Clinical trials have been started with the aim of inducing tumor immunity by blocking the immunosuppressive action of indoleamine-2,3-dioxygenase (IDO) with the IDO2-inhibitor dextro-1-methyl-tryptophan (D-1MT). Here we show that human dendritic cells (DCs) express both IDO-1 and IDO-2, but that only IDO1 mediates tryptophan catabolism; furthermore, its activity is blocked by levo-1MT, whereas D-1MT is inefficient. Consequently, in humans any possible antitumor effects of D-1MT cannot be attributed to abrogation of IDO activity in DCs as described in this study.
Introduction
IDO is an enzyme that catabolizes tryptophan and was shown to protect the fetus from rejection mediated by maternal T cells.1 Such a basic mechanism that evolved for the perpetuation of species might be expected to be used for the regulation of other biologic processes. Researchers were therefore motivated to look for a role of IDO in the regulation of immune responses in health and disease. An objective that has challenged the scientific community for decades is the unraveling of immunologic conditions that enable the development of tumors and, based on this, the design of novel therapeutic strategies for tumor prevention or treatment
Tumors are the consequence of genetic mutations leading to the expression of modified proteins.2 Despite their potential to activate the immune response, these proteins are not capable of inducing tumor-protective immunity. Many mechanisms have been made responsible for the lack of an effective immune defense against tumors.3 If IDO has the potential to prevent rejection of the fetus during pregnancy—so went the reasoning—it might also be implicated in induction of fatal tolerance to tumors. Tolerogenic mechanisms can operate in the tumor itself or in key sites where the immune system encounters tumor antigens, such as tumor-draining lymph nodes.4 Emerging evidence suggests that in lymph nodes, IDO-expressing dendritic cells (DCs) directly suppress and anergize tumor-reactive effector T cells responding to antigens presented by these DCs.5,6 In addition, through bystander suppression, IDO can inhibit T-cell responses to antigens presented by neighboring DCs.5 It was therefore appealing to trigger antitumor immunity by inhibiting the activity of IDO. Pioneering work in this field was performed by van den Eynde and colleagues. In a series of elegant experiments, they showed that the IDO-blocking agent 1-methyl-tryptophan (1MT) significantly reduces the volume of tumors in preimmunized mice (Uyttenhove et al7 ). But 1MT exists in 2 forms, the levo and the dextro isomer. In the study of van den Eynde's group the levo isoform (L-1MT) was used. Other authors found that, in animal models, the dextro stereoisomer (D-1MT) has superior antitumor activity8 and therefore chose it for phase 1 clinical trials in humans. This was a surprising choice since, on a biochemical level, in contrast to the L isomer the D form did not properly inhibit IDO.8 In recent studies, the contradiction was apparently resolved by describing, in addition to the classic IDO (dubbed IDO1), a closely related variant called IDO2,9 which is strongly inhibited by D-1MT but unaffected by L-1MT.10 Natural IDO2 was found in several tissues, including a predendritic mouse cell line.10
Taking into consideration the potential of D-1MT for cancer therapy and the already running clinical trials, it is important to know whether in humans the effect of D-1MT can be mediated by blocking the IDO activity of DCs. We tried to find an answer to this question by studying a subset of human DCs that were claimed to constitutively express IDO.11
Methods
DC generation
DCs were generated as previously described12 from blood samples of healthy volunteers. Approval was obtained from the University of Tubingen Institutional Review Board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki. Briefly, plastic adherence–purified monocytes were cultured in X-vivo 15 (Lonza, Wuppertal, Germany), with 2% autologous plasma and 50 μg/mL gentamicin supplemented with 20 ng/mL IL-4 and 800 U/mL GM-CSF (Peprotech, Hamburg, Germany) for 5 days. DCs were matured for 24 hours using a standard cytokine cocktail.12
siRNA transfection of DCs
Immature DCs were transfected with water (mock), 10 μg control siRNA (CTR siRNA; Ambion, Dresden, Germany), or 10 μg IDO1-specific siRNA (IDO1 siRNA) using standard electroporation.13 After 4 hours, DCs were matured and stimulated with 200 U/mL IFN-γ for 24 hours.
Qualitative and quantitative IDO expression
Total RNA of DCs was isolated with Trizol (InVitrogen, Karlsruhe, Germany) and 1 μg reverse transcribed using AMV Reverse Transcriptase (Promega, Mannheim, Germany). cDNA was amplified with polymerase chain reaction (PCR) using specific primers (IDO1: forward: 5′-tgccaaatccacaggaaaat-3′, reverse: 5′-gtttgccaagacacagtctg-3′; IDO2: forward: 5′-catagcaaggaaagtggtgac-3′, reverse: 5′-ctaaacacgtgggtgaaggattg-3′), and PCR products were separated on a 1.5% agarose gel; β-actin served as internal standard. For quantitative reverse-transcription (RT)–PCR, gene-specific probe and primers were used (IDO1: forward: 5′-caaatccacgatcatgtgaacc-3′, reverse: 5′-agaacccttcatacaccagac-3′, probe: 5′-ttgtctggctggaaaggcaacccc-3′) and expression levels normalized to the corresponding 18s rRNA.
IDO activity assay
IDO activity was measured by reversed-phase high-performance liquid chromatography (RP-HPLC) as previously described.12
Results and discussion
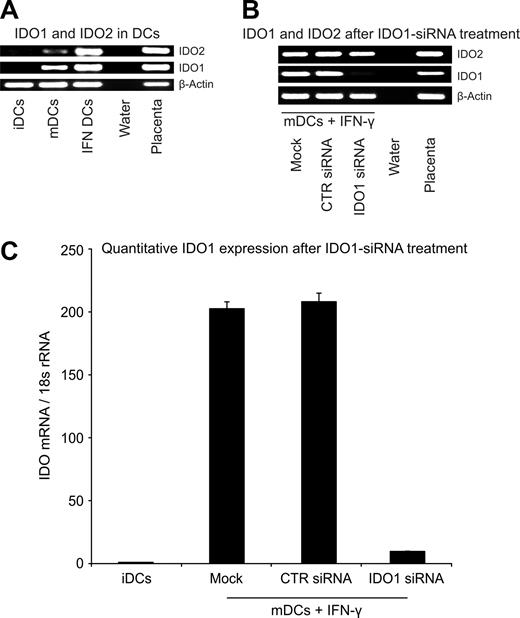
Figure 1A (lane 2) shows that mature DCs weakly express IDO1 and IDO2. To increase the IDO activity, DCs were treated with interferon-γ (Figure 1A lane 3; Figure 2A third and fourth pairs of columns). We attempted to inhibit IDO1 expression by treating these cells with IDO1-specific siRNA. Our results showed that IDO1 transcription became almost undetectable as measured by qualitative (Figure 1B lane 3) and quantitative (Figure 1C) RT-PCR. As expected, IDO2 expression was not affected by this treatment (Figure 1B lane 3).
Expression of IDO1 and IDO2 in CD123+/CCR6+ human DCs. (A) CD123+/CCR6+, nonadherent, immature human DCs (iDCs) or cytokine cocktail–matured DCs (mDCs12 ) treated or not with IFN-γ (IFN DCs) were analyzed for IDO1 and IDO2 expression by RT-PCR. Water instead of cDNA served as negative and placenta cDNA as positive control. PCR products (β-actin: 407 bp, IDO1: 321 bp, IDO2: 371 bp) were separated on agarose gel. (B) iDCs were electroporated with water instead of siRNA (mock), control siRNA (CTR siRNA), or siRNA specific for IDO1 (IDO1 siRNA) and stimulated with a maturation cocktail and IFN-γ. IDO1 and IDO2 transcription was detected as described in panel A. (C) The relative expression level of IDO1 after siRNA treatment was analyzed by qRT-PCR. IDO1 expression levels of transfected DCs were correlated to iDCs (n = 7, mean value ± SD).
Expression of IDO1 and IDO2 in CD123+/CCR6+ human DCs. (A) CD123+/CCR6+, nonadherent, immature human DCs (iDCs) or cytokine cocktail–matured DCs (mDCs12 ) treated or not with IFN-γ (IFN DCs) were analyzed for IDO1 and IDO2 expression by RT-PCR. Water instead of cDNA served as negative and placenta cDNA as positive control. PCR products (β-actin: 407 bp, IDO1: 321 bp, IDO2: 371 bp) were separated on agarose gel. (B) iDCs were electroporated with water instead of siRNA (mock), control siRNA (CTR siRNA), or siRNA specific for IDO1 (IDO1 siRNA) and stimulated with a maturation cocktail and IFN-γ. IDO1 and IDO2 transcription was detected as described in panel A. (C) The relative expression level of IDO1 after siRNA treatment was analyzed by qRT-PCR. IDO1 expression levels of transfected DCs were correlated to iDCs (n = 7, mean value ± SD).
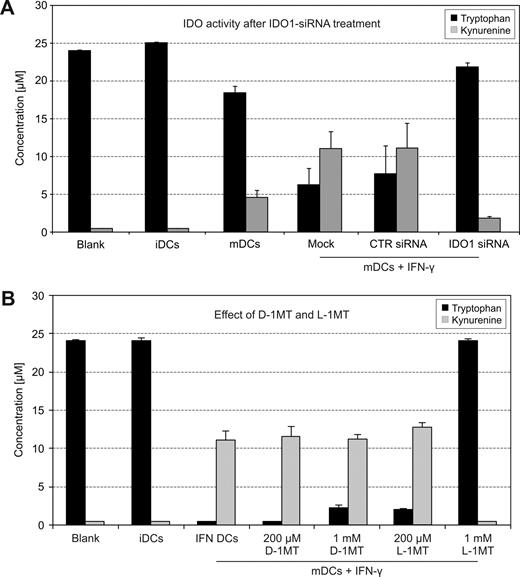
IDO activity of human DCs treated with IDO1-siRNA or 1MT stereoisomers. (A) Tryptophan degradation and kynurenine accumulation were measured in cell culture supernatants12 (n = 7). (B) iDCs were stimulated with a maturation cocktail and IFN-γ to induce IDO. D-1MT and L-1MT (Sigma) were tested at 200 μM and 1 mM for their potential to block the enzymatic activity (n = 15, mean value ± SD).
IDO activity of human DCs treated with IDO1-siRNA or 1MT stereoisomers. (A) Tryptophan degradation and kynurenine accumulation were measured in cell culture supernatants12 (n = 7). (B) iDCs were stimulated with a maturation cocktail and IFN-γ to induce IDO. D-1MT and L-1MT (Sigma) were tested at 200 μM and 1 mM for their potential to block the enzymatic activity (n = 15, mean value ± SD).
Enzymatic activity of IDO is measured by the degree of tryptophan degradation and kynurenine production. In our studies, interferon-γ–treated mature DCs had considerable enzymatic activity (Figure 2A fourth pair of columns). If IDO activity of DCs is entirely provided by IDO1, one would expect that tryptophan and kynurenine concentrations are restored following inhibition with IDO1-specific siRNA. Figure 2A (last group of columns) shows that this was indeed the case; minimal IDO1 activity is because inhibition by siRNA is never 100%. The latter finding demonstrates that IDO2, although expressed by human IDO+ DCs, is functionally inactive.
It was stated that D-1MT almost exclusively blocks IDO2, whereas L-1MT abrogates IDO1 activity.10 Because IDO2 was not expressed as an active enzyme in the DCs studied herein, we expected that D-1MT would not block their function. Figure 2B (last column) shows that D-1MT is inactive, whereas L-1MT fully inhibits the IDO activity. The slight tryptophan increase upon addition of 1 mM D-1MT is explained by the weak cross-reaction of D-1MT with IDO1.
These findings teach us 2 lessons. First, IDO2—although expressed in human DCs—is not active, that is, the tryptophan-degrading function of these cells is entirely contributed by IDO1. Second, because D-1MT almost exclusively blocks IDO2,10 it has no influence on IDO activity of human DCs. Consequently, a potential therapeutic effect of D-1MT in humans cannot be ascribed to escape of immune response from the control of IDO+ DCs. When interpreting the implications of these findings for cancer therapy, one should keep in mind that 1MT is a substance that, in addition to blocking IDO, has many other effects. It has been shown for instance that, independently of IDO, it induces drastic changes in the function of DCs,14 which might influence the immune response to tumors. 1MT is also an inhibitor of the tryptophan transporter of cell membranes15 —a key component for cell survival. A functional failure of this transporter leads to profound metabolic disturbances in eukaryotic cells. Therefore, despite mouse studies proving mediation of antitumor effects by the immune system, a direct influence of 1MT on tumor growth in humans should not be completely dismissed. We observed that 1MT has the capacity of reducing T-cell proliferation when coincubated with dendritic cells.12 Should it also stop the proliferation of malignant cells in vivo, this effect provides an alternative explanation for the described regression of tumors following treatment with 1MT.7,8
Previous animal studies showed that both L-1MT and D-1MT stop tumor growth, possibly mediated by inhibition of IDO expressed by dendritic cells.4 If in the now ongoing clinical studies with D-1MT10 the drug shows an antitumoral effect, this phenomenon cannot be explained by an abrogation of the immunosuppressive action of IDO-producing DCs with subsequent activation of the immune response.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
S.L. was supported by a Fortüne grant of the University of Tübingen (1636-0-0).
Authorship
Contribution: S.L. performed experiments and created the figures; S.L., A.K., R.S., H.-G.R., and P.T. analyzed data; S.L. and P.T. designed research; and P.T. and G.O. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Terness, Institute of Immunology, Department of Transplantation Immunology, University of Heidelberg, INF-305, 69120 Heidelberg, Germany; e-mail: peter.terness@med.uni-heidelberg.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal