To the editor:
A recent article in Blood1 reported that mitochondrial polarization to the immunologic synapse (IS) was required for cytotoxic lymphocyte (CL)–induced death of cells that overexpress Bcl-2. This potentially represented a significant advance in understanding CL-induced death because there is currently no way to determine whether a given CL has made a productive killing synapse (that is, whether any granule contents were transferred to the target cell) unless the target cell goes on to die. Polarization of mitochondria in the target cell would provide an early surrogate assay for this process.
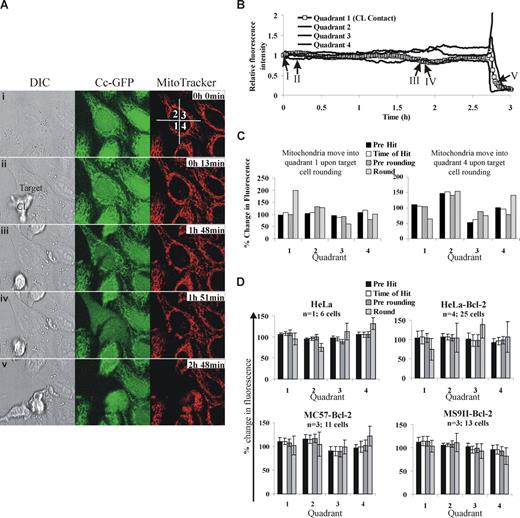
We have been following CL-induced death using time-lapse microscopy and find no evidence to support mitochondrial polarization in target cells. To ensure that our experiments had sufficient temporal and spatial resolution, we followed KHYG1-induced death of HeLa cells stained with MitoTracker Red (Molecular Probes, Eugene, OR), and acquired images every 2 minutes (Figure 1A). For analysis, we segmented the cell into 4 regions, and designated quadrant 1 as the quadrant where the effector cell made contact with its target. The other quadrants were labeled in a clockwise manner. If mitochondria polarized toward the IS, we would expect to observe increased fluorescence in quadrant 1 and a corresponding decrease in fluorescence in quadrant 3. In contrast, we observed a decrease in fluorescence in quadrants 1, 2, and 3 and a corresponding increase in fluorescence in quadrant 4 (Figure 1B). Further, these changes coincided with the target cell rounding and moving into quadrant 4, rather than polarization of the mitochondria within the cell.
Analysis of putative mitochondrial polarization in target cells during cytotoxic lymphocyte induced death. (A) HeLa cells expressing cytochrome c–green fluorescent protein (GFP) and stained with MitoTracker Red (150 nM) were mixed with KHYG1, human NK cells and images were taken every 2 minutes using a 63×/1.30 NA glycerol objective lens on a Leica SP5 confocal microscope (Leica Microsystems, Mannheim, Germany). Representative images showing (i) frame 1, (ii) KHYG1 interacting with the target, (iii) precytochrome c release, (iv) prerounding, and (v) rounding are shown. A video of this cell is available online (see the Supplemental Materials link at the top of the online article). Images were acquired by an LAS AF version 1.7.0 and processed by a MetaMorph version 7.5.5.0 (MDS Analytical Technologies, Torrance, CA). (B) To represent mitochondrial localization in the cell over time the cell was segmented into 4 quadrants (shown in panel A) where quadrant 1 was designated as the quadrant where the effector first made contact. The fluorescence of MitoTracker Red in the target cell was calculated for each frame. To account for photobleaching and random cell movement, the percentage fluorescence in each of the 4 quadrants was calculated as follows: (fluorescence intensity of the quadrant/fluorescence intensity of the entire cell) × 100. The relative fluorescence intensity was then calculated by dividing the percentage fluorescence in each quadrant by the percentage fluorescence for that quadrant in the first frame. (C) To simplify this analysis for multiple cells, we calculated the percentage fluorescence in each quadrant before any cytotoxic lymphocytes cells were added (T = 0) to establish a reference point, the time directly before CL engagement (Pre Hit), the time directly after lymphocyte engagement (Time of Hit), the time directly before the target cell rounded up (Pre rounding), and the time directly after rounding (Round). The fluorescence of each quadrant was calculated relative to that at T = 0. Data for KHYG1-induced death of 2 HeLa cells where the mitochondria move into quadrant 1 and quadrant 4, respectively, is presented. (D) To determine whether the data using KHYG1 cells were generally applicable, we followed HeLa and HeLa-Bcl-2 cells killed by NK cells isolated from human patients, and MC57-Bcl-2 or MS9II-Bcl-2 cells killed by NK cells isolated from C57BL/6 mice. The difference from T = 0 plus or minus SEM was plotted for the number of cells for each effector/target combination (n = number of individual experiements). Similar data were obtained when murine cytotoxic T cells were used as effectors (not shown).
Analysis of putative mitochondrial polarization in target cells during cytotoxic lymphocyte induced death. (A) HeLa cells expressing cytochrome c–green fluorescent protein (GFP) and stained with MitoTracker Red (150 nM) were mixed with KHYG1, human NK cells and images were taken every 2 minutes using a 63×/1.30 NA glycerol objective lens on a Leica SP5 confocal microscope (Leica Microsystems, Mannheim, Germany). Representative images showing (i) frame 1, (ii) KHYG1 interacting with the target, (iii) precytochrome c release, (iv) prerounding, and (v) rounding are shown. A video of this cell is available online (see the Supplemental Materials link at the top of the online article). Images were acquired by an LAS AF version 1.7.0 and processed by a MetaMorph version 7.5.5.0 (MDS Analytical Technologies, Torrance, CA). (B) To represent mitochondrial localization in the cell over time the cell was segmented into 4 quadrants (shown in panel A) where quadrant 1 was designated as the quadrant where the effector first made contact. The fluorescence of MitoTracker Red in the target cell was calculated for each frame. To account for photobleaching and random cell movement, the percentage fluorescence in each of the 4 quadrants was calculated as follows: (fluorescence intensity of the quadrant/fluorescence intensity of the entire cell) × 100. The relative fluorescence intensity was then calculated by dividing the percentage fluorescence in each quadrant by the percentage fluorescence for that quadrant in the first frame. (C) To simplify this analysis for multiple cells, we calculated the percentage fluorescence in each quadrant before any cytotoxic lymphocytes cells were added (T = 0) to establish a reference point, the time directly before CL engagement (Pre Hit), the time directly after lymphocyte engagement (Time of Hit), the time directly before the target cell rounded up (Pre rounding), and the time directly after rounding (Round). The fluorescence of each quadrant was calculated relative to that at T = 0. Data for KHYG1-induced death of 2 HeLa cells where the mitochondria move into quadrant 1 and quadrant 4, respectively, is presented. (D) To determine whether the data using KHYG1 cells were generally applicable, we followed HeLa and HeLa-Bcl-2 cells killed by NK cells isolated from human patients, and MC57-Bcl-2 or MS9II-Bcl-2 cells killed by NK cells isolated from C57BL/6 mice. The difference from T = 0 plus or minus SEM was plotted for the number of cells for each effector/target combination (n = number of individual experiements). Similar data were obtained when murine cytotoxic T cells were used as effectors (not shown).
To determine whether the data using KHYG1 cells was generally applicable, we followed HeLa-Bcl-2 cells killed by natural killer (NK) cells isolated from human patients, and MC57-Bcl-2 or MS9II-Bcl-2 cells killed by NK cells isolated from C57BL/6 mice. To evaluate multiple target cells in a reproducible manner, we measured MitoTracker fluorescence in each region before any NK cells were added (T = 0, to establish a reference point), directly before CL engagement (Pre-Hit), directly after CL engagement (Post-hit), directly before the target cell rounded up (Pre-rounding), and directly after the target cell rounded (Round). We clearly detected mitochondrial movement when individual target cells rounded up (Figure 1C), but we consistently found equal distribution of mitochondria in all quadrants before target cell rounding (Figure 1D). Mitochondrial polarization in the target cell is therefore not an early event, generally required for CL-induced killing of Bcl-2–overexpressing cells.
Our conclusions contrast with those of Goping et al.1 One explanation for this may be that the previous study analyzed suspension cells at only one time point. The mitochondria in these cells could not be followed over time, and it was not possible to determine whether the conjugates analyzed formed a killing synapse. Our study investigated adherent cells where mitochondria could be tracked in real time, and the conjugates analyzed verifiably involved a killing synapse; parameters that are critically important to objectively address this issue. It does remain possible, however, that mitochondrial polarization is required in some, but not all, models of CL-induced death of cells that overexpress Bcl-2.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel J. Waterhouse, Peter MacCallum Cancer Centre, Locked Bag 1, A'Beckett Street, Melbourne Victoria 8006, Australia; e-mail: nigel.waterhouse@petermac.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal