Cytotoxic T lymphocytes (CTLs) eliminate pathogenic cells in large part through the activity of the serine protease granzyme B (grB). However, while the apoptotic activity of grB is blocked by over-expression of Bcl-2, CTLs can still kill target cells through an ill-defined Bcl-2–independent pathway. In this report, we have identified key modulators of this Bcl-2–independent cell-death pathway, which is induced by CTLs and not purified components. Surprisingly, activation of this pathway is reliant on grB. Furthermore, this novel pathway requires mitochondrial contribution through triggering of permeability transition and generation of reactive oxygen species, yet is functional in the absence of Bax/Bak. This pathway stimulates movement of target cell mitochondria toward the point of contact with the CTLs and importantly, inhibition of this directed movement attenuates killing. Therefore, we propose that CTLs initiate a target cell response that activates multiple mitochondrial pathways. This ensures that CTLs can eliminate those target cells that have compromised apoptotic potential due to overexpression of Bcl-2.
Introduction
One of the major mechanisms used by cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells to destroy pathogenic cells involves directed exocytosis of lytic molecules from the effectors toward the targets.1,,,–5 Key proteins, such as perforin, a membrane-disrupting agent, and granzymes, a family of serine proteinases, are contained in cytoplasmic granules that polarize toward the contact interface of the immunologic synapse.6 Once perforin is released toward the target cell, it facilitates uptake of the granzymes into the target cell cytoplasm.7 The proteolytic activities of granzymes result in cleavage of key substrates that activate the dormant cell-death machinery of the cell under attack
The 2 major granzymes that have been extensively studied are granzyme A (grA) and granzyme B (grB). The former is a tryptic protease that acts on a number of substrates, including Ape1,8 SET,9 HMG-2,10 TREX1,11 and Ku70.12 GrB is a serine protease with an unusual substrate specificity and cleaves after aspartate residues.13,14 One key substrate for grB is the effector caspase, caspase-3.15 Initially, it was thought that grB acted to induce cell death solely through the activation of caspase-3; however, it then became clear that the situation was more complex.16
It was somewhat surprising to discover that the grB pathway was blocked by the antiapoptotic protein Bcl-2. This finding suggested that mitochondria were involved in the mechanism of action of this granzyme. Indeed, the loss of mitochondrial membrane potential17,18 and cytochrome c release19,20 occurred due to grB-mediated cleavage and activation of Bid.21 Furthermore, we and others demonstrated that mitochondrial release of Smac/DIABLO22,23 was required to relieve inhibitor of apoptosis protein (IAP)–mediated repression of caspase activation.24,25 Inhibition of Smac/DIABLO release by increased levels of Bcl-2 protected cells from grB-induced apoptosis. Therefore, these studies demonstrated the important role of mitochondrial signaling for caspase activation.24,–26
However, within the context of cell-cell killing, investigations aimed to determine whether Bcl-2 overexpression protects cells from cytotoxic cell-mediated cell death have been less clear, with studies showing both inhibition27 and lack of effect of Bcl-2.28 Since grB is one of the major effector molecules of cytotoxic lymphocytes, and because we now have a clearer understanding of the mechanism of Bcl-2 inhibition of grB-mediated cell death, we decided to clarify whether cytotoxic lymphocyte-activated cell death was indeed blocked by Bcl-2 overexpression through analysis of multiple apoptotic parameters. More notably, our aim was to elucidate the molecular mechanism of target cell response to CTL-directed attack in the presence or absence of high levels of Bcl-2. Importantly, by evaluating the role of target cell mitochondria in CTL-mediated cell death, these studies have revealed a novel CTL-directed mechanism of mitochondrial-mediated apoptosis initiation within the target cell.
Using both human and mouse target cells that expressed high levels of Bcl-2, we confirmed the study by Sutton et al28 that demonstrated target cell death even in the presence of overexpressed Bcl-2. These observations suggested that CTLs could either bypass or inactivate Bcl-2 in target cells. Indeed, we show that the Bid/Bax/Bak arm of the pathway is not required. However, this novel mechanism still appears to involve mitochondria and caspases. Most exciting, we observed that target cell mitochondria aligned along the point of contact with the cytotoxic cell. Inhibition of this directed mitochondrial movement within target cells resulted in diminished CTL-mediated apoptosis. Thus, through mobilization of mitochondria, target cells play an active role in their own demise.
Methods
Use of mice in this study was approved by the University of Alberta Review Board.
Cell lines and reagents
The human T-cell lymphoma line Jurkat and transfected JBcl-2 cells were maintained as described previously.19 Human grB was purified from the cytolytic granules of the human NK line YT-Indy (grA-deficient29 ), as previously described.30 The purified grB preparation was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) silver stain/Western blot and displayed one prominent band of expected molecular weight and immunoreactivity for grB, respectively. Human replication-deficient adenovirus (AD) type 5d170-3 was purified from infected 293 cells, as described. Human lymphocytes were prepared as described.31 Briefly, lymphocytes were incubated with irradiated, Epstein-Barr virus (EBV)–transformed RPMI-8666 cells. After 3 days of coculture, CTLs and RPMI-8666 were separated by centrifugation through Ficoll, and CTLs were maintained in RPMI 1640 media containing 10% (vol/vol) fetal bovine serum (FBS) and 20 U/mL IL-2. Mouse lymphocytes were prepared as previously described.32 The mouse lymphocytes were generated from control mice (strain ID: C57BL/6 × 12953/SV1mJ; background: C57BL/6 and 129; obtained from Dr T. J. Ley, Washington University, St Louis, MO) and mice homozygous for null mutations in the grB gene (strain ID: B6.129S Gzmb [Tm1 Ley]; background: B6/129; obtained from Dr T. J. Ley), grA gene (strain ID: C57.129SGzmA; background: C57/129; obtained from Dr T. J. Ley), or perforin gene (strain ID: C57BL/6 Pfp; background: C57BL/6; obtained from Dr W. Clark, University of California, Los Angeles). Splenocytes from these mice were activated in primary mixed lymphocyte cultures using irradiated splenocytes from C3H (H-2K) mice (shown in Figure 5), or BALB/c (H-2d) mice (shown in Figure 6) as stimulators for 3 days. CTLs and dead stimulator cells were separated by centrifugation through Ficoll, and CTLs were maintained in RPMI 1640 media containing 10% (vol/vol) FBS and 20 U/mL IL-2. Cyclosporin A, D1417, colchicine, and verapamil were purchased from Sigma-Aldrich (St Louis, MO).
Cell-death assays
GrB/AD-mediated cell death was carried out as described previously.33 Briefly, 2 × 105 cells/25 μL were treated with indicated amounts of grB and 100 pfu of AD in serum-free media supplemented with 0.1% bovine serum albumin (BSA). For experiments using higher concentrations of grB (as shown in Figure 7B), grB was initially concentrated 5-fold by centrifugation on Amicon centrifugal filters (Millipore, Billerica, MA). The cells were incubated for 3 hours at 37°C before being harvested and assessed for apoptosis. For CTL-mediated apoptosis, CTLs and target cells were incubated in the indicated effector-target (E/T) ratios in complete media before being harvested and assessed for apoptosis.
Apoptosis detection systems
The 3H-thymidine release assays have been previously described.32 The percentage of specific 3H-thymidine release was calculated as follows: [(sample cpm − spontaneous cpm) / (total cpm − spontaneous cpm)] × 100. Caspase activation and mitochondrial depolarization were determined as follows. Caspase activation was assessed using the fluorescent caspase indicator dye FITC-VAD-FMK (CaspACE; Promega, Madison, WI). Mitochondrial electrochemical potential loss (ΔΨ loss) was assessed using the dye tetramethylrhodamine, ethyl ester, perchlorate (TMRE; Invitrogen, Carlsbad, CA) which fluoresces in the FL-2 channel in cells with healthy respiring mitochondria. Briefly, target cells were initially labeled with CellTrace Far Red DDAO-SE (CTFR; Invitrogen) according to the manufacturer's directions. CTFR-labeled target cells would be detected in the FL-4 channel during flow cytometry, and thus could be distinguished from nonlabeled effector cells. After target cells were treated with grB/AD or CTLs, all cells were labeled with CaspACE and TMRE. Caspase activation in target cells was assessed by analysis of the FL1 and FL4 double-positive population. The percentage of specific caspase activation was determined as (% CaspACE+ cells of treated sample − % CaspACE+ cells of untreated control sample). Mitochondrial depolarization in target cells was assessed by analysis of the FL2− and FL4+ population. The percentage of specific TMRE loss was determined as (% TMRE− cells of treated sample − % TMRE− cells of untreated control sample). Flow cytometry was performed on a Becton Dickinson FACSCalibur (BD Biosciences, San Jose, CA) and analyzed using CellQuest software (BD Biosciences).
Target cell mitochondrial outer membrane permeablization (MOMP) was determined by analysis of loss of staining of Smac/DIABLO through a fluorescence-activated cell sorter (FACS)–based assay.34 Briefly, target cells were prestained with CTFR, as described previously and then incubated with CTLs or grB/AD. Cells were resuspended in digitonin buffer (80 mM KCl, 50 ng/mL digitonin, and 1 mM EDTA in phosphate-buffered saline [PBS]), which would cause loss of cytosolic proteins, and then processed for intracellular staining of Smac/DIABLO or the control COXIV, followed by analysis by flow cytometry.
Microscopy
For analysis of conjugates, effectors and targets in RPMI 1640 media were mixed and spotted onto glass coverslips for 5 minutes at 37°C and fixed in 4% paraformaldehyde; slides were mounted in ProLong AntiFade media (Invitrogen). Scoring of mitochondrial localization in target cells of effector–target cell conjugates was performed on a Zeiss Axiovert 100M fluorescence microscope (Jena, Germany). Representative images were acquired at room temperature on a LSM510 laser scanning confocal microscope mounted on a Zeiss Axiovert 100M microscope fitted with a Plan Neofluar objective 40×/1.3 and analyzed with LSM5 software (Zeiss).
Results
CTLs cause target cell death of Bcl-2–overexpressing cells
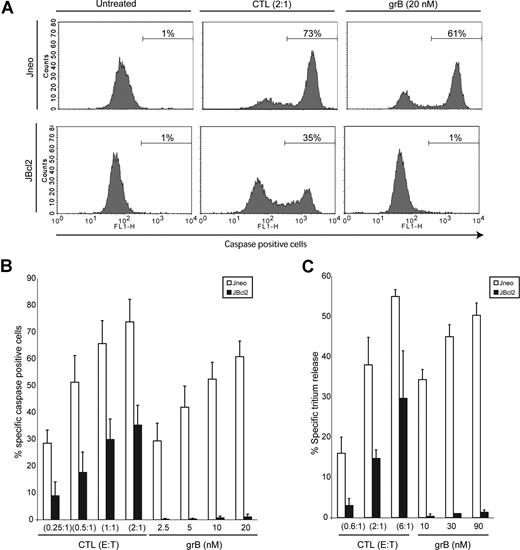
To determine the level of protection imparted by high levels of Bcl-2 on target cell death induced by cytotoxic lymphocytes, we analyzed numerous apoptotic features of target cells that had been incubated with cytotoxic cells. Control cells (Jneo) or cells overexpressing Bcl-2 (JBcl-2) were incubated with effector CTLs at increasing E/T ratios, and apoptotic features of the target cell were analyzed (Figure 1). Target cell death was also initiated by incubation with purified grB and replication deficient AD. AD is a well-characterized functional substitute for the granular pore-forming protein perforin, inducing endosomal disruption to facilitate the delivery of grB into the cytosol of the target cell.35 Figure 1A is a representative flow cytometry plot detailing caspase activation of target cells that were treated with CTLs or the control grB, and Figure 1B shows a comprehensive summary of the results. Target cells were incubated with increasing E/T ratios, or increasing grB concentrations. Jneo target cells displayed similar amounts of caspase activity when treated with increasing amounts of CTLs or purified grB (Figure 1B white bars). Both the percentage of caspase-positive cells, in addition to the mean fluorescence, as an indication of the amount of caspase activity within each cell, were similar in CTL- or grB-treated Jneo cells. In contrast, cells overexpressing Bcl-2 (Figure 1B black bars) showed a marked difference in caspase activation with respect to treatment with either CTLs or purified grB. CTLs were able to induce caspase activation in JBcl-2 target cells to levels from 30% to 50% of the levels achieved in Jneo targets, whereas treatment of those same cells with purified grB and AD resulted in little to no caspase activation. Similar results were obtained for cells that had been assessed for DNA damage as revealed by the 3H-release assay (Figure 1C), which is an indicator of DNA damage. Again, JBcl-2 target cells incubated with CTLs displayed DNA damage ranging from 20% to 50% of levels reached in control Jneo cells. As expected, treatment with purified grB and AD did not result in DNA damage of JBcl-2 cells. Therefore, similar to the results obtained when analyzing caspase activation, over-expression of Bcl-2 diminished but did not abolish CTL-mediated cytotoxicity. Nevertheless, the level of Bcl-2 expressed in these JBcl-2 clones are sufficient to abrogate apoptosis initiated by purified grB (Figures 1,2), anti-Fas treatment,24 UV treatment, and staurosporine treatment (data not shown). Therefore, while increased levels of Bcl-2 can protect cells from apoptosis initiated by purified grB/AD treatment, these same levels of Bcl-2 do not completely protect cells from CTL-mediated apoptosis.
CTLs cause target cell death of Bcl-2–overexpressing cells. (A) CTLs induce caspase activation in target cells overexpressing Bcl-2. Jneo cells (top row) and JBcl-2 cells (bottom row) were preloaded with Cell Trace Far red (FL-4) prior to incubation with CTLs (E/T ratio = 2:1) or grB/AD (20 nM grB). Cells were stained with the caspase indicator zVAD-FITC, and FL-4+ target cells were analyzed for caspase activation in the FL-1 (FITC) channel. This is a representative example of 1 of 4 independent experiments done in triplicate. (B) CTLs induce caspase activation in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.25:1, 0.5:1, 1:1, and 2:1 (CTLs), or 2.5 nM, 5 nM, 10 nM, and 20 nM grB with AD, then loaded with the caspase indicator zVAD-FITC. FL-4+ target cells were analyzed for caspase activation by flow cytometry. The numbers indicate the average of 4 independent experiments done in triplicate, with the corresponding standard deviation. (C) CTLs induce tritium release in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs at E/T ratios of 0.6:1, 2:1, and 6:1 (CTLs), or 10 nM, 30 nM, and 90 nM grB (grB). The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
CTLs cause target cell death of Bcl-2–overexpressing cells. (A) CTLs induce caspase activation in target cells overexpressing Bcl-2. Jneo cells (top row) and JBcl-2 cells (bottom row) were preloaded with Cell Trace Far red (FL-4) prior to incubation with CTLs (E/T ratio = 2:1) or grB/AD (20 nM grB). Cells were stained with the caspase indicator zVAD-FITC, and FL-4+ target cells were analyzed for caspase activation in the FL-1 (FITC) channel. This is a representative example of 1 of 4 independent experiments done in triplicate. (B) CTLs induce caspase activation in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.25:1, 0.5:1, 1:1, and 2:1 (CTLs), or 2.5 nM, 5 nM, 10 nM, and 20 nM grB with AD, then loaded with the caspase indicator zVAD-FITC. FL-4+ target cells were analyzed for caspase activation by flow cytometry. The numbers indicate the average of 4 independent experiments done in triplicate, with the corresponding standard deviation. (C) CTLs induce tritium release in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs at E/T ratios of 0.6:1, 2:1, and 6:1 (CTLs), or 10 nM, 30 nM, and 90 nM grB (grB). The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
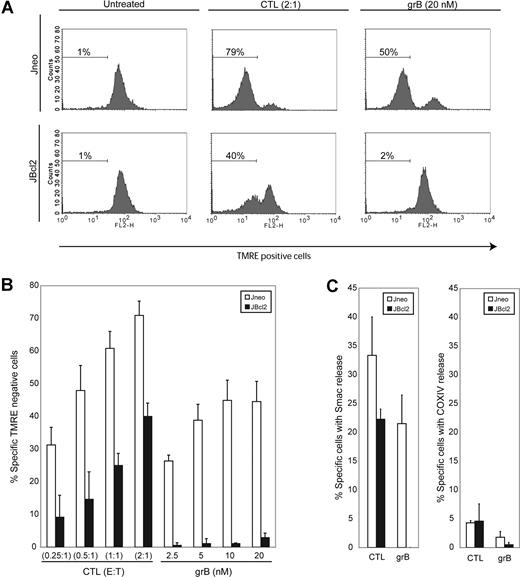
CTLs activate the mitochondrial apoptotic pathway in Bcl-2–overexpressing cells. (A) CTLs induce mitochondrial electrochemical potential loss in target cells overexpressing Bcl-2. Jneo cells (top row) and JBcl-2 cells (bottom row) were preloaded with CTFR (FL-4) prior to incubation with CTLs (E/T ratio = 2:1) or grB/AD (20 nM grB). Cells were stained with the mitochondrial potential indicator dye TMRE, and FL-4+ target cells were analyzed for TMRE loss in the FL-2 channel. This is a representative example of 1 of 4 independent experiments done in triplicate. (B) CTLs induce mitochondrial electrochemical potential loss in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.25:1, 0.5:1, 1:1, and 2:1 (CTLs), or 2.5 nM, 5 nM, 10 nM, and 20 nM grB with AD, then loaded with the mitochondrial potential indicator dye TMRE. FL-4+ target cells were analyzed for TMRE loss by flow cytometry. The numbers indicate the average of 4 independent experiments done in triplicate, with the corresponding standard deviation. (C) CTLs induce MOMP in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at an E/T ratio of 2:1 or grB/AD (10 nM grB). Cells were gently permeabilized with digitonin to release soluble cytosolic proteins prior to fixation. Cells were then completely permeabilized with saponin and processed for intracellular staining for Smac/DIABLO (left panel) or COXIV (right panel).
CTLs activate the mitochondrial apoptotic pathway in Bcl-2–overexpressing cells. (A) CTLs induce mitochondrial electrochemical potential loss in target cells overexpressing Bcl-2. Jneo cells (top row) and JBcl-2 cells (bottom row) were preloaded with CTFR (FL-4) prior to incubation with CTLs (E/T ratio = 2:1) or grB/AD (20 nM grB). Cells were stained with the mitochondrial potential indicator dye TMRE, and FL-4+ target cells were analyzed for TMRE loss in the FL-2 channel. This is a representative example of 1 of 4 independent experiments done in triplicate. (B) CTLs induce mitochondrial electrochemical potential loss in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.25:1, 0.5:1, 1:1, and 2:1 (CTLs), or 2.5 nM, 5 nM, 10 nM, and 20 nM grB with AD, then loaded with the mitochondrial potential indicator dye TMRE. FL-4+ target cells were analyzed for TMRE loss by flow cytometry. The numbers indicate the average of 4 independent experiments done in triplicate, with the corresponding standard deviation. (C) CTLs induce MOMP in target cells overexpressing Bcl-2. Jneo cells (□) and JBcl-2 cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at an E/T ratio of 2:1 or grB/AD (10 nM grB). Cells were gently permeabilized with digitonin to release soluble cytosolic proteins prior to fixation. Cells were then completely permeabilized with saponin and processed for intracellular staining for Smac/DIABLO (left panel) or COXIV (right panel).
Nevertheless, while CTL-mediated cell death is diminished in these Bcl-2–overexpressing cells, these cells are not completely protected from CTL-mediated apoptosis. In fact, a significant proportion of these target cells still die (50%-80%). Therefore, CTLs use 2 distinct pathways when activating cell death in target cells that express high levels of Bcl-2. One pathway is inhibited by Bcl-2, and the other is independent of Bcl-2. Certainly, grB-activated apoptotic mechanisms contribute to cell death in the Bcl-2–dependent apoptotic pathway. However, the signaling pathways which trigger the Bcl-2–independent CTL-mediated apoptotic pathways are completely unknown. Therefore, we resolved to characterize and decipher the molecular mechanisms of CTL-directed, Bcl-2–independent cell death.
The CTL-activated Bcl-2–independent pathway activates the mitochondrial apoptotic pathway
Bcl-2 is mainly localized to the mitochondrion and inhibits mitochondrial dysfunction initiated by various apoptotic stimuli.36 We wished to determine whether mitochondria were involved in the CTL-mediated Bcl-2–independent death pathway. First, we assessed mitochondrial dysfunction by analysis of electrochemical potential in Bcl-2–overexpressing targets that had been treated with CTLs. Nonapoptotic cells with healthy, respiring mitochondria will be fluorescent when incubated with the potentiometric dye TMRE. As cells activate the mitochondrial apoptotic program and lose the electrochemical potential (Δψ) across the inner membrane, red fluorescence will diminish. As can be seen in Figure 2A,B, JBcl-2 target cells incubated with CTLs displayed mitochondrial loss of ΔΨ ranging from 20% to 50% of levels reached in control Jneo cells. Clearly, overexpression of Bcl-2 diminished but did not completely inhibit Δψ loss as mediated by CTLs. As expected, treatment with purified grB and AD protected JBcl-2 cells from mitochondrial damage.
We also assessed mitochondrial dysfunction by determining the amount of MOMP via release of the mitochondrial proapoptotic protein Smac/DIABLO.22,23 As can be seen in Figure 2C, target cells treated with either CTLs or grB/AD displayed a robust loss of Smac/DIABLO staining (33% or 22%, respectively). However, a significant difference was seen in the Bcl-2–transfected cells. While Smac/DIABLO release was completely inhibited in response to grB/AD, CTL treatment still liberated Smac/DIABLO to the cytosol. Smac/DIABLO release was due to MOMP and not due to nonspecific destruction of mitochondria, as there was little loss of staining of the control integral membrane protein, COXIV (Figure 2C right panel). Thus, although Bcl-2 can block mitochondrial dysfunction when cells are treated with purified grB as the apoptotic initiator, in the presence of whole CTLs, proapoptotic protein Smac/DIABLO is still released from the mitochondria and presumably facilitates the activation of caspases. Therefore, target cells incubated with CTLs activate mitochondrial apoptotic pathways even in the presence of Bcl-2.
The Bcl-2–independent cell-death pathway is also independent of Bax/Bak and Bid
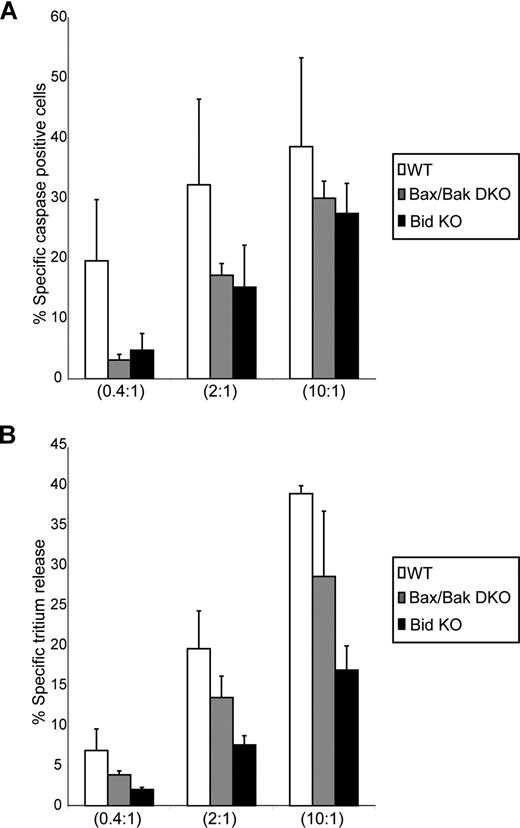
We sought to investigate the mechanism whereby CTLs activate the mitochondrial apoptotic pathway in the presence of overexpression of Bcl-2. It is possible that CTLs trigger a pathway that results in the direct inactivation of Bcl-2, and thus cells overexpressing Bcl-2 would not be protected against cell death (as is the case here). However, cells that harbor perturbations in the pathway to mitochondrial dysfunction that occur upstream or downstream of Bcl-2 action should be resistant to CTL-mediated apoptosis. We tested this hypothesis with target cells that lack the apoptotic activating molecules Bid and Bax/Bak, and assessed their sensitivity to CTL-mediated killing. A total of 2 readouts of apoptosis were monitored: caspase activation and DNA fragmentation. As seen in Figure 3, significant levels of both caspase activation and DNA fragmentation occurred when the Bid or Bax/Bak knockout (KO) cells were used as targets for CTLs. Therefore, CTLs can kill targets that not only overexpress the antiapoptotic protein Bcl-2, but can also kill cells that lack the ability to activate MOMP through the well-characterized Bid/Bax/Bak pathway. This suggests that CTLs do not overcome Bcl-2 protection simply by inhibiting Bcl-2 function, but may subvert the requirement for formation of Bax/Bak pores to facilitate Smac/DIABLO release and subsequent caspase activation and DNA damage.
CTLs cause target cell death of Bid- and Bax/Bak-null target cells. (A) CTLs induce caspase activation in target cells lacking expression of Bid and Bax/Bak. Wild-type (WT) cells (□) and Bax/Bak double-KO cells (Bax Bak DKO; ▩) and Bid KO cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1, then loaded with the caspase indicator zVAD-FITC. FL-4+ target cells were analyzed for caspase activation by flow cytometry. The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation. (B) CTLs induce tritium release in target cells lacking expression of Bid and Bax/Bak. WT cells (□) and Bax Bak DKO (▩) and Bid KO cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1. The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
CTLs cause target cell death of Bid- and Bax/Bak-null target cells. (A) CTLs induce caspase activation in target cells lacking expression of Bid and Bax/Bak. Wild-type (WT) cells (□) and Bax/Bak double-KO cells (Bax Bak DKO; ▩) and Bid KO cells (■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1, then loaded with the caspase indicator zVAD-FITC. FL-4+ target cells were analyzed for caspase activation by flow cytometry. The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation. (B) CTLs induce tritium release in target cells lacking expression of Bid and Bax/Bak. WT cells (□) and Bax Bak DKO (▩) and Bid KO cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1. The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
Mitochondria are involved in the CTL-mediated Bcl-2–independent cell-death pathway
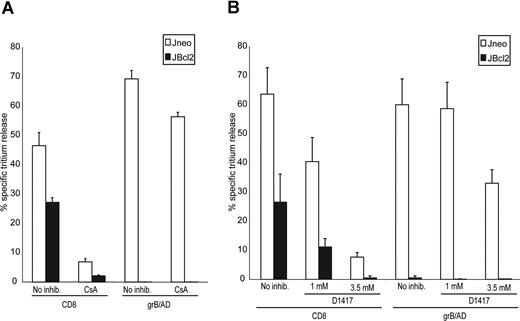
An alternative mechanism that causes the release of proapoptotic molecules from the mitochondria is characterized by the sustained opening of the mitochondrial permeability transition pore (PTP). Therefore, we tested whether the opening of the PTP was a required step in CTL-mediated apoptosis. Cyclosporine A (CsA) is a potent inhibitor of the PTP,37 and has been used to test the involvement of the PTP in apoptosis.38,,–41 To control for loading conditions in experiments where CsA was used,42 we simultaneously treated the cells with verapamil,43 which is an inhibitor of the plasma membrane drug resistance pump,44 but not an inhibitor of PTP.45 We have previously shown that CsA does not inhibit grB/AD-mediated death.18 In contrast, CsA treatment repressed CTL-mediated killing of Jneo cells (Figure 4A). This CsA-dependent protection was also evident in JBcl-2 cells, suggesting that activation of the PTP is a requirement for both the Bcl-2–dependent and – independent pathways. Furthermore, these results demonstrate that the activation of the mitochondrial pathway as initiated by CTLs is not merely a consequence of the cell dying, but is required for execution of apoptosis. Therefore, although purified grB activates apoptosis independent of PTP opening, CTLs can also trigger target cell death through a pathway that requires PTP signaling.
CTL-induced apoptosis is inhibited by the PTP inhibitor CsA and the ROS scavenger D1417. (A) CTL-induced tritium release in target cells overexpressing Bcl-2 is dependent on the PTP. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs (E/T ratio = 2:1) or grB/AD in the presence or absence of 100 μM CsA and 5 μM verapamil. The percentage of tritium release, which is an indication of DNA fragmentation, was determined as is outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation. (B) CTL-induced tritium release in target cells overexpressing Bcl-2 is dependent on the production of ROS. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs (E/T ratio = 2:1) or grB/AD in the presence or absence of 1 mM or 3.5 mM D1417, a ROS scavenger. The percentage of tritium release, which is an indication of DNA fragmentation, was determined as outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
CTL-induced apoptosis is inhibited by the PTP inhibitor CsA and the ROS scavenger D1417. (A) CTL-induced tritium release in target cells overexpressing Bcl-2 is dependent on the PTP. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs (E/T ratio = 2:1) or grB/AD in the presence or absence of 100 μM CsA and 5 μM verapamil. The percentage of tritium release, which is an indication of DNA fragmentation, was determined as is outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation. (B) CTL-induced tritium release in target cells overexpressing Bcl-2 is dependent on the production of ROS. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs (E/T ratio = 2:1) or grB/AD in the presence or absence of 1 mM or 3.5 mM D1417, a ROS scavenger. The percentage of tritium release, which is an indication of DNA fragmentation, was determined as outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
Another way to assess the importance of mitochondria in CTL-mediated cell death is to analyze the role played by reactive oxygen species (ROS). ROS are produced in mitochondria and accumulate in apoptotic cells. Increased levels of ROS cause cellular damage, including lipid modifications and DNA damage, which can then, in turn, sensitize mitochondria to PTP.46 If this aspect of mitochondrial involvement was required for the CTL-mediated Bcl-2–independent cell-death pathway, then incubation with ROS scavengers would attenuate Bcl-2–independent cell death. The testing of this hypothesis is shown in Figure 4B. Target cells overexpressing Bcl-2 were incubated with CTLs in the presence and absence of the ROS scavenger D1417. Figure 4B demonstrated that sequestration of ROS caused a marked reduction in CTL-mediated cell death as assessed by tritium release. In contrast, grB/AD-mediated cell death was less reliant on ROS production. This result, in addition to the requirement for the PTP, demonstrated that mitochondria were indeed required for the execution of the CTL-mediated Bcl-2–independent apoptotic pathway.
CTL-mediated Bcl-2–independent pathway is dependent on grB
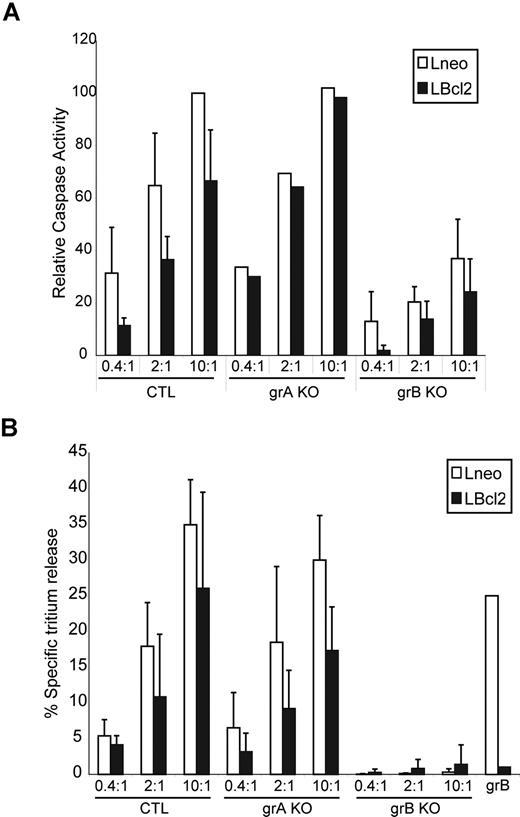
Purified grB delivered into the target cell cytoplasm is not capable of activating a Bcl-2–independent cell-death program (Figure 1). grA, on the other hand, can activate mitochondrial damage through a Bcl-2–independent cell death pathway, although grA-mediated cell death cannot be detected by tritium release or caspase activation.47 However, tritium release and caspase activation are the parameters that are activated in the observed CTL-dependent, Bcl-2–independent pathway described here. Thus, it appeared unlikely that grA would contribute to the apoptotic changes we observed in the Bcl-2–independent pathway, although we decided to specifically address the role of grA by using grA-deficient CTLs. CTLs derived from mice carrying targeted deletions in Gzma and Gzmb were isolated, and their ability to initiate apoptosis in target cells overexpressing Bcl-2 were assessed (Figure 5). Mouse target cells (L cells) were generated that overexpressed Bcl-2. Similar to the Jurkat cells that overexpressed Bcl-2 (JBcl-2), the mouse L cells that were transfected with Bcl-2 (LBcl-2) did not display caspase activation and tritium release when incubated with purified grB/AD, but were still killed by incubation with wild-type (WT) CTLs (Figure 5). Furthermore, grA-null CTLs also initiated caspase activation and tritium release in Bcl-2–overexpressing targets. In contrast, grB-null CTLs showed reduced caspase activation and nearly complete loss of tritium release in both control and Bcl-2–overexpressing targets. Thus, grB and not grA was required for the execution of the Bcl-2–dependent and – independent cell death pathways. The important result is that the CTLs can overcome the Bcl-2 block in the absence of grA. Therefore, this Bcl-2–independent killing is dependent on grB, probably in conjunction with other unknown factors.
CTL target cell apoptosis is dependent on grB, and the Bcl-2 block is still reversed in the absence of grA. CTLs were derived from splenocytes of control WT mice (WT), grA KO mice (grA KO), and grB-cluster KO mice (grB KO). (A) Control mouse L cells (Lneo; □) and Bcl-2–overexpressing L cells (LBcl-2; ■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1. Cells were then loaded with the caspase indicator zVAD-FITC (FL-1), and caspase activation of target cells (FL-4+ cells) were assessed by flow cytometry. Results of each treatment are graphed as the percentage of specific caspase-positive cells relative to the percentage of specific caspase-positive target cells treated with WT CTLs at an E/T ratio of 10:1. (B) Lneo cells (□) and LBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1. The percentage of specific tritium release was determined as outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
CTL target cell apoptosis is dependent on grB, and the Bcl-2 block is still reversed in the absence of grA. CTLs were derived from splenocytes of control WT mice (WT), grA KO mice (grA KO), and grB-cluster KO mice (grB KO). (A) Control mouse L cells (Lneo; □) and Bcl-2–overexpressing L cells (LBcl-2; ■) were preloaded with CTFR (FL-4) and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1. Cells were then loaded with the caspase indicator zVAD-FITC (FL-1), and caspase activation of target cells (FL-4+ cells) were assessed by flow cytometry. Results of each treatment are graphed as the percentage of specific caspase-positive cells relative to the percentage of specific caspase-positive target cells treated with WT CTLs at an E/T ratio of 10:1. (B) Lneo cells (□) and LBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs at E/T ratios of 0.4:1, 2:1, and 10:1. The percentage of specific tritium release was determined as outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation.
Target cell mitochondria migrate to point of contact between effector cell and target cell
The observation that grB is required for the Bcl-2–independent pathway raised the question of why purified grB could not activate the Bcl-2–independent pathway. It is possible that once released into the target cell cytoplasm, grB cooperates with another CTL-delivered molecule to induce target cell apoptosis despite increased levels of Bcl-2. Alternatively, grB could cooperate with another signaling pathway that has been activated by CTLs, possibly through CTL–target cell contact. Clearly, CTL interaction with target cells activates a number of signaling pathways that are not triggered by purified grB alone.
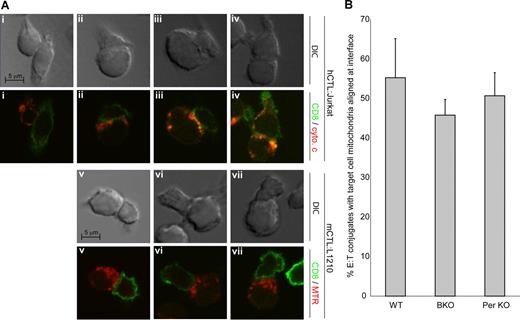
We decided to address the question of whether the Bcl-2–independent pathway was triggered by signaling pathways that were activated upon CTL–target cell contact. Knowing that CTLs activate the mitochondrial apoptotic program within the target cell, we observed the target cell mitochondria of human CTL–target cell conjugates using microscopy. Human Jurkat target cells and human CTLs were mixed and examined by immunofluorescence using anti-CD8 to identify the CTL and anti–cytochrome c antibodies to determine the localization of mitochondria. Analysis of conjugates was done within 5 minutes of cell mixing so that mitochondrial release of cytochrome c had not yet occurred. Conjugates between CTLs and target cells (1 CTL in contact with 1 target cell) were identified. We observed that unconjugated target cells and 75% of the target cells that were conjugated with CTLs had mitochondrial localization that was distributed throughout the cytoplasm, as shown in Figure 6Ai. However, in 25% of conjugates, the target cells displayed a mitochondrial distribution, which was polarized toward the effector cell (Figure 6Aii-iv). To determine whether mitochondrial polarization also occurred in mouse cell conjugates, we repeated conjugate analyses with mouse CTLs incubated with mouse L1210 targets. We prelabeled L1210 target cells with Mitotracker Red and repeated conjugate experiments as described, identifying CTLs with anti-CD8. We observed similar mitochondrial polarization in 54% of mouse target cell conjugates (Figure 6Av-vii). Thus, mitochondria from both human and mouse target cells were clustered at the point of contact between the 2 cells and were more closely aligned toward the membranes near the predicted immunologic synapse. This suggested that upon contact with CTLs, the mitochondria within the target cell are transported toward the CTLs.
CTLs cause target cell–mitochondria polarization to point of contact. (A) Representative microscope images of target cell mitochondrial localization in E/T conjugates. Target Jurkat cells (i-iv) or target mouse L1210 (v-vii) were incubated with human CTLs (i-iv) or mouse CTLs (v-vii) for 5 minutes at 37°C prior to fixation. Samples were processed for immunofluorescence using anti-CD8 antibody (secondary anti-mouse 488) to identify CTLs, as described in “Microscopy.” Effector–target cell conjugates were identified, and locations of target cell mitochondria were scored. Mitochondrial localization was determined by immunofluorescence using anti–cytochrome c (Jurkat, anti-mouse 555) or Mitotracker Red (L1210). Shown are representative DIC (top) and fluorescent (bottom) images of mitochondria not aligned at conjugate interface (i), or aligned at conjugate interface (ii-vii) of both human (ii-iv) and mouse (v-vii) cells. Only conjugates formed from a 1:1 interaction between target and effector cells were scored, with an average 150 conjugates per experiment scored. The hCD8-Jurkat experiment was performed 2 times, and the mCTL-L1210 experiment was performed 7 times. (B) Target cell mitochondrial polarization to point of contact is not dependent on grB or perforin. The percentage of cells displaying target cell mitochondrial alignment to conjugate interface with WT mCTLs (WT), grB-cluster KO mCTLs (grB KO), and perforin KO mCTLs (Per KO) are shown. Shown are the average values of 4 independent experiments plus or minus SD.
CTLs cause target cell–mitochondria polarization to point of contact. (A) Representative microscope images of target cell mitochondrial localization in E/T conjugates. Target Jurkat cells (i-iv) or target mouse L1210 (v-vii) were incubated with human CTLs (i-iv) or mouse CTLs (v-vii) for 5 minutes at 37°C prior to fixation. Samples were processed for immunofluorescence using anti-CD8 antibody (secondary anti-mouse 488) to identify CTLs, as described in “Microscopy.” Effector–target cell conjugates were identified, and locations of target cell mitochondria were scored. Mitochondrial localization was determined by immunofluorescence using anti–cytochrome c (Jurkat, anti-mouse 555) or Mitotracker Red (L1210). Shown are representative DIC (top) and fluorescent (bottom) images of mitochondria not aligned at conjugate interface (i), or aligned at conjugate interface (ii-vii) of both human (ii-iv) and mouse (v-vii) cells. Only conjugates formed from a 1:1 interaction between target and effector cells were scored, with an average 150 conjugates per experiment scored. The hCD8-Jurkat experiment was performed 2 times, and the mCTL-L1210 experiment was performed 7 times. (B) Target cell mitochondrial polarization to point of contact is not dependent on grB or perforin. The percentage of cells displaying target cell mitochondrial alignment to conjugate interface with WT mCTLs (WT), grB-cluster KO mCTLs (grB KO), and perforin KO mCTLs (Per KO) are shown. Shown are the average values of 4 independent experiments plus or minus SD.
Importantly, this polarized mobilization of intracellular organelles to the point of contact between target and effector cells has been observed for target cell lysosomes and endosomes.7 This organeller movement is triggered by damaged membranes that cause a rapid rise in intracellular Ca2+ concentrations and cause endomembranes to mobilize to the site of damage and reseal the damaged plasma membrane.48 CTL-mediated lysosomal movement was required to repair membrane damage that was caused by perforin, and allows the target cell time to initiate the apoptotic program and avoid death by necrosis. We wanted to determine if this same mechanism for movement of endomembranes caused mitochondrial mobilization, so we assessed whether mitochondrial movement was ablated in conjugates formed between target cells and CTLs that were deficient for perforin (Per). We observed that mitochondrial polarization occurred not only due to interaction with WT CTLs, but also due to interaction with Per KO CTLs and grB KO CTLs (Figure 6B). Therefore, the mitochondrial movement is activated by another as yet unknown mechanism.
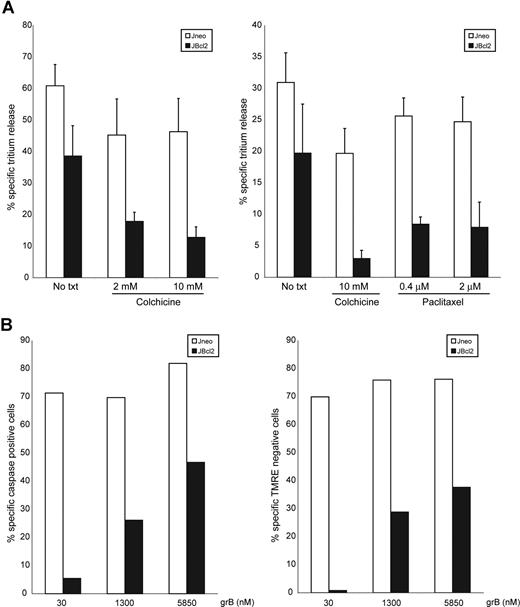
A critical prediction from these results is that the movement of mitochondria to the synapse would be required for killing. We tested whether this migration would have functional relevance to the CTL-mediated apoptotic pathway. In higher eukaryotes, mitochondria migrate via microtubules.49 We disrupted the microtubular network with colchicine and determined whether this affected the CTL-mediated Bcl-2–independent pathway (Figure 7A). Colchicine treatment of cells alone did not induce mitochondrial depolarization or ROS production (data not shown). In the presence of colchicine, there was repression of cell death of Jneo cells and importantly, a stronger repression of cell death in JBcl-2 cells. To ensure that these effects were not due to a non–microtubule-dependent effect from colchicine treatment, we repeated the experiments with both the microtubule-disrupting drug colchicine and the microtubule-stabilizing drug paclitaxel. Both of these drugs inhibit microtubule dynamics, and Figure 7A (right panel) demonstrates that both drugs inhibited the Bcl-2–independent apoptotic pathway. This suggests that execution of CTL-mediated cell death requires functional microtubules. Specifically, since the Bcl-2–independent pathway displayed a greater sensitivity to microtubule disruption, these results support our hypothesis that mitochondrial migration toward the immunologic synapse is a necessary step toward CTL-mediated apoptosis of Bcl-2–expressing cells.
Colchicine inhibits CTL-mediated cell death, and high concentrations of grB can overcome Bcl-2 block. (A) CTL-induced tritium release in target cells overexpressing Bcl-2 is dependent on the presence of assembled microtubules. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs (E/T ratio = 2:1) in the presence or absence of colchicines or paclitaxel. The percentage of specific tritium release, which is an indication of DNA fragmentation, was determined as outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation. (B) High concentrations of grB can induce apoptosis of JBcl-2 cells. Jneo cells (□) and JBcl-2 cells (■) were incubated with increasing concentrations of 30 nM, 1300 nM, or 5850 nM grB with AD, then loaded with the caspase indicator zVAD-FITC (left panel) and mitochondrial potential indicator dye (TMRE; right panel). Caspase activation and TMRE loss was determined by flow cytometry. The numbers indicate a representative of 2 independent experiments.
Colchicine inhibits CTL-mediated cell death, and high concentrations of grB can overcome Bcl-2 block. (A) CTL-induced tritium release in target cells overexpressing Bcl-2 is dependent on the presence of assembled microtubules. Jneo cells (□) and JBcl-2 cells (■) were preloaded with 3H-thymidine and incubated for 2 hours with CTLs (E/T ratio = 2:1) in the presence or absence of colchicines or paclitaxel. The percentage of specific tritium release, which is an indication of DNA fragmentation, was determined as outlined in “Apoptosis detection systems.” The numbers indicate the average of 3 independent experiments done in triplicate, with the corresponding standard deviation. (B) High concentrations of grB can induce apoptosis of JBcl-2 cells. Jneo cells (□) and JBcl-2 cells (■) were incubated with increasing concentrations of 30 nM, 1300 nM, or 5850 nM grB with AD, then loaded with the caspase indicator zVAD-FITC (left panel) and mitochondrial potential indicator dye (TMRE; right panel). Caspase activation and TMRE loss was determined by flow cytometry. The numbers indicate a representative of 2 independent experiments.
One possible mechanism of activation of the Bcl-2–independent pathway is that CTLs deliver a highly concentrated pulse of grB within an area of the target cell that has increased concentration of the target organelles: mitochondria. If this is true, then it may be possible to activate the Bcl-2–independent pathway by in vitro delivery of very high doses of grB. To test this hypothesis, we incubated Bcl-2–expressing cells with higher doses of grB (nearly 200-fold higher than that of standard conditions) and assessed cell death (Figure 7B). Indeed, addition of high levels of grB (compare 30 nM with 1.3 μM or 5.9 μM) did result in activation of mitochondrial dysfunction and caspase activation in Bcl-2–expressing cells. This suggests that CTL delivery of high local concentrations of grB in the vicinity of aggregated mitochondria may induce cell death independent of elevated levels of Bcl-2.
Discussion
Extensive investigations have demonstrated that grB cannot kill Bcl-2–overexpressing cells, but the situation with CTLs was unclear. We show that CTLs activate a Bcl-2–independent apoptotic pathway, which is characterized by caspase activation, double-strand DNA damage, and mitochondrial loss of Δψ and Smac/DIABLO. This pathway is independent of grA and Bid/Bax/Bak, but is dependent on activation of the mitochondrial permeability transition.
The observation that CTLs eliminate target cells in a Bcl-2–independent manner could be due to the cytotoxic properties of grA, since in contrast to grB, grA activates cell death that is not inhibited by Bcl-2.47,50 However, we demonstrated that Bcl-2–expressing target cells treated with CTLs displayed caspase activation and double-strand DNA breaks, which are typically induced by grB and are not induced by grA.47 Although grA does induce DNA damage, unlike grB, grA treatment causes single-strand DNA damage, producing large nicked DNA fragments that are not detectable by the 3H-release assays that are presented here.9,50,51 By analyzing apoptotic features that are not induced by grA (3H-release assays and caspase activation), and by using CTLs that were derived from grA KO mice, we have identified a grA-independent cell-death pathway that contributes to the elimination of target cells overexpressing Bcl-2. Therefore, although grA contributes to the CTL-mediated cell death of Bcl-2–expressing target cells, we have focused on grA-independent apoptotic features in order to dissect the mechanisms of caspase activation and DNA double-strand breaks that are induced by CTLs even in the presence of high levels of Bcl-2.
In this report, we show that grB plays a major role in this CTL-mediated, Bcl-2–independent cell-death pathway, since CTLs lacking grB did not induce DNA damage in either control target cells or targets with elevated levels of Bcl-2. This was surprising, because initial in vitro studies using purified grB demonstrated that grB only activates a Bcl-2–dependent mitochondrial apoptotic pathway.24,25,28 However, Thomas et al42 have provided evidence for a grB pathway that is independent of the Bcl-2 family members Bid, Bax, and Bak,42 although in those in vitro experiments, quite high concentrations of grB (1 μM) were used. We routinely perform our killing assays with relatively low amounts of grB (2.5 to 90 nM), and at these concentrations, Bcl-2 is an effective block. Taken together, one possible implication from these in vitro experiments is that CTLs actually deliver a high local concentration of grB to the target. As we discuss later, the delivery of a concentrated pulse of grB may be quite relevant to our model of CTL-mediated killing.
Although CTLs induce a Bid-Bax/Bak–independent killing pathway, mitochondrial events still occur and are required to induce cell death. These include mitochondrial loss of Δψ, and MOMP with the resultant loss of Smac/DIABLO from mitochondria. How CTLs trigger the mitochondrial apoptotic program in the presence of elevated levels of Bcl-2 is not clear. The proapoptotic members of the Bcl-2 family can initiate MOMP.52 Both proapoptotic Bax and Bid are activated by grB and induce release of mitochondrial cytochrome c.19 However, CTL-mediated mitochondrial dysfunction is not entirely dependent on the Bid-Bax/Bak pathway, since although Bax/Bak- and Bid-null target cells showed decreased sensitivity, these cells were still eliminated by CTLs (Figure 3). Therefore, MOMP and Δψ loss of the Bcl-2–independent CTL-mediated apoptotic program is triggered both through a Bid-Bax/Bak–dependent mechanism and through another separate pathway.
In support of the presence of an alternative mitochondrial activation pathway, it has been shown in the mouse system that the Bid pathway is not directly involved in mitochondrial dysfunction.53 Recently, it has also been demonstrated that human and mouse grB exhibit different abilities to cleave other substrates in addition to Bid.54 These differences are undoubtedly important in the mechanisms used by mice and human CTLs and illustrate the dangers of crossing species. They also offer an explanation for the discrepancies in the literature regarding the importance of Bid.42,55 The lack of a “Bid-amplification arm” also contributes to the low cytotoxicity of mouse grB relative to human.56 However, despite the differences in the in vitro potency of grB, CTLs from mice and humans have quite similar cytolytic and apoptotic activities at equal E/T ratios. It is intriguing to speculate that the mechanism described in the present work, which was revealed in human targets transfected with Bcl-2, might be the major pathway used by the mouse. The localized delivery of a highly concentrated pulse of grB by CTLs may be the physiologically relevant context that is mimicked by the need to use large doses of mouse grB on target cells in vitro.42,56
Activation of the mitochondrial PTP can also induce MOMP and loss of Δψ. Independent of the Bid-Bax/Bak pathway, apoptotic stimuli induce mitochondrial permeability through the opening of inner mitochondrial membrane pores that cause osmotic swelling of the mitochondrial matrix, rupture of the outer mitochondrial membrane, and release of mitochondrial intermembrane space proteins.57,58 We determined whether the PTP was induced in target cells treated with CTLs by analyzing cell death in the presence of the PTP inhibitor CsA. Inhibition of PTP decreased the levels of apoptosis in target cells (Figure 4A), demonstrating that PTP opening facilitated CTL-mediated cell death and importantly, that the activation of the mitochondrial apoptotic pathway was a functional requirement for apoptosis induction of Bcl-2–overexpressing cells and not merely a consequence of the activation of cell-death signaling pathways. Furthermore, in the healthy cellular state, mitochondrial production of the ROS superoxide (O2−) is countered by an antioxidant defense mechanism. Under apoptotic conditions, mitochondrial damage causes a net increase in ROS, which in turn can further damage mitochondria, thereby contributing to the progression of the cellular apoptotic pathway.59 ROS production is a required step for activation of both grA- and grB-mediated cell death.47 We showed that ROS production was required for CTL-mediated cell death in Bcl-2–expressing cells, since scavenging of ROS resulted in a decrease in apoptosis. Therefore, it may be that activation of this Bcl-2–independent mitochondrial pathway is a initiated through PTP opening.
Given that the mitochondria played a critical role in the grB-dependent, Bcl-2–independent apoptotic pathway, we were puzzled as to why this pathway was activated by CTLs and not by treatment with purified grB. We observed that CTL–target cell conjugates showed an accumulation of mitochondria at the target face of the immunologic synapse (Figure 6). Mitochondria are highly motile organelles; through interactions with microtubule motor proteins, they move along microtubule tracks within the cell.49 Thus, mitochondria migrate to areas with the cell which have a high demand for ATP production or where other mitochondrial metabolic functions, such as buffering of Ca2+ transients, are required. Examples of this include mitochondrial transport along neuronal axons60 and mitochondrial migration to the uropod of lymphocytes to facilitate chemoattractant-induced lymphocyte migration.61 If the observation that mitochondria movement was a requirement for activation of target cell apoptosis, target cells that had disassembled microtubules due to treatment with the microtubule poison colchicine should show a decrease in the Bcl-2–dependent apoptotic program. That is indeed what we observed (Figure 7). This suggested that this polarized accumulation of mitochondria contributed to the activation of mitochondrial dysfunction, possibly allowing direct activation of PTP by grB.
In support of direct activation of grB on the mitochondrial apoptotic pathway, MacDonald et al17 showed that grB can induce mitochondrial dysfunction of isolated mitochondria. Possibly, the accumulation of target cell mitochondria at sites of high local intracellular concentrations of grB facilitate direct activation of the mitochondrial apoptotic pathway independent of Bcl-2. In support of the idea that high concentrations of grB can overcome a Bcl-2 block, we observed that increased levels of grB delivered in an in vitro system could indeed induce apoptosis of Bcl-2–expressing cells (Figure 7B).
Therefore, we suggest a model whereby contact with CTLs signals target cell mobilization of mitochondria to the point of contact. The accumulation of mitochondria in the region where grB enters the cell, which may be an area of high local grB concentration, could set the stage for the activation of the (Bid-Bax/Bak)/Bcl-2–independent killing pathway. Therefore, this mitochondrial polarization may provide a means to overcome Bcl-2 protection in target cells. By facilitating this alternative mitochondrial apoptotic pathway, the target cell plays an active role in its own demise. This has long-ranging implications in the treatment of cancer given that Bcl-2 is overexpressed in a broad range of malignancies.62 As a result, identifying the molecular mechanisms of cytotoxic T-cell–mediated apoptosis may make it possible to enhance the immune system's ability to eliminate tumors.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Ms Catherine Ewen and Dr Simonetta Sipione for helpful discussions.
This work was supported by grants from the Canadian Institute of Health Research (CIHR) and the National Cancer Institute of Canada. R.C.B. is a CIHR Distinguished Scientist, a Medical Scientist of the Alberta Heritage Foundation for Medical Research (AHFMR), and a Canada Research Chair.
Authorship
Contribution: I.S.G., T.S., A.R., and I.S. performed experiments; I.S.G. analyzed results and made the figures; and I.S.G. and R.C.B. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: R. Chris Bleackley, Department of Biochemistry, University of Alberta, Edmonton, AB, Canada T6G 2H7; e-mail: chris.bleackley@ualberta.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal