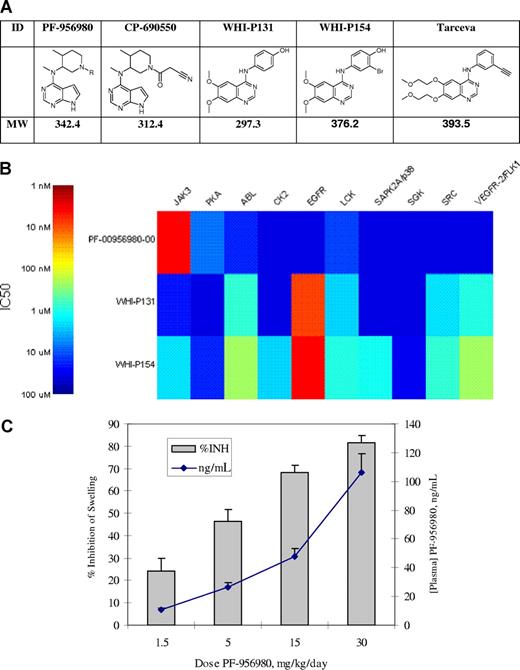
PF-956980 is a selective inhibitor of JAK3, related in structure to CP-690550, a compound being evaluated in clinical trials for rheumatoid arthritis and prevention of allograft rejection. PF-956980 has been evaluated against a panel of 30 kinases, and found to have nanomolar potency against only JAK3. Cellular and whole blood activity of this compound parallels its potency and selectivity in enzyme assays. It was effective in vivo at inhibiting the delayed type hypersensivity reaction in mice. We compared 2 commercially available JAK3 inhibitors (WHI-P131 and WHI-P154) in the same panel of biochemical and cellular assays and found them to be neither potent nor selective for JAK3. Both were found to be nanomolar inhibitors of the EGF receptor family of kinases. As these compounds have been used in numerous publications in the transplant and autoimmune disease literature, their specificity should be considered when interpreting these results.
Introduction
We have previously described an inhibitor of JAK3, CP-690550,1 which has recently demonstrated efficacy in phase 2 clinical trials for the treatment of rheumatoid arthritis2 and prevention of renal transplant rejection.3 We now describe a related analog, PF-956980, which has equivalent potency and selectivity for JAK3. We have made this compound available to the academic community for selected studies, and provide comparative data with 2 other widely used and commercially available JAK3 inhibitors, WHI-P131 and WHI-P154. The structures of the compounds studied here are shown in Figure 1, along with the structure of erlotinib (Tarceva), a clinically approved EGFR inhibitor that is structurally related to WHI-P131 and WHI-P154.
Compounds, enzyme inhibition, and in vivo activity of kinase inhibitors studied. (A) Compound structures and molecular weights. (B) Heat map of kinase inhibition, IC50 (nM). (C) Efficacy of PF-956980 in murine delayed type hypersensitivity. Error bars represent SD.
Compounds, enzyme inhibition, and in vivo activity of kinase inhibitors studied. (A) Compound structures and molecular weights. (B) Heat map of kinase inhibition, IC50 (nM). (C) Efficacy of PF-956980 in murine delayed type hypersensitivity. Error bars represent SD.
Methods
Kinase assays
Compounds were tested in kinase assays developed at Pfizer. The panel of kinases was selected to broadly cover the kinome, providing a good approximation of specificity. For all kinases, recombinant rat (IKKβ) or human (all others), full-length or GST-kinase domain fusion proteins, were used. Assays were performed using either Caliper (Hopkinton, MA) mobility-shift assay format or Perkin Elmer (Waltham, MA) Streptavidin coated Flashplate radiometric assay format. All compounds were inactive (concentration that inhibits response by 50% [IC50] > 30 μM) for the following kinases: AKT, AuroraA, cdk2, cdk6, CHK1, FGFR1, GSK3b, IKKb, IKKi, INSR, MAPK1, MAPKAP-K2, MASK, MET, PAK4, PDK1, PKCb, ROCK1, TaoK3, TrkA. Specific details of each assay are available on request.
Cellular assays
Protocols for the HUO3, HFF, EGF, and DND39 cell assays were described previously.1,4 Human whole blood assay: Anti-CD3ϵ (R&D Systems, Minneapolis, MN; 1 μg/mL final concentration), anti-CD28 (BD Biosciences, San Jose, CA; 10 ng/mL final concentration) IL-2 (R&D Systems, 10 ng/mL final concentration) were added to heparinized whole blood diluted 1:1 in RPMI 1640 and incubated at 37°C for 18 hours. Production of IFNγ was measured using a Quantikine kit (R&D Systems). For the delayed type hypersensitivity assay, mice received intravenous injections of 6 × 105 sheep red blood cells in a volume of 200 μL sterile isotonic saline. Five days later, sensitized mice received an injection of the same number of sheep red blood cells in a volume of 30 μL into the left footpad. Twenty-four hours later, footpad swelling was measured in both the right and left footpads using a dial thickness gauge. The swelling response was calculated by subtracting the right footpad thickness (baseline) from that of the left footpad (experimental). PF-956980 was administered using osmotic minipump infusion (Alza Model #2004; Durect, Cupertino, CA) in a vehicle of PEG300. Osmotic minipumps delivered at a flow rate of 0.5 μL/h for 14 days. Two days before sensitization, mice were anesthetized and their dorsal surface shaved. An incision was made 1 cm from the base of the tail, a subcutaneous pocket created, and pumps inserted. The incision site was closed with wound clips. Approval for these studies was obtained from the Pfizer Animal Care and Use Committee.
Results and discussion
The potency and specificity of these compounds were evaluated in a series of 30 in vitro kinase assays developed at Pfizer. Similar to CP-690550, PF-956980 was found to be highly specific for JAK3 (IC50 of 4 nM), with only modest inhibition of PKA (6.7 μM) and LCK (10.9 μM) in vitro. To expand our earlier findings,1 WHI-P131 and WHI-P154 had modest activity against JAK3 in the current enzyme assays (IC50 of 19.2 and 1.8 μM, respectively). They were, however, extremely potent against the EGFR kinase(9 and 4 nM, respectively), results consistent with the close structurally similarity (Figure 1A) of these 2 compounds to erlotinib (IC50 = 3 nM in the same EGFR assay). They also had significant activity against several other kinases, including ABL, LCK, SRC, and VEGFR. A representation of the kinase potencies is shown in Figure 1B for 10 of the 30 kinases tested. The remaining 20 kinases tested were not significantly affected by any of the compounds, and are listed in “Kinase assays.”
While kinase data can provide a useful, first approximation for the potency of a compound, we have found that the artificial environment of in vitro assays can be misleading. Cellular assays, which involve full-length kinase proteins in their native environment with respect to ATP and substrate concentration, are more relevant to in vivo activity. Cell-based assays designed to measure effects of compounds on JAK2, EGFR, and nonspecific antiproliferation have previously been described.1 We have also used a cellular assay (DND39) designed to measure inhibition of IL-4 receptor signaling, which involves signaling through both JAK1 and JAK3.4 In this assay, the DND39 cells have been stably transfected with a luciferase gene under control of the germ line ϵ promoter. The interaction of IL-4 with its receptor leads to activation and binding of STAT6 to the promoter, and production of the luciferase protein. Finally, as most tissue culture-based assays use only 5% to 10% fetal calf serum, we developed a whole blood assay that provides a more reliable estimate of exposures required for in vivo efficacy. The whole blood, which contains circulating T lymphocytes, is stimulated with antibodies to CD3 and CD28, mimicking antigen receptor signaling. This results in epigenetic changes to the IFNG gene locus, such that when IL-2 is added, IFNγ is produced in a JAK3-dependent manner.5
Table 1 displays the potencies for these compounds in various cellular assays. PF-956980 exhibited nanomolar potency in the primary JAK1/3-dependent cellular assay (DND39), with approximately 8-fold less potency in the JAK2-dependent cellular assay (HUO3). For comparison, CP-690550 has a cellular selectivity of approximately 15-fold in these same JAK1/3 versus JAK2 assays. There was no effect of PF-956980 in the HFF assay, a measure of nonspecific effects on cellular proliferation. This result is consistent with its excellent specificity profile. In the JAK1/3-dependent whole blood assay, the IC50 was 121 nM, consistent with the modest protein binding found for PF-956980 (data not shown). Based on its molecular weight, this IC50 corresponds to 39 ng/mL. We have found the number derived from this assay to be a reasonable initial target for the exposure required for in vivo immunosuppressive activity.
Potency of compounds in various cellular assay
| Cell assay . | Kinases . | Compounds . | |||
|---|---|---|---|---|---|
| CP-690550 . | PF-956980 . | WHI-P131 . | WHI-P154 . | ||
| DND39 | JAK1, JAK3 | 21 | 23 | >10 000 | >10 000 |
| HUO3 | JAK2 | 324 | 188 | >10 000 | NT |
| HFF | Nonspecific | >10 000 | >10 000 | >10 000 | 6343 |
| Whole blood | JAK1, JAK3 | 25 | 121 | NT | NT |
| EGF | EGFR | NT | NT | 405 | <100 |
| Cell assay . | Kinases . | Compounds . | |||
|---|---|---|---|---|---|
| CP-690550 . | PF-956980 . | WHI-P131 . | WHI-P154 . | ||
| DND39 | JAK1, JAK3 | 21 | 23 | >10 000 | >10 000 |
| HUO3 | JAK2 | 324 | 188 | >10 000 | NT |
| HFF | Nonspecific | >10 000 | >10 000 | >10 000 | 6343 |
| Whole blood | JAK1, JAK3 | 25 | 121 | NT | NT |
| EGF | EGFR | NT | NT | 405 | <100 |
All potencies are shown as IC50 in nM, and are the mean values of at least three independent determinations.
NT indicates not tested.
In contrast to PF-956980, WHI-P131 and WHI-P154 were completely inactive (> 10 000 nM) in the DND39 cellular assay, consistent with their micromolar potency in the JAK3 enzyme assay. Further, consistent with their enzyme activity, they each display activity in the EGFR-dependent cell assay. These data, along with the broad kinase activity displayed by these compounds, and coupled with their lack of activity against JAK3, suggest careful consideration be taken when interpreting publications that use these compounds.
Given the activity of PF-956980 in the whole blood assay, we evaluated its activity in a short term model of the inflammatory response. As previously described, delayed-type hypersensitivity (DTH) swelling responses have been used to follow the activity of immunosuppressive molecules in vivo,6 including CP-690550.7 It is an in vivo T cell–dependent immune response manifested as an inflammatory reaction that peaks in intensity 24 to 48 hours after antigen challenge in previously sensitized animals. As shown in Figure 1C, when dosed via osmotic minipump, PF-956980 dose-dependently inhibited the DTH response in mice with an efficacious concentration of 50% (EC50) of approximately 5 mg/kg. This corresponded to an exposure of approximately 30 ng/mL, and is consistent with the IC50 of PF-956980 in the whole blood assay (39 ng/mL).
We have provided evidence that PF-956980 is a potent and specific inhibitor of JAK3, with in vivo activity consistent with in vitro enzyme and whole blood assays. We have also provided further evidence that WHI-P131 and WHI-P154, 2 commercially available and widely used putative JAK3 inhibitors, are neither specific nor potent. This issue has been recently addressed in a letter to the editor8 citing our original work and that of others, which we have now expanded upon. The widespread use of poorly characterized signal transduction reagents has the potential to result in misleading conclusions. Of note, in vivo efficacy results for WHI-P131 and WHI-P154 have only rarely been accompanied by pharmacokinetic measurements of drug plasma concentrations.9 For example, in a recent paper,10 WHI-P131 (JANEX-1) is claimed to inhibit diabetes development in the NOD mouse due to blockade of JAK3. Even at the extraordinary doses used in this study (100 mg/kg), the rapid clearance of this compound would result in exposures that would be unlikely to inhibit JAK3 beyond 1 hour after dose. Based on our enzyme data, the only kinases that are likely to be inhibited at even 4 hours after dose would be EGFR, VEGFR, ABL, and SRC. Potential inhibition of these other kinases should be considered when interpreting the disease models in which this compound has shown efficacy.11 We believe it to be the responsibility of vendors and investigators to ensure that compounds are adequately evaluated for specificity and pharmacokinetics before in vivo use. Further, we believe it to be the responsibility of companies that develop inhibitors of key signaling molecules to make representative compounds available to the academic community, whenever possible, as we have done with PF-956980. The signal transduction literature depends on such tools to be carefully profiled, to allow others to build on their results.
Note: Investigators interested in obtaining aliquots of PF-956980 for in vitro use should contact donnie.w.owens@pfizer.com.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.S.C. conceived the studies and wrote the paper; D.M. and K.S.M. performed kinase assays; C.F.K. cowrote the paper; M.E.F. and M.J.M. conceived the studies: T.M.H., J.L.D., D.A.W., and J.S. synthesized compounds; C.R.K. and D.G.P. performed cellular assays; P.S. performed in vivo assays; and E.M.K. conceived the studies and cowrote the paper.
Conflict-of-interest disclosure: All authors were employees of Pfizer at the time of these studies.
Correspondence: Paul S. Changelian, PhD, 1009 Glenhill Drive, Northville, MI 48167; e-mail: changelianps@gmail.com.