Abstract
Catabolism of free heme by heme oxygenase-1 (HO-1) generates carbon monoxide, biliverdin, and free iron (Fe). These end-products are responsible for much of the biologic activity of HO-1, including anti-inflammatory, antiapo-ptotic, antiproliferative, and antioxidant effects. We have identified an additional cytoprotective action, the regulation of complement activation, mediated via induction of decay-accelerating factor (DAF). Pharmacologic inhibition or short-interfering RNA (siRNA) depletion of HO-1 prevented induction of DAF expression in human endothelial cells. In contrast, HO-1 agonists hemin and cobalt protoporphyrin IX significantly increased DAF protein expression, reflecting an increase in transcription and steady-state mRNA. Adenoviral-mediated overexpression of HO-1 increased DAF expression, enhancing protection against C3 deposition and complement-mediated lysis, and this was reversed by DAF inhibitory monoclonal antibody (mAb) 1H4. Likewise, bilirubin, Fe chelation, and overexpression of heavy-chain ferritin all induced DAF expression in endothelial cells (EC). Analysis of cardiac endothelial cells isolated from Hmox1−/− mice revealed a 60% reduction in DAF expression compared with Hmox1+/+ EC, and Hmox1−/− cells showed enhanced sensitivity to complement. We propose that modulation of complement activation through induction of DAF represents an important component of the cytoprotective effects of HO-1 against vascular injury, such as that associated with posttransplant vasculopathy, allograft rejection, and ischemia reperfusion.
Introduction
Vascular endothelial cells (ECs) express constitutive and inducible cytoprotective genes, which exert cytoprotective and anti-inflammatory effects, maintain vascular integrity, and assist in endothelial repair.1 Vascular endothelial growth factor (VEGF) plays an important role in the maintenance of these pathways in the normal adult vasculature. This notion is supported by the observation that VEGF up-regulates expression of several protective genes in EC, including endothelial nitric oxide synthase (eNOS), prostacyclin, and Bcl-2, and protects EC against oxidative stress.2 Furthermore, we have reported that VEGF induces the expression of the cytoprotective, anti-inflammatory enzyme heme oxygenase-1(HO-1)3 and the complement inhibitory protein decay-accelerating factor (DAF).4
HO-1 is the inducible form of the heme oxygenase system, acting as the rate-limiting factor in the catabolism of heme into biliverdin, releasing free iron (Fe) and carbon monoxide (CO).5 Biliverdin is subsequently converted to bilirubin by biliverdin reductase, whereas intracellular Fe induces expression of the iron-binding protein heavy-chain ferritin6 and the opening of Fe2+-export channels.7 The importance of HO-1 in vasculoprotection is demonstrated by the severe and persistent endothelial damage observed in human HO-1 deficiency8 and Hmox1−/− mice.9 HO-1 exerts a potent protective effect against atherogenesis,10 cardiac ischemia/reperfusion injury,11 and both graft rejection and accelerated arteriosclerosis after transplantation.12-14
The complement cascade provides an innate defense mechanism against bacterial infection, bridging innate and adaptive immunity while affording clearance of antibody immune complexes. However, by the nature of its cytolytic effects, complement has the potential to inflict injury on bystander host tissues, including vascular endothelium. C3a, C5a, and the C5b-9 membrane attack complex (MAC) exert a variety of effects, including cytokine release, induction of cellular adhesion molecules, leukocyte adhesion, and generation of a prothrombotic endothelial surface.15,16 Hence, complement activation has been implicated in the pathogenesis of atherosclerosis and the rejection of transplanted organs.
Mechanisms for the control of complement activation on the surface of human cells include membrane-bound regulatory proteins: DAF (CD55), membrane cofactor protein (MCP; CD46), and CD59. In addition, murine cells express complement receptor-related protein-Y (Crry/p65), which combines the functions of DAF and MCP. Membrane-bound human DAF prevents the formation and accelerates the decay of C3 and C5 convertases, exerting potent cytoprotective and anti-inflammatory effects by minimizing deposition of C3 and C5b-9, and the generation of the anaphylatoxin C5a.17 DAF expression on EC is regulated via distinct agonist-specific pathways, suggesting that induction may be important in the maintenance of vascular integrity during inflammation.18 This concept is supported by the demonstration that DAF-deficient mice are particularly susceptible to C5b-9–induced microvascular injury after ischemia/reperfusion.19
Induction of HO-1 mediates the cytoprotective effects of interleukin-1020 and rapamycin,21 a phenomenon referred to as the HO-1 therapeutic funnel.22 In some instances, HO-1 may amplify these actions via a positive feedback loop.22 We have shown that VEGF up-regulates HO-1 expression in EC,3 which in turn increases VEGF synthesis.23 A similar positive feedback loop has been identified for interleukin-10 and HO-1 in monocytes/macrophages.20 In light of our data demonstrating that VEGF induces EC expression of both HO-1 and DAF and studies demonstrating that HO-1 activity attenuates complement-mediated acute inflammation and Forssman anaphylaxis,24,25 we explored the role of HO-1 in the regulation of DAF expression. We show, for the first time, that HO-1 and its products induce DAF expression and protect human EC against complement-mediated injury. We demonstrate that ECs derived from Hmox1−/− mice have reduced DAF expression and increased susceptibility to complement-mediated injury. These data suggest that inhibition of complement activation represents an important component of the vasculoprotective effects of HO-1.
Methods
Reagents
Anti–human DAF monoclonal antibody (mAb) 1H4 and antihuman MCP mAb TRA-2-10 were gifts from D. Lublin and J. Atkinson, respectively (Washington University School of Medicine, St Louis, MO). Anti–human DAF mAb IA10 and anti-Crry/p65 (BD Biosciences, Oxford, United Kingdom), anti–human CD59 mAb Bric 229 (Blood Group Reference Laboratory, Bristol, United Kingdom), anti–murine DAF mAbs MD-1 and 3D5, anti–mouse CD59 mAb MEL-4 (gifts from C. Harris and B. P. Morgan, University of Wales College of Medicine, Cardiff, United Kingdom), anti–human endoglin RMAC8 (gift from A. D'Apice, St Vincent's Hospital, Fitzroy, Australia), and anti–mouse endoglin MJ17/8 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). HO-1 inhibitor zinc (II) protoporphyrinIX (ZnPPIX) and HO-1 activator cobalt (III) protoporphyrin IX chloride (CoPPIX) were from Frontier Scientific (Logan, UT), bilirubin, biliverdin, N-acetylcysteine, and desferrioxamine mesylate were from Sigma-Aldrich (Poole, United Kingdom). Bilirubin and metalloporphyrins were dissolved in 0.1 M NaOH and the pH adjusted to 7.4. Salicylaldehyde isonicotinoyl hydrazone was a gift from P. Ponka (McGill University, Montreal, QC). VEGF165, tumor necrosis factor-α (TNF-α), and interferon-γ (IFN-γ) were from PeproTech (London, United Kingdom). Normal human serum (NHS) was prepared as previously described18 ; normal mouse serum (NMS) was from Dako (Ely, United Kingdom). Sera were stored at −70°C and, when required, heat-inactivated by incubation at 56°C for 45 minutes. In all experiments, ECs were treated with the appropriate drug vehicle controls.
Animals
Hmox1−/− mice (backcrossed 10 generations into the C57BL/6 background) were generated by Shaw-Fang Yet (Pulmonary and Critical Care Division, Brigham and Women's Hospital, Boston, MA) and are currently maintained at the Instituto Gulbenkian de Ciência by Hmox1+/− mating as described.26 Mice were bred and maintained under specific pathogen-free conditions in accordance with guidelines from the Animal User and Institutional Ethical Committees of the Instituto Gulbenkian de Ciência.
EC isolation and culture
Human umbilical vein ECs (HUVECs) were isolated and cultured in M199/20% fetal bovine serum (FBS), 2 mM l-glutamine, 100 IU/mL penicillin, and 0.1 mg/mL streptomycin (Invitrogen, Paisley, United Kingdom) and 30 μg/mL endothelial growth factor (Sigma-Aldrich) as described.18 The use of human ECs was approved by Hammersmith Hospitals Research Ethics Committee (reference 06/Q0406/21).
Murine ECs (MECs) were isolated from Hmox1−/− and Hmox1+/+ littermate controls as described.27,28 Hearts from three 6- to 8-week-old mice were mechanically minced, digested (collagenase A 2.2 U/mL, 37°C, 30 minutes; Sigma-Aldrich), washed, and labeled with rat anti–mouse PECAM-1 antibody (CD31; BD Biosciences). Cells were incubated with goat anti–rat IgG magnetic microbeads (Miltenyi Biotec, Auburn, CA) and CD31-positive cells purified in magnetic separation columns (Miltenyi Biotec), as per the manufacturer's instructions. MECs were cultured in MCDB-131/20% FBS, 10 mM l-glutamine, 100 IU/mL penicillin, 0.1 mg/mL streptomycin, and 30 μg/mL endothelial growth factor. MEC phenotype was confirmed by characteristic cobblestone morphology and by uniform expression of endoglin (CD105) in more than 95% of cells, demonstrated by flow cytometry with mAb MJ7/18.
Exposure of ECs to CO
HUVECs maintained at 37°C were cultured either in a standard cell-culture incubator containing 95% air plus 5% CO2, or in a Plexiglas gas-tight 10-L capacity chamber containing 94% air, 5% CO2, and 1% CO, corresponding to 10 000 ppm of CO for up to 24 hours.29,30 CO concentration was monitored using a CO analyzer (Interscan, Chatsworth, CA).
Recombinant adenoviruses
Recombinant adenoviruses expressing human H-ferritin, rat HO-1, or β-galactosidase (β-gal) have been described elsewhere.31 The empty vector Ad0 control virus was a gift from E. Paleolog (Kennedy Institute, Imperial College London, London, United Kingdom). HO-1, β-gal, and Ad0 adenoviruses were amplified in HEK-293A cells, purified, and titered using BD Adeno-X Purification and Rapid Titer Kits (BD Biosciences). HUVECs were infected by incubation with adenovirus in serum-free M199 for 2 hours at 37°C. The media was changed to M199/10% FBS and HUVECs incubated overnight before experimentation. Infection of HUVECs with a β-gal control adenovirus demonstrated a transfection efficiency of more than or equal to 95%.
Flow cytometry
Flow cytometry was performed as described using a Beckman Coulter flow cytometer (Luton, United Kingdom).18 The results are expressed as the relative fluorescent intensity (RFI), representing mean fluorescent intensity (MFI) with test mAb divided by the MFI using an isotype-matched irrelevant mAb. Cell viability was assessed by examination of EC monolayers using phase-contrast microscopy, cell counting, and estimation of trypan blue exclusion.
Western blotting
Immunoblot analysis was performed as described.4 Nonreduced EC lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), which were probed with anti-DAF mAbs IA10, MD-1, or 3D5, detected with horseradish peroxidase–conjugated secondary antibodies and visualized with a chemiluminescence substrate (Pierce Chemical, Rockford, IL). For analysis of DAF expression in the liver, tissue from Hmox1−/− mice or Hmox1+/+ littermates (n = 3) was homogenized and proteins resolved by electrophoresis. Anti-DAF mAbs MD1 and 3D5 were used in combination (1 μg/mL) and detected with biotinylated rabbit anti–rat immunoglobulin (Dako), followed by streptavidin–horseradish peroxidase (BD Biosciences). To demonstrate equivalent sample loading, membranes were stripped and reprobed with control Abs. Relative levels of protein expression were quantified using ImageJ 1.29 Software (National Institutes of Health, Bethesda, MD).
Northern blotting and real-time PCR
RNA was extracted from HUVECs using the RNeasy kit (QIAGEN, Dorking, United Kingdom). Total RNA was separated on a 1% agarose/formaldehyde gel, transferred overnight to Hybond-N nylon membranes (GE Healthcare, Little Chalfont, United Kingdom), and analyzed by specific hybridization to a radiolabeled cDNA probe for human DAF as described.18 Integrated density values were obtained with an Alpha Innotech ChemiImager 5500 (San Leandro, CA), normalized with respect to the ethidium bromide–stained 28S bands on the nylon membrane, and expressed as percentage change from control.
Quantitative real-time polymerase chain reaction (PCR) was carried out using an iCycler Bio-Rad (Hercules, CA). DNase-1–digested total RNA (1 μg) was reverse-transcribed using 1 μM oligo-dT and Superscript reverse transcriptase (Invitrogen), according to the manufacturer's instructions. cDNA was amplified in a 25-μL reaction containing 5 μL cDNA template, 12.5 μL iSYBR supermix (Bio-Rad), 0.5 pmol of each sense and antisense gene-specific primer. β-Actin, GAPDH, and HPRT were used as housekeeping genes, with data calculated in relation to β-actin and verified with GAPDH and HPRT. The primer sequences used were: DAF forward 5′-CCCTCAAACAGCCTTATATCACTC-3′, DAF reverse 5′-AATATGCCACCTGGTACATCAATC-3′ and ferritin forward 5′-CGACCGCGTCCACCTCG-3′, ferritin reverse 5′-CTTTCATTATCACTGTCTCCC-3′. The cycling parameters were: 3 minutes at 95°C followed by 40 cycles of 56°C for 45 seconds, 95°C for 60 seconds, and 56°C for 60 seconds.
Transfection of endothelial cells
HUVECs were plated at 3 × 105 cells per well in 6-well plates. Short-interfering RNA (siRNA; 50 nM) was transfected into HUVECs using oligofectamine-siRNA complex-based transfection in Optimem (Invitrogen). ECs were subsequently cultured for 48 hours in endothelial cell basal medium (EBM2; Lonza Wokingham, Wokingham, United Kingdom). The siRNA duplexes for HO-1 (sense: 5′-UGCUGAGUUCAUGAGGAACUU-3′ and antisense: 5′-GUUCCUCAUGAACUCAGCAUU-3′), and negative control sequences, were synthesized by Dharmacon RNA Technologies (Lafayette, CO). Previous validation of the HO-1 sequences confirmed they reduced HO-1 mRNA by 90% and protein expression by 70% in HUVECs.32 The DAF promoter luciferase reporter plasmid pGL3-DAF A was a gift from Dr V. R. Holla (University of Texas, Austin, TX).33 ECs were transfected in triplicate with pGL3-basic or pGL3-DAF A using microporation technology (Digital Bio, Seoul, Korea) as described previously.32
Complement deposition and lysis assays
For analysis of C3 binding, HUVECs were opsonized with antiendoglin mAb RMAC8 before incubation for up to 90 minutes at 37°C with 10% to 20% NHS or heat-inactivated human serum (HIHS) in veronal-buffered saline containing 0.1% gelatin (VBSG). C3 deposition was detected with fluorescein isothiocyanate (FITC)–conjugated anti-C3 (Dako) and quantified by flow cytometry. Similar experiments were performed with MECs opsonized with antiendoglin mAb MJ7/18 and incubated with 50 μL 5% to 10% NMS in VBSG for 90 minutes at 37°C, before analysis of C3 deposition by flow cytometry using FITC-labeled antimouse C3 (ICN Biomedicals, Irvine, CA).
To estimate complement-mediated cell lysis, human and murine ECs were opsonized with RMAC8 and incubated with normal or heat-inactivated human or mouse serum (20%-50%), respectively, for up to 3 hours at 37°C. After washing, ECs were resuspended in VBSG and propidium iodide added, final concentration 50 μg/mL. ECs were analyzed by flow cytometry and lysis calculated, in triplicate samples, as the number of propidium iodide–positive cells expressed as a percentage of the total number of cells. For DAF inhibition studies, anti-DAF inhibitory mAb 1H4 was added to achieve a final concentration of 15 μg/mL.
Statistical analysis
Data are expressed as the mean of individual experiments plus or minus SEM. Data were grouped according to treatment and analyzed using GraphPad Prism Software (San Diego, CA) and the analysis of variance with Bonferroni correction or an unpaired Student t test. Differences were considered significant at values of P less than .05.
Results
HO-1 regulates endothelial cell DAF expression
VEGF and TNF-α/IFN-γ protect ECs against complement-mediated injury by up-regulating cell-surface DAF expression.4,18 To investigate the role of HO-1 in DAF induction, HUVECs were treated with VEGF or TNF-α/IFN-γ for 48 hours in the presence or absence of HO antagonist ZnPPIX. HO-1 inhibition reduced constitutive expression of DAF, whereas TNF-α/IFN-γ and VEGF both led to a 2- to 3-fold increase in expression (P < .01 vs untreated controls), an effect abrogated when HO activity was inhibited by ZnPPIX, as analyzed by flow cytometry (Figure 1A,B). Inclusion of actinomycin D or cycloheximide abrogated induction of DAF (not shown), suggesting that up-regulation under conditions of HO-1 excess requires gene transcription and increased steady-state DAF mRNA.18 The dependence on transcription was further supported by showing that hemin significantly increased DAF promoter activity after transfection of a luciferase reporter construct pGL3-DAF A (Figure 1C).
HO-1 regulates human and murine DAF expression on vascular ECs. HUVECs were treated for 48 hours with (A) VEGF (25 ng/mL), (B) TNF-α (10 ng/mL), and IFN-γ (500 U/mL) (T/I), in the presence or absence of ZnPPIX (15 μM) or with vehicle alone (UT). DAF expression was measured by flow cytometry using mAb 1H4 and presented as RFI (mean ± SEM), derived by dividing the mean fluorescent intensity (MFI) with test mAb by the MFI obtained with an isotype-matched irrelevant mAb (n = 3 experiments). (C) HUVECs were transfected with a DAF promoter luciferase reporter construct pGL3-DAF or pGL3-basic before treatment with hemin (0.2 μM) and analysis of luciferase activity. Data are expressed as relative to untreated ECs. (D) Murine cardiac ECs isolated from Hmox1−/− and Hmox1+/+ mice were lysed, transblotted to polyvinylidene difluoride membranes, and immunoblotted with rat anti–mouse DAF mAb MD1. Equal loading was confirmed by reprobing with anti–α-tubulin. (E) Aortic ECs from Hmox1−/− and Hmox1+/+ mice were lysed and immunoblotted for expression of HO-1 and HO-2. (F) Livers from Hmox1−/− and Hmox1+/+ mice were homogenized, and expression of DAF was detected by immunoblotting using rat anti–mouse DAF mAbs MD1 and 3D5. Equal loading was confirmed by reprobing with antiactin. In panels D and F, relative levels of protein expression were quantified using image analysis and expressed as the DAF/control Ab ratio (n = 4 experiments). (G) HUVECs were left untreated (UT) or transfected with control siRNA (Ctrl) or validated HO-1–specific siRNA. DAF mRNA levels were quantified by real-time PCR after 48 hours. *P < .05; **P < .01.
HO-1 regulates human and murine DAF expression on vascular ECs. HUVECs were treated for 48 hours with (A) VEGF (25 ng/mL), (B) TNF-α (10 ng/mL), and IFN-γ (500 U/mL) (T/I), in the presence or absence of ZnPPIX (15 μM) or with vehicle alone (UT). DAF expression was measured by flow cytometry using mAb 1H4 and presented as RFI (mean ± SEM), derived by dividing the mean fluorescent intensity (MFI) with test mAb by the MFI obtained with an isotype-matched irrelevant mAb (n = 3 experiments). (C) HUVECs were transfected with a DAF promoter luciferase reporter construct pGL3-DAF or pGL3-basic before treatment with hemin (0.2 μM) and analysis of luciferase activity. Data are expressed as relative to untreated ECs. (D) Murine cardiac ECs isolated from Hmox1−/− and Hmox1+/+ mice were lysed, transblotted to polyvinylidene difluoride membranes, and immunoblotted with rat anti–mouse DAF mAb MD1. Equal loading was confirmed by reprobing with anti–α-tubulin. (E) Aortic ECs from Hmox1−/− and Hmox1+/+ mice were lysed and immunoblotted for expression of HO-1 and HO-2. (F) Livers from Hmox1−/− and Hmox1+/+ mice were homogenized, and expression of DAF was detected by immunoblotting using rat anti–mouse DAF mAbs MD1 and 3D5. Equal loading was confirmed by reprobing with antiactin. In panels D and F, relative levels of protein expression were quantified using image analysis and expressed as the DAF/control Ab ratio (n = 4 experiments). (G) HUVECs were left untreated (UT) or transfected with control siRNA (Ctrl) or validated HO-1–specific siRNA. DAF mRNA levels were quantified by real-time PCR after 48 hours. *P < .05; **P < .01.
To dissect further the role of HO-1 in DAF regulation, primary ECs were isolated from Hmox1−/− mice and Hmox1+/+ littermates and DAF expression evaluated by immunoblotting. As seen in Figure 1D, Hmox1+/+ EC express DAF and expression is significantly reduced in Hmox1-deficient ECs. Quantification of 4 separate experiments confirmed that Hmox1−/− ECs expressed 60% less DAF than Hmox1+/+ ECs (P < .01). In contrast, analysis of cell-surface CD59 and Crry demonstrated no significant difference in expression between Hmox1−/− and Hmox1+/+ ECs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Immunoblotting confirmed that HO-1 was not detectable in Hmox1−/− ECs, whereas HO-2 was equally expressed in both Hmox1−/− and Hmox1+/+ ECs (Figure 1E). Likewise, comparison of DAF expression by immunoblotting liver homogenates from Hmox1−/− and Hmox1+/+ mice revealed 50% less DAF in HO-1–deficient mice (Figure 1F) (P = .02). The importance of HO-1 for DAF expression in human ECs was demonstrated after siRNA depletion of HO-1, using oligonucleotides previously shown to reduce HO-1 expression by up to 80%.32 DAF expression was reduced by 60% in HUVECs treated with HO-1 siRNA, a response that was not seen with control siRNA (Figure 1G).
Pharmacologic induction of HO-1 increases cell-surface DAF expression
The role of HO-1 in the regulation of DAF expression was further investigated using the HO-1 agonist CoPPIX. Initial dose-response studies confirmed that a concentration of 50 to 100 μM CoPPIX induced HO-1 expression in HUVECs (Figure 2A). Northern blotting demonstrated that treatment of HUVECs with 50 μM CoPPIX also led to a sustained increase in steady-state DAF mRNA, first detectable at 8 hours and maximal at 16 to 24 hours after treatment, with levels increasing 2-fold (Figure 2B). Analysis of EC DAF protein expression by flow cytometry and immunoblotting demonstrated a significant increase above that in untreated control ECs after treatment with CoPPIX for 48 hours (Figure 2C,D).
CoPPIX induces DAF expression on vascular ECs. (A) HUVECs were treated for 24 hours with CoPPIX (up to 100 μM), and HO-1 expression was detected by immunoblotting. (B). HUVECs were treated for up to 24 hours with CoPPIX (50 μM). Total RNA was isolated; Northern blots prepared and probed for DAF mRNA. The fold change in DAF mRNA was calculated after densitometric scanning, normalized with respect to the ethidium bromide–stained 28S band. (C,D) HUVECs were cultured for 24 hours in the presence of CoPPIX or vehicle alone, and DAF expression was measured by (C) flow cytometry with results expressed as percentage increase in RFI above control ECs, treated with plain culture medium alone (mean ± SEM; n = 3 experiments) and (D) by immunoblotting. (E) HUVECs were infected with a HO-1 recombinant adenovirus (Adv–HO-1) at an MOI of up to 200 virus particles per cell or an empty vector control adenovirus (Ad0; MOI 200). ECs were lysed 24 hours after infection, and expression of HO-1, DAF, and α-tubulin was analyzed by immunoblotting. Relative levels of DAF protein expression were quantified using image analysis densitometry and expressed as the fold change above that in Ad0-infected ECs. (F) HUVECs were infected with Adv–HO-1 or Ad0 (MOI 200), and DAF expression was measured by flow cytometry 24 hours after infection, with results expressed as percentage increase in RFI above noninfected control ECs (mean ± SEM; n = 3 experiments). (G) HUVECs were treated for 8 hours with hemin (0.2 μM) in the presence or absence of ZnPPIX (15 μM) or vehicle alone (UT). DAF mRNA levels were quantified by real-time PCR, and data are mean plus or minus SEM (n =2 experiments) relative to untreated ECs. (H) HUVECs were left untreated (UT) or transfected with control siRNA (Ctrl) or HO-1 siRNA before treatment with hemin (Hem; 0.2 μM), CoPP (50 μM), or vehicle and DAF mRNA quantified by real-time PCR as above. *P < .05; **P < .01.
CoPPIX induces DAF expression on vascular ECs. (A) HUVECs were treated for 24 hours with CoPPIX (up to 100 μM), and HO-1 expression was detected by immunoblotting. (B). HUVECs were treated for up to 24 hours with CoPPIX (50 μM). Total RNA was isolated; Northern blots prepared and probed for DAF mRNA. The fold change in DAF mRNA was calculated after densitometric scanning, normalized with respect to the ethidium bromide–stained 28S band. (C,D) HUVECs were cultured for 24 hours in the presence of CoPPIX or vehicle alone, and DAF expression was measured by (C) flow cytometry with results expressed as percentage increase in RFI above control ECs, treated with plain culture medium alone (mean ± SEM; n = 3 experiments) and (D) by immunoblotting. (E) HUVECs were infected with a HO-1 recombinant adenovirus (Adv–HO-1) at an MOI of up to 200 virus particles per cell or an empty vector control adenovirus (Ad0; MOI 200). ECs were lysed 24 hours after infection, and expression of HO-1, DAF, and α-tubulin was analyzed by immunoblotting. Relative levels of DAF protein expression were quantified using image analysis densitometry and expressed as the fold change above that in Ad0-infected ECs. (F) HUVECs were infected with Adv–HO-1 or Ad0 (MOI 200), and DAF expression was measured by flow cytometry 24 hours after infection, with results expressed as percentage increase in RFI above noninfected control ECs (mean ± SEM; n = 3 experiments). (G) HUVECs were treated for 8 hours with hemin (0.2 μM) in the presence or absence of ZnPPIX (15 μM) or vehicle alone (UT). DAF mRNA levels were quantified by real-time PCR, and data are mean plus or minus SEM (n =2 experiments) relative to untreated ECs. (H) HUVECs were left untreated (UT) or transfected with control siRNA (Ctrl) or HO-1 siRNA before treatment with hemin (Hem; 0.2 μM), CoPP (50 μM), or vehicle and DAF mRNA quantified by real-time PCR as above. *P < .05; **P < .01.
An adenoviral approach was used to overexpress HO-1 in cultured ECs. Immunoblotting demonstrated optimal expression of HO-1 24 hours after transduction of ECs with an HO-1 recombinant adenovirus (multiplicity of infection [MOI] 200 virus particles per cell), compared with an empty vector control adenovirus Ad0 (MOI 200) (Figure 2E).31 Likewise, overexpression of HO-1 increased total cell DAF expression by 2-fold and cell-surface DAF by up to 60% above that in untreated control ECs, compared with Ad0-transduced ECs (Figure 2E,F), and this was sustained at 48 hours (data not shown). Further analysis confirmed that DAF was induced by HO-1 agonist hemin, a response inhibited by ZnPPIX (Figure 2G). The dependence on HO-1 for DAF up-regulation was further supported by the observation that siRNA-mediated depletion of HO-1 significantly reduced DAF induction by both CoPP and hemin (Figure 2H).
We next considered whether a change in the cellular redox environment might influence DAF regulation in response to VEGF and TNF-α/IFN-γ. ECs were pretreated with the antioxidant N-acetylcysteine before exposure to either VEGF or TNF-α/IFN-γ for 48 hours. As shown, the presence of the alternative antioxidant failed to significantly influence DAF induction by these cytokines (Figure 3A), suggesting that up-regulation is independent of reactive oxygen species. Moreover, the effect of HO-1 on membrane-bound complement inhibitory proteins was specific to DAF, as overexpression of HO-1 had no effect on expression of MCP or CD59 (MFI ± SD for CD59 after infection with Ad0 Adv 297.3 + 9.5 and HO-1 Adv 307.0 + 6.1; MFI for MCP with Ad0 Adv 8.3 + 1.6 and HO-1 Adv 9.2 + 2.5) (Figure 3B).
HO-1–induced DAF expression is independent of ROS. (A) HUVECs were treated for 48 hours with VEGF (25 ng/mL) or TNF-α (10 ng/mL) and IFN-γ (500 U/mL) (T/I), in the presence or absence of N-acetylcysteine (5 mM) or vehicle alone (UT). DAF expression was measured by flow cytometry using mAb 1H4 and presented as mean RFI plus or minus SEM (n = 2 experiments). (B) HUVECs were left untreated (UT), infected with Adv–HO-1 or empty vector control adenovirus Ad0 (both MOI 200), and surface antigen expression was measured by flow cytometry at 24 hours after infection using mAbs 1H4 (DAF), BRIC 229 (CD59), and TRA-2-10 (MCP). Results are RFI (mean ± SEM; n = 3 experiments). *P < .05.
HO-1–induced DAF expression is independent of ROS. (A) HUVECs were treated for 48 hours with VEGF (25 ng/mL) or TNF-α (10 ng/mL) and IFN-γ (500 U/mL) (T/I), in the presence or absence of N-acetylcysteine (5 mM) or vehicle alone (UT). DAF expression was measured by flow cytometry using mAb 1H4 and presented as mean RFI plus or minus SEM (n = 2 experiments). (B) HUVECs were left untreated (UT), infected with Adv–HO-1 or empty vector control adenovirus Ad0 (both MOI 200), and surface antigen expression was measured by flow cytometry at 24 hours after infection using mAbs 1H4 (DAF), BRIC 229 (CD59), and TRA-2-10 (MCP). Results are RFI (mean ± SEM; n = 3 experiments). *P < .05.
Increased HO-1 expression enhances EC protection against complement
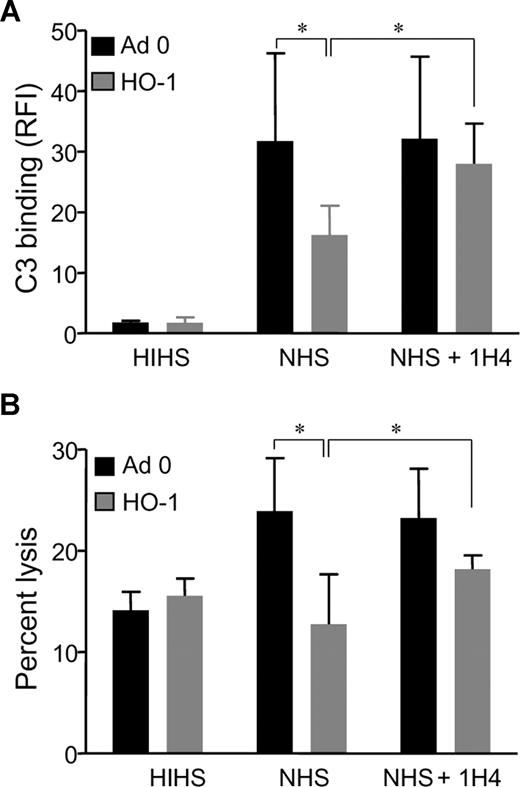
To address the functional significance of HO-1–induced DAF expression, HO-1 was overexpressed by adenoviral transfection, after which complement factor C3 deposition on the EC surface was quantified. HUVECs were opsonized, 24 hours after infection with adenoviruses, with antiendoglin mAb RMAC8, and exposed to NHS or heat-inactivated NHS. Analysis of C3 deposition by flow cytometry confirmed that HO-1 overexpression resulted in a 50% decrease in cell-surface C3, compared with Ad0 control virus-treated cells (P < .05; Figure 4A). The inclusion of inhibitory anti-DAF mAb 1H4 reversed the protective effects of HO-1 (P < .05), confirming a role for HO-1–induced DAF in cytoprotection.
HO-1 protects ECs against complement-mediated injury. HUVECs were infected with Adv-HO-1 ( ) or Ad0 (■) (MOI 200); and 24 hours after infection, ECs were harvested and (A) opsonized with mAb RMAC8 before exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 4 experiments). *P < .05. (B) To measure complement-mediated lysis, ECs were opsonized and exposed to NHS or HIHS. Propidium iodide (PI; 50 μg/mL) was added to the cell suspension, and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM, n = 4 experiments). *P < .05.
) or Ad0 (■) (MOI 200); and 24 hours after infection, ECs were harvested and (A) opsonized with mAb RMAC8 before exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 4 experiments). *P < .05. (B) To measure complement-mediated lysis, ECs were opsonized and exposed to NHS or HIHS. Propidium iodide (PI; 50 μg/mL) was added to the cell suspension, and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM, n = 4 experiments). *P < .05.
HO-1 protects ECs against complement-mediated injury. HUVECs were infected with Adv-HO-1 ( ) or Ad0 (■) (MOI 200); and 24 hours after infection, ECs were harvested and (A) opsonized with mAb RMAC8 before exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 4 experiments). *P < .05. (B) To measure complement-mediated lysis, ECs were opsonized and exposed to NHS or HIHS. Propidium iodide (PI; 50 μg/mL) was added to the cell suspension, and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM, n = 4 experiments). *P < .05.
) or Ad0 (■) (MOI 200); and 24 hours after infection, ECs were harvested and (A) opsonized with mAb RMAC8 before exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 4 experiments). *P < .05. (B) To measure complement-mediated lysis, ECs were opsonized and exposed to NHS or HIHS. Propidium iodide (PI; 50 μg/mL) was added to the cell suspension, and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM, n = 4 experiments). *P < .05.
To assess the physiologic relevance of reduced C3 binding, a validated propidium iodide cell-lysis assay was used,4 in which ECs were infected with Adv–HO-1 or Ad0, opsonized, and exposed to serum. A significant reduction in complement-mediated cell lysis was observed in ECs overexpressing HO-1 (P < .05; Figure 4B), and inclusion of mAb 1H4 significantly reversed the effect of HO-1 overexpression (P < .05). Furthermore, the effect of HO-1 could not be ascribed to changes in either CD59 or MCP (Figure 3B).
HO-1 products and DAF expression
The catabolism of heme results in the generation of biliverdin, free Fe, and CO, with biliverdin subsequently converted to bilirubin by biliverdin reductase.5 Initial experiments investigated the effect of bilirubin on EC DAF expression in vitro. Bilirubin increased by 2-fold the expression of steady-state DAF mRNA at 6 to 8 hours (Figure 5A). Immunoblotting of total cell DAF demonstrated a 3-fold increase in DAF protein expression, after treatment with bilirubin for 24 hours (Figure 5B), whereas flow cytometric analysis of the change in cell-surface expression revealed a dose-dependent increase of up to 70%, which was maximal after treatment with 10 μM bilirubin at 24 hours compared with untreated ECs (P < .05; Figure 5C). Subsequent experiments investigated the potential role of CO in HO-1–mediated DAF expression. Although a transient increase in DAF expression was seen, which peaked at 4 to 8 hours after exposure (Figure 5D), returning to baseline at 24 hours (not shown), this did not reach significance, suggesting that CO is not predominant in HO-1–mediated DAF induction.
Bilirubin induces DAF expression on vascular ECs. (A) HUVECs were treated for up to 8 hours with bilirubin (10 μM). Total RNA was isolated; Northern blots prepared and probed for DAF mRNA. The fold change in DAF mRNA was calculated after densitometric scanning of each band, normalized with respect to the ethidium bromide–stained 28S bands. (B) HUVECs were treated with bilirubin (0-10 μM) for 24 hours, before lysis and analysis of DAF expression by immunoblotting. The fold change in DAF expression was calculated by densitometry, normalized with respect to α-tubulin (n = 3). (C) HUVECs were treated with bilirubin (up to 10 μM) or vehicle alone for 24 hours before analysis of DAF expression by flow cytometry using mAb 1H4. Results are percentage increase in RFI above control ECs treated with plain EC culture medium (mean ± SEM; n = 6 experiments). *P < .05; **P < .01. (D) HUVECs were exposed to 95% air plus 5% CO2 or 94% air, 5% CO2 plus 1% CO (corresponding to 10 000 ppm of CO) for up to 8 hours, before lysis and quantification of DAF expression by immunoblotting with mAb IA10. The membrane was stripped and reprobed with antitubulin. Relative levels of protein expression were quantified using image analysis and expressed as percentage above air-treated control.
Bilirubin induces DAF expression on vascular ECs. (A) HUVECs were treated for up to 8 hours with bilirubin (10 μM). Total RNA was isolated; Northern blots prepared and probed for DAF mRNA. The fold change in DAF mRNA was calculated after densitometric scanning of each band, normalized with respect to the ethidium bromide–stained 28S bands. (B) HUVECs were treated with bilirubin (0-10 μM) for 24 hours, before lysis and analysis of DAF expression by immunoblotting. The fold change in DAF expression was calculated by densitometry, normalized with respect to α-tubulin (n = 3). (C) HUVECs were treated with bilirubin (up to 10 μM) or vehicle alone for 24 hours before analysis of DAF expression by flow cytometry using mAb 1H4. Results are percentage increase in RFI above control ECs treated with plain EC culture medium (mean ± SEM; n = 6 experiments). *P < .05; **P < .01. (D) HUVECs were exposed to 95% air plus 5% CO2 or 94% air, 5% CO2 plus 1% CO (corresponding to 10 000 ppm of CO) for up to 8 hours, before lysis and quantification of DAF expression by immunoblotting with mAb IA10. The membrane was stripped and reprobed with antitubulin. Relative levels of protein expression were quantified using image analysis and expressed as percentage above air-treated control.
Deletion of Hmox1 by homologous recombination increases the cellular pool of labile Fe in ECs,28 whereas HO-1 overexpression increases levels of the Fe-sequestering protein H-ferritin and opens Fe2+ export channels, reducing cellular free Fe2+.7 CoPP, which induces DAF (Figure 2), also significantly increases expression of ferritin (Figure 6A). To explore a potential mechanistic link, we initially used the iron chelators desferrioxamine (DFO) and salicyl-aldehyde isonicotinoyl hydrazone (SIH) to reduce intracellular free Fe, as well as adenoviral-mediated expression of H-ferritin, to create an in vitro model of the effects of HO-1–derived Fe, as previously described.31 Exposure of HUVECs to DFO or SIH resulted in a significant increase of up to 120% in cell-surface DAF expression, first detectable at 24 hours and maximal at 48 hours after treatment. Adenoviral-mediated overexpression of H-ferritin also induced DAF expression by 55% (P < .01 vs βgal control; Figure 6B). We next explored the effect of iron chelation on DAF induction by VEGF, TNF-α/IFN-γ, and hemin (Figure 6C,D). In all cases, the presence of DFO further increased DAF induction, although this only reached significance with TNF-α/IFN-γ.
DFO, SIH, and ferritin increase DAF expression on vascular ECs. (A) HUVECs were left untreated or treated for up to 24 hours with CoPPIX (50 μM), and DAF mRNA levels were quantified by real-time PCR. (B) HUVECs were treated with desferrioxamine (DFO; 100 μM), SIH (100 μM), vehicle (UT), or transfected with adenoviruses expressing either heavy-chain ferritin (FHC) or βgal (MOI 200), for up to 48 hours before analysis of DAF expression by flow cytometry using mAb 1H4. (C) HUVECs were treated for 48 hours with VEGF (25 ng/mL) or TNF-α (10 ng/mL) and IFN-γ (500 U/mL) (T/I), in the presence or absence of DFO or vehicle alone (UT), and DAF expression was quantified by flow cytometry. Flow cytometric data are expressed as percentage increase in RFI above that of ECs treated with plain EC culture medium alone. (D) HUVECs were left untreated or treated for 6 hours with hemin (0.2 μM) in the presence or absence of DFO or vehicle alone (UT). DAF mRNA levels were quantified by real-time PCR. (E) HUVECs were left untreated or treated for up to 24 hours with VEGF (25 ng/mL), and DAF (▨) and ferritin (▩) mRNA levels were quantified by real-time PCR. Data are mean plus or minus SEM (n = 2-4 experiments). *P < .05; **P < .01.
DFO, SIH, and ferritin increase DAF expression on vascular ECs. (A) HUVECs were left untreated or treated for up to 24 hours with CoPPIX (50 μM), and DAF mRNA levels were quantified by real-time PCR. (B) HUVECs were treated with desferrioxamine (DFO; 100 μM), SIH (100 μM), vehicle (UT), or transfected with adenoviruses expressing either heavy-chain ferritin (FHC) or βgal (MOI 200), for up to 48 hours before analysis of DAF expression by flow cytometry using mAb 1H4. (C) HUVECs were treated for 48 hours with VEGF (25 ng/mL) or TNF-α (10 ng/mL) and IFN-γ (500 U/mL) (T/I), in the presence or absence of DFO or vehicle alone (UT), and DAF expression was quantified by flow cytometry. Flow cytometric data are expressed as percentage increase in RFI above that of ECs treated with plain EC culture medium alone. (D) HUVECs were left untreated or treated for 6 hours with hemin (0.2 μM) in the presence or absence of DFO or vehicle alone (UT). DAF mRNA levels were quantified by real-time PCR. (E) HUVECs were left untreated or treated for up to 24 hours with VEGF (25 ng/mL), and DAF (▨) and ferritin (▩) mRNA levels were quantified by real-time PCR. Data are mean plus or minus SEM (n = 2-4 experiments). *P < .05; **P < .01.
To further define the relationship between ferritin induction and DAF up-regulation, we compared their expression kinetics after treatment of ECs with VEGF (Figure 6E). An increase in ferritin mRNA was apparent 8 hours after treatment and was maintained at 24 hours. In comparison, DAF expression was delayed and maximal 24 hours after treatment. Likewise, after exposure to CoPP, ferritin mRNA induction was maximal by 8 hours (Figure 6A), compared with DAF mRNA levels peaking from 8 to 24 hours (Figure 2B). A similar relationship was seen in response to TNF-α/IFN-γ (not shown). Thus, the combined action of increased intracellular bilirubin and Fe2+-induced ferritin synthesis appears to underpin the ability of HO-1 activity to increase cell-surface DAF expression.
Bilirubin enhances EC protection against complement-mediated cytotoxicity
To determine whether bilirubin-induced DAF expression accounted, at least in part, for the cytoprotective effects of HO-1 against complement activation, HUVECs were treated for 24 hours with bilirubin, before exposure to NHS and analysis of C3 deposition by flow cytometry. Bilirubin reduced C3 deposition by 40% (P < .05 vs untreated controls; Figure 7A). Likewise, bilirubin-induced DAF expression protected against complement-mediated EC lysis (Figure 7B). Inclusion of mAb 1H4 reversed the protective of bilirubin against both C3 deposition and cell lysis (Figure 7A,B).
HO-1 and bilirubin protect ECs against complement-mediated injury. HUVECs were treated for 24 hours with bilirubin (10 μM;  ) or vehicle alone (UT; ■) before harvesting, opsonization with mAb RMAC8, and exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. (A) C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 6 experiments). *P < .05. (B) To measure complement-mediated lysis, propidium iodide (PI; 50 μg/mL) was added to the cell suspension and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM; n = 6 experiments). *P < .05. (C) Murine cardiac ECs isolated from Hmox-1−/− and Hmox-1+/+ mice were opsonized with antiendoglin mAb MJ7/18 and incubated with 5% to 10% normal mouse serum (NMS) or heat-inactivated NMS (HIMS) for 90 minutes at 37°C, before analysis of C3 deposition by flow cytometry using FITC-labeled antimouse C3. Results are RFI (mean ± SEM; n = 3 experiments). *P < .05. (D) To estimate complement-mediated cell lysis, murine ECs were opsonized with MJ7/18 and incubated with NMS or HIMS, respectively, for 2 hours at 37°C. After washing, ECs were resuspended in veronal-buffered saline and propidium iodide added (50 μg/mL). ECs were analyzed by flow cytometry and percentage EC lysis calculated as in panel B (mean ± SEM; n = 3). *P < .05.
) or vehicle alone (UT; ■) before harvesting, opsonization with mAb RMAC8, and exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. (A) C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 6 experiments). *P < .05. (B) To measure complement-mediated lysis, propidium iodide (PI; 50 μg/mL) was added to the cell suspension and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM; n = 6 experiments). *P < .05. (C) Murine cardiac ECs isolated from Hmox-1−/− and Hmox-1+/+ mice were opsonized with antiendoglin mAb MJ7/18 and incubated with 5% to 10% normal mouse serum (NMS) or heat-inactivated NMS (HIMS) for 90 minutes at 37°C, before analysis of C3 deposition by flow cytometry using FITC-labeled antimouse C3. Results are RFI (mean ± SEM; n = 3 experiments). *P < .05. (D) To estimate complement-mediated cell lysis, murine ECs were opsonized with MJ7/18 and incubated with NMS or HIMS, respectively, for 2 hours at 37°C. After washing, ECs were resuspended in veronal-buffered saline and propidium iodide added (50 μg/mL). ECs were analyzed by flow cytometry and percentage EC lysis calculated as in panel B (mean ± SEM; n = 3). *P < .05.
HO-1 and bilirubin protect ECs against complement-mediated injury. HUVECs were treated for 24 hours with bilirubin (10 μM;  ) or vehicle alone (UT; ■) before harvesting, opsonization with mAb RMAC8, and exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. (A) C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 6 experiments). *P < .05. (B) To measure complement-mediated lysis, propidium iodide (PI; 50 μg/mL) was added to the cell suspension and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM; n = 6 experiments). *P < .05. (C) Murine cardiac ECs isolated from Hmox-1−/− and Hmox-1+/+ mice were opsonized with antiendoglin mAb MJ7/18 and incubated with 5% to 10% normal mouse serum (NMS) or heat-inactivated NMS (HIMS) for 90 minutes at 37°C, before analysis of C3 deposition by flow cytometry using FITC-labeled antimouse C3. Results are RFI (mean ± SEM; n = 3 experiments). *P < .05. (D) To estimate complement-mediated cell lysis, murine ECs were opsonized with MJ7/18 and incubated with NMS or HIMS, respectively, for 2 hours at 37°C. After washing, ECs were resuspended in veronal-buffered saline and propidium iodide added (50 μg/mL). ECs were analyzed by flow cytometry and percentage EC lysis calculated as in panel B (mean ± SEM; n = 3). *P < .05.
) or vehicle alone (UT; ■) before harvesting, opsonization with mAb RMAC8, and exposure to NHS or heat-inactivated NHS (HIHS) for up to 3 hours. To inhibit DAF, mAb 1H4 was added to the assay with RMAC8. (A) C3 deposition was analyzed by flow cytometry using FITC-conjugated anti–human C3, and results are RFI (mean ± SEM; n = 6 experiments). *P < .05. (B) To measure complement-mediated lysis, propidium iodide (PI; 50 μg/mL) was added to the cell suspension and analysis was by flow cytometry. Percentage EC lysis was calculated as the number of PI-positive cells expressed as a percentage of the total number of cells (mean ± SEM; n = 6 experiments). *P < .05. (C) Murine cardiac ECs isolated from Hmox-1−/− and Hmox-1+/+ mice were opsonized with antiendoglin mAb MJ7/18 and incubated with 5% to 10% normal mouse serum (NMS) or heat-inactivated NMS (HIMS) for 90 minutes at 37°C, before analysis of C3 deposition by flow cytometry using FITC-labeled antimouse C3. Results are RFI (mean ± SEM; n = 3 experiments). *P < .05. (D) To estimate complement-mediated cell lysis, murine ECs were opsonized with MJ7/18 and incubated with NMS or HIMS, respectively, for 2 hours at 37°C. After washing, ECs were resuspended in veronal-buffered saline and propidium iodide added (50 μg/mL). ECs were analyzed by flow cytometry and percentage EC lysis calculated as in panel B (mean ± SEM; n = 3). *P < .05.
HO-1–deficient ECs are susceptible to complement-mediated cytotoxicity
The ability of HO-1–deficient and Hmox1+/+ EC to resist complement activation was assessed by opsonizing ECs with antiendoglin mAb MJ17/8 and exposing them to NMS. Flow cytometric analysis of C3 demonstrated cell-surface deposition on wild-type Hmox1+/+ ECs exposed to 7.5% and 10% NMS, with significantly more C3 deposited on Hmox1−/− ECs (P < .05; Figure 7C). Likewise, when the functional significance was assessed by estimating cell lysis, HO-1–deficient ECs displayed enhanced susceptibility to complement-mediated injury (percentage lysis ± SEM Hmox1+/+ EC 52.6 ± 4.6, Hmox1−/− EC 83.4 ± 3.1, P < .01; Figure 7D).
Discussion
The association between vascular injury, EC dysfunction, and atherogenesis has highlighted the importance of understanding mechanisms associated with vasculoprotection. HO-1 is a cytoprotective protein that activates antiapoptotic,13,34 anti-inflammatory,29 and antiproliferative signaling pathways (reviewed by Ryter et al35 ). The significance of these actions is illustrated by the widespread vascular injury associated with HO-1 deficiency8,9 and by the protective effects against ischemic heart disease and inflammatory aortic aneurysms afforded by HO-1 gene polymorphisms associated with enhanced induction.35 However, although the products of HO-1 are capable of activating an array of downstream cytoprotective responses, the mechanisms underlying these remain poorly understood. We have now shown that modulation of complement activation, through induction of DAF, is an important component of the cytoprotective armamentarium of HO-1.
Adult vascular endothelial homeostasis is dependent on survival signals, many of which are regulated by VEGF.2 Thus, withdrawal of VEGF can lead to vessel regression, and treatment with the anti-VEGF mAb bevacizumab may be complicated by thrombosis and hypertension.36 We have reported that VEGF induces EC expression of HO-1 and the complement inhibitory protein DAF,3,4 leading to the current hypothesis that HO-1 is an important intermediate in VEGF-induced cytoprotection and specifically in DAF expression. This mechanistic link was supported by the demonstration that HO antagonist ZnPPIX reduced constitutive expression of DAF and abrogated VEGF-induced up-regulation. The role of HO-1 in constitutive DAF expression may be driven by VEGF, reflecting the importance of autocrine VEGF signaling in adult endothelial homeostasis.37 Likewise, Hmox1 deletion, or siRNA-mediated HO-1 knockdown, resulted in decreased DAF expression and inhibition of DAF induction, suggesting that HO-1 is a physiologic regulator of DAF. This novel role of HO-1 was reinforced by data demonstrating that Hmox1−/− cardiac EC are more susceptible to complement-induced injury than those isolated from Hmox1+/+ mice.
A reporter construct revealed increased DAF promoter activity in response to hemin, whereas both actinomycin D and cycloheximide inhibit DAF induction under conditions of HO-1 excess, and CoPPIX, hemin, and bilirubin increase DAF mRNA. These data suggest that DAF induction requires gene transcription, increased steady-state DAF mRNA, and de novo protein synthesis. However, although we did not find evidence for increased DAF mRNA stability (data not shown), we cannot exclude a role for posttranslational processes, such as reduced loss of cell-surface DAF, which might occur through increased stability of the glycosylphosphatidyl-inositol anchor.
Although a 60% increase in cell-surface DAF expression after induction of HO-1 appears relatively modest, its biologic significance was revealed by reduction in C3 deposition after complement activation. Reduced cell-surface C3 is especially relevant in view of the relative inefficiency of terminal complement pathway activation, which is critically dependent on excess C3. Furthermore, HO-1 overexpression and up-regulation of DAF protected ECs against complement-mediated lysis independently of any effect on MCP or CD59. Although DAF, MCP, and CD59 are constitutively expressed on vascular endothelium, their functions are complementary and regulation distinct. DAF expression is readily induced by cytokines and growth factors, providing enhanced resistance against complement-mediated injury, whereas MCP and CD59 are unaffected.4,18 In contrast, laminar shear stress induces CD59 expression via activation of Kruppel-like factor 2, suggesting that specificity in regulation is conferred at the level of transcription.38
In addition to DAF induction, HO-1 may activate alternative mechanisms capable of enhancing protection against complement. Thus, C5a and the C5b-9 MAC may increase generation of cytotoxic reactive oxygen species by ECs,39 and these may be countered by the known antioxidant properties of HO-1. Moreover, the antiapoptotic actions of HO-1 are probably important. Although the role of the MAC in modulating apoptosis is cell type– and dose-dependent, in excess, C5b-9 may trigger apoptosis. Hence, the ability of HO-1, and in particular CO, to protect ECs against apoptosis may contribute to EC cytoprotection against the deleterious effects of complement fixation.34
The efficacy of HO-1 is thought to reflect the complementary actions of its products biliverdin, free Fe, and CO, which may use distinct mechanisms.31 Thus, although CO and biliverdin minimize ischemia reperfusion injury, they influence different aspects of the pathophysiology and act synergistically in combination.40 We found that bilirubin and iron chelation with SIH or DFO were capable of inducing sustained DAF expression. In addition, DFO enhanced DAF up-regulation in response to VEGF and TNF-α/IFN-γ. In contrast, the effect of CO was more transient and did not reach statistical significance. Heme catabolism liberates free Fe, which induces expression of the iron-binding protein H-ferritin6 and opening of Fe2+ export channels.7 Our data suggest that, in addition to bilirubin, increased intracellular ferritin plays an important role in DAF induction, a concept supported by the observation that increased H-ferritin mRNA preceded DAF mRNA induction after treatment with VEGF and CoPP, whereas H-ferritin overexpression resulted in DAF up-regulation.
Functional analysis confirmed that bilirubin-induced DAF expression restricted C3 deposition and complement-mediated cell lysis, and this was reversed by inhibitory DAF mAb 1H4. To the best of our knowledge, this is the first demonstration that bile pigments inhibit complement activation at the level of the C3 and C5 convertases. It should be noted that biliverdin and bilirubin have previously been reported to inhibit the classic pathway at the level of C1 and to reduce complement-mediated hemolysis and inhibit the Forsmann reaction.24,41 Complement activation has been implicated in the pathogenesis of cardiovascular diseases, including atherosclerosis, myocardial infarction, vein graft arteriosclerosis, and posttransplant vasculopathy.42,43 Further protective actions of HO-1 in these settings may include inhibition of cellular adhesion molecule expression,31 antioxidant effects,44 and enhanced resistance to apoptosis.13 In addition, we propose that interference with the classic complement pathway at the level of C1, and the C3 and C5 convertases of both alternative and classic pathways, represents an important additional and unsuspected mechanism by which HO-1 and its products mediate their potent cytoprotective actions.
Posttransplant vasculopathy is in part the result of antibody-mediated complement activation, which induces proinflammatory and prothrombotic gene expression by ECs, leading to arterial remodeling and the development of fixed irreversible lesions, so compromising graft function. Accordingly, C6 deficiency protects against posttransplantation vasculopathy,42 as does expression of the cytoprotective genes HO-1, A20, and Bcl-XL.12 We have shown that HO-1 expression is essential for prolonged allograft survival.13,45 Similarly, both HO-1 expression and complement regulation are important in accommodation, that is, resistance of a transplanted organ to the effects of graft-specific antibodies and complement fixation. In light of this, our observation of a link between the activity of HO-1 and expression of the complement inhibitor DAF is probably important in accommodation, resistance to posttransplantation vasculopathy, and prolonged graft survival.46,47
The potent cytoprotective actions of HO-1 suggest that modulation of its expression or delivery of its products may have therapeutic potential. However, such an approach is not straightforward in light of the potential toxicity of CO, free iron, and bilirubin. Notwithstanding, several epidemiologic studies demonstrate that high normal or mildly raised serum bilirubin significantly protect against coronary artery disease.48 Induction of HO-1 promotes tolerance and favors long-term allograft survival.12,45,47 Exogenous CO can substitute for HO-1, conferring protection against ischemia reperfusion,49 restenosis injury, and allograft rejection.14 Likewise, biliverdin and bilirubin exert similar effects.14,50 Thus, clinical trials investigating the manipulation of HO-1 and its products will be of interest. Evidence demonstrating that activation of HO-1 may play an important role in the therapeutic benefits associated with rapamycin21 suggests that such an approach may prove fruitful.
In conclusion, HO-1 activity protects against posttransplantation vasculopathy, allograft rejection, and ischemia reperfusion injury, in which complement activation plays a significant role. We propose that modulation of complement activity through induction of DAF expression is an important component of the cytoprotective effects of HO-1 and its products, alongside their recognized anti-inflammatory, antiapoptotic, antioxidant, and antiproliferative actions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by fellowships from the Arthritis Research Campaign: KO566 (A.R.K.) and 13616 (J.C.M.). M.P.S. was partially supported by European Community, 6th Framework Grant LSH-2005-1.2.5-1 (Xenome), Phillip Morris External Research Program, POCTI/SAU-MNO/56066/2004, and POCTI/BIA-BCM/56829/2004 by Fundaçao para a Ciencia e Tecnologia (Lisbon, Portugal). I.P.G. and G.S. are supported by grants SFRH/BPD/9380/2002 and SFRH/BPD/21072/2004 from Fundaçãopara a Ciencia e Tecnologia (Lisbon, Portugal).
Authorship
Contribution: A.R.K. helped design the study and performed the research; I.P.G. and S.S.H. performed immunoblotting and flow cytometric studies; R.S., F.A., G.S., B.W., and N.A. performed some of the experimental work; D.O.H. contributed to study design and editing of the manuscript; M.P.S. helped design some of the experiments and contributed to writing of the manuscript; and J.C.M. conceived the study, participated in the research, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Justin C. Mason, Bywaters Center for Vascular Inflammation, Imperial College, Hammersmith Hospital, Du Cane Road, London, W12 ONN, United Kingdom; e-mail: justin.mason@imperial.ac.uk.
References
Author notes
*I.P.G. and S.S.H. contributed equally to this study.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal