CiVentiChem, and sunitinib was custom synthesized by Sai Advantium Ltd. Sorafenib, PKC-412, CGP-52421, CEP-701, and AC220 were synthesized at Ambit Biosciences.
Biochemical kinase binding assays
KinomeScan kinase binding assays were performed as previously described.43,44 For the FLT3 assay, we used a kinase construct that spanned the catalytic domain only (amino acids 592 to 969 in NP_004110.2). This construct does not include the juxtamembrane domain and is designed to measure the intrinsic binding affinity of the open FLT3 active site for inhibitors.
Cellular assays
MV4-11 and RS4;11 cells were cultured in Iscove media with 10% fetal bovine serum (FBS) and RPMI complete with 10% FBS, respectively. For proliferation assays, cells were cultured overnight in low serum media (0.5% FBS), then seeded in a 96-well plate at 40 000 cells per well. Inhibitors were added to the cells and incubated at 37°C for 72 hours. Cell viability was measured using the Cell Titer-Blue Cell Viability Assay from Promega. To measure inhibition of FLT3 autophosphorylation, cells were cultured in low serum media (0.5% FBS) overnight and seeded at a density of 400 000 cells per well in a 96-well plate the following day. The cells were incubated with inhibitors for 2 hours at 37°C. To induce FLT3 autophosphorylation in RS4;11 cells, 100 ng/mL FLT3 ligand (R&D Systems) was added for 15 minutes after the 2-hour compound incubation. Cell lysates were prepared and incubated in 96-well plates precoated with a total FLT3 capture antibody (R&D Systems). The coated plates were incubated with either a biotinylated antibody against FLT3 (R&D Systems) to detect total FLT3 or an antibody against phosphotyrosines (Millipore) to detect FLT3 autophosphorylation. In both cases, a SULFO-tagged streptavidin secondary antibody was used for electrochemiluminescence detection on the Meso Scale Discovery platform.
Pharmacokinetic studies
Female NU/NU or severe combined immunodeficient mice were purchased from Charles River Laboratories or Harlan. AC220 (hydrochloride salt) was formulated in 22% hydroxypropyl-β-cyclodextrin, CEP-701 was formulated in 20% gelucire 44/14 in water (vol/vol), MLN-518 and sunitinib were formulated in 10 mM sodium citrate (pH 3.5), PKC-412 was formulated in 3:1 gelucire 44/14–propylene glycol (vol/vol), and sorafenib (toluene sulfonate salt) was formulated in 80% PEG-400. Compound concentrations were chosen to deliver the desired dose in a volume of 10 mL/kg. Compounds were administered by oral gavage and plasma samples collected 0.25, 0.5, 1, 2, 4, 6, and 24 hours after dosing. To collect plasma samples, eye bleeds (150 μL) were taken semilongitudinally using 3 groups of 3 animals each, taking 2 to 3 time points per animal to obtain a total of 3 independent plasma concentration time courses. Plasma samples and controls (25 μL) were extracted with 4 volumes of acetonitrile containing an internal standard and analyzed by liquid chromatography tandem mass spectrometry. Pharmacokinetic parameters were obtained by fitting the normalized liquid chromatography tandem mass spectrometry peak areas to a noncompartmental model using the linear trapezoidal estimation method in the WinNonlin software package (Pharsight). Mouse studies at Ambit complied with the recommendations of the “Guide for Care and Use of Laboratory Animals”45 with respect to restraint, husbandry, surgical procedures, feed and fluid regulation, and veterinary care. The mouse studies at Piedmont Research Center likewise complied with the recommendations of the “Guide for Care and Use of Laboratory Animals”45 with respect to restraint, husbandry, surgical procedures, feed and fluid regulation, and veterinary care, and the animal care and use program at Piedmont Research Center is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), which assures compliance with accepted standards for the care and use of laboratory animals.
Animal efficacy studies
Subcutaneous xenograft model.
This model was performed at Ambit to measure in vivo inhibition of FLT3, and by Piedmont Research Center LLC to determine antitumor efficacy, following published procedures.20 Compounds were formulated and administered as described for pharmacokinetic studies. To measure FLT3 inhibition, tumors were harvested at 2 or 24 hours after compound administration, weighed, and lysed by mechanical dissociation. Tumor lysates were cleared of protein and tissue fragments by centrifugation at 835g for 15 minutes. Cleared lysates were assayed for total and phosphorylated FLT3 using the electrochemiluminescence-based enzyme-linked immunoassay (ELISA) described in “Cellular assays.”
Bone marrow engraftment model.
The model was performed according to published procedures.20 For intravenous bone marrow engraftment, nonobese diabetic/severe combined immunodeficient mice were acclimated for 2 weeks before pretreatment with 150 mg/kg cyclophosphamide (Sigma Life Science) delivered intraperitoneally once a day for 2 days. After a 48-hour rest period, animals were given an intravenous injection of 5 × 106 MV4-11 cells into the tail vein. AC220 was formulated and delivered as described for pharmacokinetic studies.
Primary cell assays
Leukemia cell specimens were provided by the Sidney Kimmel Cancer Center at the Johns Hopkins Tumor and Cell Procurement Bank, supported by the Regional Oncology Research Center Grant no. 2 P30 CA 006973-44. All patients gave informed consent according to the Declaration of Helsinki and institutional review board (IRB) approval was obtained from Johns Hopkins University. Mononuclear cells were isolated from whole blood or marrow using density gradient centrifugation with Ficoll-Hypaque (Amersham) and stored in liquid nitrogen in FBS with 10% dimethyl sulfoxide. When used, frozen samples were thawed rapidly, incubated in culture medium overnight, then subjected to another round of density centrifugation (with added DNAse, obtained from Amersham) to eliminate cells that had undergone apoptosis from the freeze-thaw cycle. The FLT3 mutation status was determined as described.46 Cytotoxicity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay.19 To assess FLT3 phosphorylation by Western blot, patient-derived leukemia blasts were washed in phosphate-buffered saline, then lysed by resuspending them in lysis buffer (20 mM Tris, pH 7.4, 100 mM NaCl, 1% Igepal [Sigma Life Science], 1 mM EDTA, 2 mM NaVO4, plus Complete Protease Inhibitor Cocktail [Roche]) for 30 minutes while rocking. The lysate was clarified by centrifugation at 18 000 g and the supernatant was assayed for protein (Bio-Rad). Anti-FLT3 (S18) antibody (Santa Cruz Biotechnology) was added to the extract for overnight incubation; then protein A sepharose (Upstate Biotechnology) was added for 2 additional hours. After sodium dodecylsulfate polyacrylamide electrophoresis and transfer to Immobilon membranes (Millipore), immunoblotting was performed with antiphosphotyrosine antibody (4G10; Millipore) to detect phosphorylated FLT3, then stripped and reprobed with anti-FLT3 antibody to measure total FLT3. Proteins were visualized using enhanced chemiluminescence (Amersham). To quantitate phospho-FLT3 levels, cell lysates were assayed for phospho-FLT3 and total FLT3 by ELISA as described for “Cellular assays.”
Results
Potency of FLT3 inhibition
To identify promising starting points for kinase inhibitor drug discovery, we screened a scaffold-focused library of compounds against a panel of kinases.47 Among the hits were compounds with binding affinity for FLT3 below 100 nanomolar and excellent kinase selectivity that also were druglike and for which analogs to explore structure activity relationships could be secured by standard synthetic chemistry procedures. Optimization of the initial hits yielded AC220, a novel bis-aryl urea FLT3 inhibitor (Figure 1). The chemistry of optimization and structure activity relationships will be described in detail elsewhere (Q.C., K.G.S., R. M. Grotzfeld, A. G. Lai, T. A. Carter, A. M. Velasco, R.N.G., M.D.C., M.F.G., J.J., P.P.Z., H.K.P., and S.S.B., manuscript submitted).
To compare the potency of AC220 with that of other FLT3 inhibitors in clinical development, we first used a previously described biochemical binding assay.43 We included in this comparison CEP-701, MLN-518, PKC-412, sorafenib, sunitinib, as well as CGP-52421, a metabolite of PKC-412 that may be the major active species in patients (Figure 1).26 All compounds tested had high affinity for FLT3 in the binding assay (Table 1), with sunitinib exhibiting the greatest potency, followed by AC220. To determine the ability of compounds to inhibit FLT3 in the cellular environment, we measured inhibition of FLT3 autophosphorylation in the human leukemia cell lines MV4-11, which harbors a homozygous FLT3-ITD mutation and is FLT3 dependent,19,48 and RS4;11, which expresses wild-type FLT3.49 AC220 was the most potent cellular FLT3-ITD inhibitor tested. MLN-518 and sunitinib, in contrast, were more than 10-fold less potent in the cellular autophosphorylation assays compared with the binding assay, and consequently were, along with the PKC-412 metabolite CGP-52421, the least potent compounds in cells (Table 1).
Potency of FLT3 inhibitors in biochemical and cellular assays
| Compound . | Biochemical assay . | Cellular assays . | |||
|---|---|---|---|---|---|
| FLT3 binding affinity, Kd, nM . | FLT3 autophosphorylation . | Cell proliferation . | |||
| ITD, IC50, nM . | Wild type, IC50, nM . | MV4-11, IC50, nM . | A375, IC50, nM . | ||
| AC220 | 1.6 ± 0.7 | 1.1 ± 0.1 | 4.2 ± 0.3 | 0.56 ± 0.3 | > 10 000 |
| CEP-701 | 8.5 ± 2 | 1.5 ± 0.3 | 2.6 ± 2 | 2.1 ± 0.2 | 32 ± 9 |
| MLN-518 | 3.0 ± 0.3 | 33 ± 20 | 170 ± 50 | 60 ± 8 | > 10 000 |
| PKC-412 | 11 ± 8 | 13 ± 2 | 15 ± 9 | 12 ± 1 | 180 ± 50 |
| CGP-52421 | 68 ± 20 | 160 ± 40 | 350 ± 200 | 64 ± 8 | 530 ± 100 |
| Sorafenib | 13 ± 6 | 2.0 ± 0.3 | 3.2 ± 1 | 0.87 ± 0.6 | 2200 ± 100 |
| Sunitinib | 0.47 ± 0.2 | 34 ± 20 | 9.9 ± 6 | 4.3 ± 0.4 | 5400 ± 1000 |
| Compound . | Biochemical assay . | Cellular assays . | |||
|---|---|---|---|---|---|
| FLT3 binding affinity, Kd, nM . | FLT3 autophosphorylation . | Cell proliferation . | |||
| ITD, IC50, nM . | Wild type, IC50, nM . | MV4-11, IC50, nM . | A375, IC50, nM . | ||
| AC220 | 1.6 ± 0.7 | 1.1 ± 0.1 | 4.2 ± 0.3 | 0.56 ± 0.3 | > 10 000 |
| CEP-701 | 8.5 ± 2 | 1.5 ± 0.3 | 2.6 ± 2 | 2.1 ± 0.2 | 32 ± 9 |
| MLN-518 | 3.0 ± 0.3 | 33 ± 20 | 170 ± 50 | 60 ± 8 | > 10 000 |
| PKC-412 | 11 ± 8 | 13 ± 2 | 15 ± 9 | 12 ± 1 | 180 ± 50 |
| CGP-52421 | 68 ± 20 | 160 ± 40 | 350 ± 200 | 64 ± 8 | 530 ± 100 |
| Sorafenib | 13 ± 6 | 2.0 ± 0.3 | 3.2 ± 1 | 0.87 ± 0.6 | 2200 ± 100 |
| Sunitinib | 0.47 ± 0.2 | 34 ± 20 | 9.9 ± 6 | 4.3 ± 0.4 | 5400 ± 1000 |
Values shown are averages from at least 3 independent experiments (± SD).
To determine the effect of FLT3-ITD inhibition on cell growth, we measured MV4-11 cell proliferation in the presence of each FLT3 inhibitor. The IC50s in this assay tracked very well with FLT3 autophosphorylation inhibition, with AC220 and sorafenib the most potent compounds (Table 1). One major exception was sunitinib, which inhibited MV4-11 cell proliferation with almost 8-fold greater potency than it inhibited FLT3-ITD autophosphorylation. Inhibition of other targets by sunitinib, which is capable of interacting with a large number of kinases,44 may be contributing to the effect on MV4-11 cell proliferation. As a cellular selectivity control, we measured the effect on proliferation of A375 cells, which harbor an activating mutation in BRAF and are not FLT3 dependent. There was no evidence of inhibition of the growth of A375 cells by AC220 (Table 1), indicating a large window between FLT3 inhibition and general cytotoxic effects. The staurosporine analogs CEP-701, PKC-412, as well as the PKC-412 metabolite CGP-52421, however, inhibited A375 cell proliferation with IC50s within 20-fold of those for MV4-11 growth inhibition. The effect of sorafenib on A375 cells is likely mediated by inhibition of BRAF, a known target of sorafenib.41 These results establish that AC220 has strong activity against FLT3 in biochemical and cellular assays, is not generally cytotoxic, and is among the most potent FLT3 inhibitors known.
Kinase selectivity
To assess the potential of AC220 to inhibit off-target kinases, and to compare its selectivity with that of the other FLT3 inhibitors, we screened the compounds against a KinomeScan panel of 402 kinase binding assays,43,44 representing almost 80% of “typical” human protein kinases.50 Each compound was screened against the panel at a single concentration of 10 μM in a primary screen, and dissociation constants (Kds) were determined for all kinases identified as targets in the primary screen. For AC220, we also measured a Kd for every kinase not found to bind in the primary screen to ensure that no targets were missed.
The highest affinity target identified for AC220 was FLT3 (supplemental Table 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The only other kinases with binding constants within 10-fold that for FLT3 were the closely related receptor tyrosine kinases (RTKs) KIT, PDGFRA, PDGFRB, RET, and CSF1R, and only 4 additional kinases, also related RTKs (FLT1, FLT4, DDR1, VEGFR2), bound with Kds within 100-fold of that for FLT3. In follow-up cellular assays the activity of AC220 against KIT, RET, CSF1R, and PDGFR was at least 10-fold less potent than the cellular activity against FLT3, confirming the selectivity observed in the biochemical assays (data not shown). The kinase interaction pattern for AC220 was therefore highly focused (Figure 2). In contrast, CEP-701, PKC-412, CGP-52421, and sunitinib cross-reacted extensively with kinases of all groups and subfamilies, and even sorafenib had a substantially broader interaction pattern (Figure 2 and supplemental Table 1). Broad interaction patterns have also previously been reported for NVP-AST487, ABT-869, and TKI-258.44 The only compound among those tested that had a similar profile to AC220 was MLN-518, which also predominantly targeted class III RTKs (Figure 2 and supplemental Table 1). To quantitate the selectivity of each compound, we calculated absolute selectivity scores as well as selectivity scores relative to FLT3 (Table 2).44 The absolute and the relative scores for AC220 and MLN-518 were comparable with each other, and lower than for the other compounds. They were in the same range as scores previously determined for other selective inhibitors such as imatinib and gefitinib.44 PKC-412, CGP-52421, and CEP-701 are close analogs of staurosporine and their broad cross-reactivity is therefore not surprising. These results show that AC220 is the only compound among those tested that is both highly potent as a FLT3 inhibitor in in vitro and cellular assays, and has a highly focused and selective interaction pattern across the human protein kinome. The biochemical profile of AC220 therefore is unique among the FLT3 inhibitors tested and closely matches the desirable profile for a clinical FLT3 inhibitor.
Small molecule kinase interaction maps for FLT3 inhibitors. Compounds were screened against a KinomeScan (http://www.kinomescan.com) panel of 402 kinase assays. Red circles indicate kinases bound, and circle size indicates binding affinity. Interactions with Kd < 3 μM are shown. The complete dataset is shown in supplemental Table 1, and is also available through an interactive website (http://www.ambitbio.com/technology/publications). The data for MLN-518, PKC-412, sunitinib, and sorafenib against a subset of 317 of the 402 assays were previously published,44 and are reproduced here together with new data for assays not represented in the earlier panel. The kinase dendrogram was adapted and is reproduced with permission from Cell Signaling Technology Inc (http://www.cellsignal.com).
Small molecule kinase interaction maps for FLT3 inhibitors. Compounds were screened against a KinomeScan (http://www.kinomescan.com) panel of 402 kinase assays. Red circles indicate kinases bound, and circle size indicates binding affinity. Interactions with Kd < 3 μM are shown. The complete dataset is shown in supplemental Table 1, and is also available through an interactive website (http://www.ambitbio.com/technology/publications). The data for MLN-518, PKC-412, sunitinib, and sorafenib against a subset of 317 of the 402 assays were previously published,44 and are reproduced here together with new data for assays not represented in the earlier panel. The kinase dendrogram was adapted and is reproduced with permission from Cell Signaling Technology Inc (http://www.cellsignal.com).
Quantitative selectivity of FLT3 inhibitors
| Compound . | S (3 μM) . | S (100 nM) . | S ([Kdoff-target/Kd FLT3] < 10) . |
|---|---|---|---|
| AC220 | 0.067 | 0.028 | 0.014 |
| CEP-701 | 0.81 | 0.46 | 0.43 |
| MLN-518 | 0.056 | 0.014 | 0.011 |
| PKC-412 | 0.44 | 0.084 | 0.087 |
| CGP-52421 | 0.19 | 0.031 | 0.089 |
| Sorafenib | 0.18 | 0.045 | 0.042 |
| Sunitinib | 0.58 | 0.19 | 0.020 |
| Compound . | S (3 μM) . | S (100 nM) . | S ([Kdoff-target/Kd FLT3] < 10) . |
|---|---|---|---|
| AC220 | 0.067 | 0.028 | 0.014 |
| CEP-701 | 0.81 | 0.46 | 0.43 |
| MLN-518 | 0.056 | 0.014 | 0.011 |
| PKC-412 | 0.44 | 0.084 | 0.087 |
| CGP-52421 | 0.19 | 0.031 | 0.089 |
| Sorafenib | 0.18 | 0.045 | 0.042 |
| Sunitinib | 0.58 | 0.19 | 0.020 |
Selectivity scores were calculated as described,44 based on the data shown in supplemental Table 1 for 359 distinct kinases. Lower scores indicate greater selectivity. Mutant variants were not included in the calculation. S ([Kd off-target/Kd FLT3] < 10) is the fraction of kinases screened that bind with affinities within 10-fold or better of the affinity for FLT3.
Pharmacokinetics
To assess the pharmacokinetic properties of AC220, a single dose of 10 mg/kg was administered to mice by oral gavage and plasma levels were measured over a 24-hour period (Figure 3A and Table 3). The compound was well absorbed, achieving a maximum plasma level (Cmax) of 3.8 μM (2100 ng/mL) within 2 hours of dosing. The apparent plasma half-life was approximately 4 hours. The peak concentration of free AC220 (taking into account plasma protein binding of approximately 99%; data not shown) after a single 10-mg/kg dose therefore is more than 30-fold above the IC50 for FLT3-ITD inhibition in cellular assays. After 24 hours, estimated free AC220 is still present at a concentration approximately at the cellular IC50, indicating that once a day oral dosing is sufficient for continuous inhibition of FLT3 activity in mice. To determine whether varying the dose of AC220 resulted in a corresponding change in plasma levels, we measured single-dose pharmacokinetics at doses ranging from 0.1 to 300 mg/kg (Figure 3B and Table 3). Total exposure (AUC0-24 hours) as well as Cmax correlated well with the administered dose between 0.1 and approximately 30 mg/kg. At higher doses, both Cmax and AUC0-24 hours continued to increase, but approached a plateau above 100 mg/kg. The oral bioavailability of AC220, determined in rats by comparing oral and intravenous pharmacokinetics at 3 mg/kg, was approximately 40%. To ensure that the pharmacokinetic properties did not change with repeated dosing, we administered AC220 to rats at 10 mg/kg orally once a day for 5 days, and measured plasma levels after the final dose. The concentration of AC220 in plasma was consistent after single and repeat dosing (data not shown). The pharmacokinetics of AC220 therefore are dose proportional in the dose range required for inhibition of FLT3, and appropriate plasma levels of AC220 can be maintained through repeat dosing.
Pharmacokinetics of AC220. (A) Time course of AC220 plasma levels in NU/NU mice after a single oral administration at 10 mg/kg. The average values from 3 independent time courses are shown in black and individual values, in red. (B) Dose dependence of AC220 peak plasma levels (Cmax) and exposure (AUC0-24 hours). The insets show a zoomed view of the data at dosages of 10 mg/kg and below. Average values from 3 independent time courses are shown in black and individual values, in red.
Pharmacokinetics of AC220. (A) Time course of AC220 plasma levels in NU/NU mice after a single oral administration at 10 mg/kg. The average values from 3 independent time courses are shown in black and individual values, in red. (B) Dose dependence of AC220 peak plasma levels (Cmax) and exposure (AUC0-24 hours). The insets show a zoomed view of the data at dosages of 10 mg/kg and below. Average values from 3 independent time courses are shown in black and individual values, in red.
Oral pharmacokinetics of FLT3 inhibitors
| Compound . | Dose, mg/kg . | Cmax, μM . | Tmax, h . | AUC0-24 h, μM×h . |
|---|---|---|---|---|
| AC220 | 10 | 3.8 ± 0.4 | 1.5 ± 0.9 | 35 ± 4 |
| CEP-701 | 10 | 0.14 ± 0.06 | 0.92 ± 0.9 | < 0.88† |
| MLN-518 | 10 | 0.081 ± 0.003 | 1.0 ± 0 | 0.28 ± 0.03 |
| PKC-412* | 10 | 0.77 ± 0.04 | 0.67 ± 0.3 | 2.5 ± 0.4 |
| CGP-52421 | From PKC-412* | 2.3 ± 0.3 | 0.67 ± 0.3 | 8.4 ± 1 |
| Sorafenib | 10 | 12 ± 2 | 3.0 ± 3 | 240 ± 50 |
| Sunitinib | 10 | 0.32 ± 0.06 | 1.2 ± 0.8 | 1.1 ± 0.3 |
| Compound . | Dose, mg/kg . | Cmax, μM . | Tmax, h . | AUC0-24 h, μM×h . |
|---|---|---|---|---|
| AC220 | 10 | 3.8 ± 0.4 | 1.5 ± 0.9 | 35 ± 4 |
| CEP-701 | 10 | 0.14 ± 0.06 | 0.92 ± 0.9 | < 0.88† |
| MLN-518 | 10 | 0.081 ± 0.003 | 1.0 ± 0 | 0.28 ± 0.03 |
| PKC-412* | 10 | 0.77 ± 0.04 | 0.67 ± 0.3 | 2.5 ± 0.4 |
| CGP-52421 | From PKC-412* | 2.3 ± 0.3 | 0.67 ± 0.3 | 8.4 ± 1 |
| Sorafenib | 10 | 12 ± 2 | 3.0 ± 3 | 240 ± 50 |
| Sunitinib | 10 | 0.32 ± 0.06 | 1.2 ± 0.8 | 1.1 ± 0.3 |
Values shown are averages from 3 independent time courses (± SD).
PKC-412 and CGP-52421 were quantitated independently after dosing PKC-412.
The plasma concentration of CEP-701 was below the limit of detection at 24 hours.
To compare the pharmacokinetics of AC220 with those of other FLT3 inhibitors, we measured plasma levels of CEP-701, MLN-518, PKC-412 and CGP-52421, sunitinib, and sorafenib in mice after a single oral dose of 10 mg/kg (Table 3). The Cmax and AUC0-24 hours for CEP-701, MLN-518, PKC-412, and sunitinib were 5- to 100-fold lower than for AC220. The peak free plasma concentration (taking into account mouse plasma protein binding of 90% for sunitinib and 80% for MLN-518 [data not shown] as well as 98% for CEP-701 and approximately 99% for PKC-412 [data not shown] and consistent with the reported high plasma protein binding for CEP-701 and PKC-41226 ) therefore is estimated to be only approximately at the cellular IC50 for CEP-701 and sunitinib, and below the cellular IC50 for MLN-518 and PKC-412, compared with more than 30-fold above the cellular IC50 for AC220. Free plasma levels of all 4 compounds are well below the cellular IC50 for FLT3 inhibition after 24 hours. Plasma levels of CGP-52421, the PKC-412 metabolite, were determined independently after dosing PKC-412, and at their peak were also well below the concentrations required to inhibit FLT3 in plasma.26 It is therefore likely that much higher doses of these compounds, as well as more frequent administration, would be required, in mice, to achieve the same level and duration of FLT3 inhibition as for AC220. In contrast, the free plasma concentration of sorafenib (again taking into account mouse plasma protein binding of approximately 99%) is more than 50-fold above the cellular IC50 at Cmax, and still approximately 10-fold above IC50 after 24 hours. The pharmacokinetic properties of sorafenib relative to FLT3 inhibition therefore are somewhat better than those of AC220. Together these results show that the pharmacokinetic properties in animals of AC220 relative to FLT3 inhibition are, with the exception of sorafenib, substantially better than those of other FLT3 inhibitors.
In vivo efficacy in tumor models
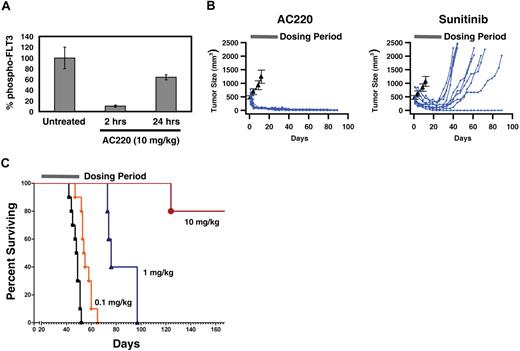
To determine whether AC220 could inhibit FLT3 activity in vivo, we used the FLT3-ITD–dependent MV4-11 tumor xenograft model.17,20 MV4-11 cells were implanted subcutaneously in mice and tumors allowed to grow to a size of 200 to 250 mm3. A single 10-mg/kg dose of AC220 was administered by oral gavage, and mice were killed at 2 time points after dosing, using groups of 4 animals each. Quantitation of total FLT3 and phospho-FLT3 in tumor samples revealed time-dependent inhibition of FLT3 autophosphorylation (Figure 4A). FLT3 activity was inhibited by 90% at 2 hours, and 40% at 24 hours after administration. The extent of inhibition therefore correlated well with the expected free AC220 plasma levels, based on pharmacokinetic experiments (Figure 3A).
In vivo efficacy of AC220 in animal tumor models. (A) Inhibition of FLT3 activity in subcutaneous tumor xenografts. Total and phospho-FLT3 were quantitated in lysates from tumors harvested at each time point, and phospho-FLT3 levels normalized relative to the total amount of FLT3. Normalized phospho-FLT3 levels are shown as a percentage of levels in untreated animals. Each group contained 4 animals. (B) Comparison of antitumor efficacy of AC220 and sunitinib in the subcutaneous tumor model at a dose of 10 mg/kg. Day 1 of the study was the first day of treatment, when tumors had reached a size of 450 to 600 mm3. The solid bar indicates the 28-day treatment period. Tumor size was monitored for an additional 61 days after treatment was discontinued, and total study length was 89 days. Each treatment group contained 10 animals, and the time course of tumor size for each individual animal is shown in blue. The average tumor size in the vehicle control groups is shown in black (± SEM) for comparison. Animals were killed if tumor volume reached 1000 mm3. (C) Efficacy of AC220 in a bone marrow transplant model. Kaplan-Meier plots of survival. Day 1 of the study was the day after cells were implanted. The solid bar indicates the treatment period of 30 days. Each treatment group contained 5 animals. Data for the vehicle control group are shown as ■. The study was terminated on day 172.
In vivo efficacy of AC220 in animal tumor models. (A) Inhibition of FLT3 activity in subcutaneous tumor xenografts. Total and phospho-FLT3 were quantitated in lysates from tumors harvested at each time point, and phospho-FLT3 levels normalized relative to the total amount of FLT3. Normalized phospho-FLT3 levels are shown as a percentage of levels in untreated animals. Each group contained 4 animals. (B) Comparison of antitumor efficacy of AC220 and sunitinib in the subcutaneous tumor model at a dose of 10 mg/kg. Day 1 of the study was the first day of treatment, when tumors had reached a size of 450 to 600 mm3. The solid bar indicates the 28-day treatment period. Tumor size was monitored for an additional 61 days after treatment was discontinued, and total study length was 89 days. Each treatment group contained 10 animals, and the time course of tumor size for each individual animal is shown in blue. The average tumor size in the vehicle control groups is shown in black (± SEM) for comparison. Animals were killed if tumor volume reached 1000 mm3. (C) Efficacy of AC220 in a bone marrow transplant model. Kaplan-Meier plots of survival. Day 1 of the study was the day after cells were implanted. The solid bar indicates the treatment period of 30 days. Each treatment group contained 5 animals. Data for the vehicle control group are shown as ■. The study was terminated on day 172.
To test the antitumor efficacy of AC220 in the MV4-11 tumor xenograft model and to compare the activity of AC220 to that of sunitinib,20 animals were treated once daily orally for 28 days, and tumor size was monitored for an additional 60 days after discontinuation of treatment. Treatment with AC220 at 10 mg/kg resulted in rapid and complete regression of tumors in all animals, and no tumor regrowth was observed during the 60-day posttreatment observation period (Figure 4B). There was no weight loss or any other obvious signs of toxicity. At the end of the study all animals remained essentially tumor free. Treatment with sunitinib also caused tumors to shrink, but not as rapidly as with AC220, and tumor growth resumed immediately upon discontinuation of treatment in all but one of the animals (Figure 4B). There was also substantially more variability in the response of individual tumors than there was for AC220 (Figure 4B). These results are consistent with previous observations with sunitinib in this model.20 Published results with sorafenib in the MV4-11 tumor xenograft model also show substantial activity with once a day oral dosing at 10 mg/kg, with complete regressions observed in 9 of 10 treated animals,17 comparable with the activity of AC220 demonstrated here.
To determine whether AC220 is also efficacious in a more physiologically relevant context that mimics some of the pathology of human leukemia, we used a mouse bone marrow engraftment model where the tumor cells establish in the bone marrow and disseminate.20 MV4-11 cells were injected into the tail vein of mice that had been previously treated with cyclophosphamide to ablate the bone marrow. After 23 days to allow the disease to establish, treatment was initiated with 0.1, 1, and 10 mg/kg AC220 or vehicle alone, using groups of 5 mice each. With once a day dosing at 10 mg/kg the free plasma concentration of AC220 should remain at or above the cellular IC50 at all times during the entire treatment period, at 1 mg/kg it likely is below the cellular IC50 during a substantial fraction of each 24-hour period, and at 0.1 mg/kg the free plasma concentration may approach the cellular IC50 only briefly after each daily dose. AC220 was administered once a day orally for 30 days. No obvious toxicity or body weight loss was attributed to treatment with AC220. In the vehicle control group animals experienced hind limb paralysis, and the mean survival time was 49 days, with all animals expired by day 52 (Figure 4C). AC220 prolonged survival in a dose-dependent manner. At 10 mg/kg, 80% of animals treated survived until the study was terminated on day 172, 119 days after discontinuation of treatment, corresponding to at least a 250% increase in life span (ILS; P < .001). At the time the study was terminated the animals did not exhibit any signs of disease. At 1 mg/kg a significant increase in the mean survival time was observed, to 77 days (55% ILS; P = .004). At the lowest dose tested of 0.1 mg/kg, a marginal 10% ILS relative to vehicle was observed (P = .94). Together, these results clearly demonstrate that AC220 is efficacious in in vivo models of FLT3-dependent disease at very modest doses.
Activity in primary AML cells
To test whether AC220 has activity in primary cells, we obtained peripheral blood blasts from a 55-year-old male with relapsed acute myeloid leukemia. The blasts harbored a 33–base pair FLT3-ITD mutation in the juxtamembrane domain. Cells were treated with AC220 for 1 hour, and the phosphorylation state of FLT3 was determined by Western blot and enzyme-linked immunoassay (ELISA; Figure 5A). FLT3 autophosphorylation was inhibited with an IC50 of 2 nM, comparable with the activity observed in the MV4-11 cell line (Table 1). To determine the effect on blast cell survival, we measured cell viability after exposure to AC220. The primary cells were sensitive to AC220, with an IC50 of 0.3 nM (Figure 5B), again comparable with the activity observed in the MV4-11 cell line (Table 1). AC220 activity against blast cells from 4 additional patients harboring FLT3-ITD mutations was similar, with IC50s ranging from 0.8 to 2 nM (Figure 5C). The potency of AC220 in blasts observed here is comparable with or better than what has been reported for other FLT3 inhibitors in primary cells.19,32,36,51 The fraction of cells sensitive to AC220 was variable among the samples tested, and a more comprehensive characterization of the effects of AC220 on primary cells will be published separately. The present results do demonstrate that AC220 is a highly potent inhibitor of FLT3 in primary patient cells.
Activity of AC220 in primary AML cells. (A) Inhibition of FLT3 phosphorylation. Blasts were incubated in increasing concentrations of AC220 with 10% FBS for 1 hour at 37°C. For the Western blot, cell lysates were subjected to immunoprecipitation, sodium dodecylsulfate polyacrylamide electrophoresis, and immunoblotting for phosphorylated and total FLT3. As is frequently observed in patient samples for which limited numbers of cells are available, phospho-FLT3 (pFLT3) levels are relatively low and only weakly detected by Western blot. Robust quantitation of phospho-FLT3 was achieved by a more sensitive ELISA (“Cellular assays”). The graph shows phospho-FLT3 levels, normalized for total FLT3, obtained by ELISA. The line represents a fit of the data to the Hill equation. (B) Effect of AC220 on cell viability. In parallel to the phosphorylation assay, using the same AC220 medium preparation, blasts from the same patient and the same thawing were incubated at 37°C in 5% CO2. After 72 hours, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay was performed, and the results were plotted as a fraction of untreated control. The line represents a fit of the data to the Hill equation. (C) Effect of AC220 on cell viability in primary cell samples from additional patients. The experiments were performed and the data are plotted as in panel B. The primary cells were obtained from a 65-year-old male (■; IC50 = 0.8 nM), a 53-year-old male (▲; IC50 = 1 nM), a 56-year-old female (●; IC50 = 1 nM), and a 34-year-old male (▼; IC50 = 2 nM), all with relapsed AML harboring FLT3-ITD mutations.
Activity of AC220 in primary AML cells. (A) Inhibition of FLT3 phosphorylation. Blasts were incubated in increasing concentrations of AC220 with 10% FBS for 1 hour at 37°C. For the Western blot, cell lysates were subjected to immunoprecipitation, sodium dodecylsulfate polyacrylamide electrophoresis, and immunoblotting for phosphorylated and total FLT3. As is frequently observed in patient samples for which limited numbers of cells are available, phospho-FLT3 (pFLT3) levels are relatively low and only weakly detected by Western blot. Robust quantitation of phospho-FLT3 was achieved by a more sensitive ELISA (“Cellular assays”). The graph shows phospho-FLT3 levels, normalized for total FLT3, obtained by ELISA. The line represents a fit of the data to the Hill equation. (B) Effect of AC220 on cell viability. In parallel to the phosphorylation assay, using the same AC220 medium preparation, blasts from the same patient and the same thawing were incubated at 37°C in 5% CO2. After 72 hours, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium assay was performed, and the results were plotted as a fraction of untreated control. The line represents a fit of the data to the Hill equation. (C) Effect of AC220 on cell viability in primary cell samples from additional patients. The experiments were performed and the data are plotted as in panel B. The primary cells were obtained from a 65-year-old male (■; IC50 = 0.8 nM), a 53-year-old male (▲; IC50 = 1 nM), a 56-year-old female (●; IC50 = 1 nM), and a 34-year-old male (▼; IC50 = 2 nM), all with relapsed AML harboring FLT3-ITD mutations.
Discussion
The association of FLT3 mutations with poor clinical outcome7,–9,11,12,15 and the therapeutic effects observed in early stage clinical trials with first-generation FLT3 inhibitors23,,,,,,,–31 strongly implicate mutant FLT3 as an important target for small molecule intervention in AML. A key question is whether the relatively modest clinical activity of first-generation FLT3 inhibitors can be improved upon, or whether the potential benefits of treatment with FLT3 inhibitors are inherently limited. The available evidence suggests that obtaining the maximum therapeutic benefit from FLT3 inhibitors will require sustained and near-complete inhibition of FLT3 kinase activity.19,–21,25,26,32 This is most likely achieved with a compound that is an extremely potent inhibitor of FLT3, that has pharmacokinetic properties compatible with maintaining free plasma levels above the IC50 for FLT3 inhibition, and that is sufficiently well tolerated to allow continuous dosing.
We present here an initial characterization of the biochemical and pharmacologic activity of the novel FLT3 inhibitor AC220. AC220 is unique among FLT3 inhibitors currently in development in that it combines high potency, excellent kinase selectivity, and favorable pharmacokinetic properties. Among the compounds tested, only CEP-701 and sorafenib were comparably potent in cellular assays for FLT3 inhibition. CEP-701 is a nonselective staurosporine analog. Sorafenib is highly potent and also has pharmacokinetic properties in animals at least as favorable as AC220, but is not as selective. Sorafenib is known to inhibit kinases other than FLT3 and class III RTKs at clinical doses, and is not currently approved for treatment of AML, or for continuous dosing in patients. Only MLN-518 has kinase selectivity comparable with AC220, however MLN-518 is 30- to 100-fold less potent than AC220 in cellular assays. In humans, both sorafenib and MLN-518 have been given with twice-a-day dosing schedules, and responses with MLN-518 generally have been transient.23,31 AC220 therefore is a second-generation FLT3 inhibitor that has been explicitly optimized for the combination of properties believed to be required for the successful treatment of AML, and specifically to test the hypothesis that selective FLT3 inhibition will result in clinical benefit. AC220 is being evaluated as a treatment for AML in clinical trials.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Paul Gallant, Antonio Torres, and the Ambit Screening Team for kinase profiling; Raffaella Faraoni, Michael Hocker, Brian Campbell, and Warren Lewis for synthesis of reference compounds; Wendell Wierenga for critical reading of the paper; and Gary Gilliland for helpful discussions.
This work was supported by grants from the National Cancer Institute (NCI; NCI Leukemia SPORE P50 CA100632-06, R01 CA128864) and the American Society of Clinical Oncology (M.L.). M.L. is a Clinical Scholar of the Leukemia & Lymphoma Society. The phylogenetic tree of the human kinome is reproduced with permission from Cell Signaling Technology Inc (http://www.cellsignal.com). With sponsorship by Cell Signaling Technology and Sugen, the figure was originally presented as a poster in Science to accompany the first analysis of the complete human kinome in a paper by Manning et al.50
National Institutes of Health
Authorship
Contribution: P.P.Z. directed research and wrote the paper; R.N.G., M.D.C., M.F.G., D.B., B.B., M.W.K., K.W.P., and G.P. conducted experiments and analyzed results; Q.C. and K.G.S. synthesized compounds; H.K.P. directed compound synthesis and the optimization of FLT3 leads to obtain AC220; M.L. directed and performed experiments on primary patient cells and analyzed results; R.C.A. directed cellular and animal studies and analyzed results; J.J. directed pharmacokinetic studies and analyzed results; and S.S.B. directed the AC220 drug discovery project.
The current address for H.K.P. is Sai Advantium Pharma Ltd, Hyderabad, India.
Conflict-of-interest disclosure: P.P.Z., R.N.G., M.D.C., M.F.G., D.B., B.B., M.W.K., G.P., Q.C., K.G.S., H.K.P., R.C.A., J.J., and S.S.B. are current or former employees and shareholders of Ambit Biosciences. M.L. received research funding from Kyowa Hakko Kogyo and is a member of the Clinical Advisory Board of Ambit Biosciences. K.W.P. declares no competing financial interests.
Correspondence: Patrick Zarrinkar, Ambit Biosciences, 4215 Sorrento Valley Blvd, San Diego, CA 92121; e-mail: pzarrinkar@ambitbio.com.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal