Abstract
Monocytes and macrophages are an important reservoir of human immunodeficiency virus (HIV) and may represent the largest reservoir of this virus in tissues. Differentiation of monocytes into macrophages leads to cell attachment and susceptibility to infection and replication of HIV. Among other cell-surface molecules, integrins are overexpressed during monocyte-macrophage differentiation and may play a role in the replication cycle of envelope viruses including HIV. Here, we show that inhibition of αV integrin in monocyte-derived macrophages, by RNA interference or their inhibition by a selective small heterocyclic RGD-mimetic nonpeptide compound, inhibited the replication of HIV in the absence of cytotoxicity. Interference or inhibition of αV integrins triggered a signal transduction pathway, leading to down-regulation of nuclear factor-κB–dependent HIV-1 transcription. Such inhibition was mediated by a MAP-kinase signaling cascade, probably involving ERK1/2, p38-mitogen–activated protein kinases, and HSP27. In conclusion, our results reveal a significant role of integrin αV-mediated adhesion in HIV-1 infection of macrophages.
Introduction
Integrins are a family of transmembrane cell adhesion receptors that recognize cell-surface and extracellular matrix ligands.1 All integrins are heterodimers composed of noncovalently linked α and β subunits. In humans, at least 18 α and 8 β subunits have been identified, forming more than 20 heterodimers, each of them with different cell expression patterns and ligand specificities.2 Integrin-mediated interactions are vital to the maintenance of normal cell functioning, as they mediate the interaction between cells and with the extracellular matrix, having therefore important implications for cell adhesion, migration, and proliferation. Integrins function as mediators for cell-cell interaction and adhesion but may also elicit signal transduction events leading to cell activation and response to the extracellular stimulus.1
Different enveloped and nonenveloped viruses use integrins to enter and replicate in host cells.3 Integrins may act as viral receptors that mediate virus attachment to the cell, a process that may also promote signaling responses influencing viral replication at a later step. In the case of human cytomegalovirus, αVβ3 has been described as an entry coreceptor but also as a trigger-mediator of intracellular signaling pathways.4 The α4β7 integrin, preferentially expressed in activated T cells homing to the gut, may interact with the HIV-1 envelope glycoprotein gp120, leading to increased virus replication.5
Human blood monocytes, originated from hematopoietic precursors in the bone marrow, give rise to mature macrophages, which are distributed ubiquitously in all tissues.6,7 Adhesion molecules, particularly integrins, have been shown to up-regulate when monocytes differentiate into macrophages,8-10 suggesting that integrins play an important role in macrophage adhesion, migration, and tissue infiltration. Blood monocytes and tissue resident macrophages are important in vivo cell targets of HIV-1 infection.11-13 Macrophages may become chronically infected or serve as reservoirs of latent virus. Furthermore, HIV may sequester macrophage immunoregulatory function, that is, inducing secretion of proinflammatory cytokines that lead to recruitment and activation of new target cells for HIV, including CD4+ T cells.14,15
Unlike monocyte-derived macrophages (MDMs), undifferentiated monocytes are not readily infected by HIV-1 in cell culture.16,17 A productive viral infection may only occur after monocytes are differentiated after serum or cytokine stimulation,18 a process that leads to the up-regulation of different cell-surface markers and receptors, including αV integrins19,20 and, more specifically, an increase in the vitronectin αVβ3 receptor during the first 3 to 6 hours after infection.14
We and others have shown the antiviral effect of specific anti-αV antibodies in MDMs.19,20 Here, we describe the involvement of αV integrin-mediated adhesion in HIV-1 replication of MDMs and HeLa cells. Our data support the idea that blocking integrin αV interaction with its ligands initiates a signaling cascade that finally compromises HIV-1 replication by down-regulating nuclear factor-κB (NF-κB)–dependent HIV-1 transcription.
Methods
Cells
HeLa-P4R5-MAGI cells (AIDS Reagent Program, National Institutes of Health, Bethesda, MD), containing an HIV-1 long terminal repeat (LTR) driving expression of a LacZ reporter gene integrated into the genome, were cultured in Dulbecco modified Eagle medium (Invitrogen, Carlsbad, CA), supplemented with 10% heat-inactivated fetal calf serum (FCS; Lonza Walkersville, Walkersville, MD) and 1 μg/mL of puromycin (Sigma-Aldrich, St Louis, MO).
Peripheral blood mononuclear cells (PBMCs) were obtained from the blood of healthy donors using a Ficoll-Hypaque density gradient centrifugation. To obtain the monocyte population, 300 million PMBCs were blocked with an anti-CD32 antibody (StemCell Technologies, Vancouver, BC), and monocytes were separated by a negative selection antibody cocktail (StemCell Technologies) supplemented with an anti-CD41 antibody (StemCell Technologies) to eliminate platelets. Cells from monocyte/macrophage lineage, identified by flow cytometry as CD14+, were obtained with a purity more than or equal to 85%. Monocytes were resuspended in complete culture medium: RPMI 1640 medium (Invitrogen) supplemented with 10% heat-inactivated FCS, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and monocyte-colony stimulating factor (M-CSF; PeproTech, Rocky Hill, NJ) at 20 U/mL (100 ng/mL). Then, monocytes were differentiated for 3 days at 50 000 cells/well in 96-well plates for viability and acute infection experiments. This study received Institutional Review Board approval from the Scientific Research Committee of the Hospital Germans Trias i Pujol.
For the experiments under nonadhesive conditions, HeLa cells were maintained in sterile cryotubes rolling at 37°C in culture media supplemented with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffer. Low adhesive conditions in MDMs were achieved by plating in ultra low attachment plates (Corning Life Sciences, Acton, MA).
Vitronectin (Sigma-Aldrich) coatings were performed at the indicated concentrations on standard culture plates overnight at 4°C, followed by intensive washes with phosphate-buffered saline (PBS) before monocyte plating.
Drugs
S36578 (5-(S)-7-{[4-(pyridin-2-ylamino)-butyrylamino]-methyl}-6,9-dihydro-5H-benzocyclohepten-5-yl) acetic acid (compound 1.2, synthesized as described by Perron-Sierra et al21 ) is a selective small heterocyclic RGD-mimetic nonpeptide compound targeting αVβ3 and αVβ5 integrins.21,22 The RT inhibitor 3-azido-3-deoxythymidine (zidovudine; AZT) was purchased from Sigma-Aldrich, CCR5 antagonist TAK-779 (reviewed by Este and Telenti23 ) and AMD3100 were received from the National Institutes of Health AIDS Reagent Program, and the HIV integrase inhibitor L-731988 was obtained from Merck (Rahway, NJ).24 Phorbol 12-myristate 13-acetate (PMA) was purchased from Sigma-Aldrich and tumor necrosis factor α (TNF-α) from PeproTech.
Western blots and array hybridization
HeLa-P4R5-MAGI or MDMs were harvested and lysed in chilled Cell Extraction Buffer (Invitrogen) supplemented with protease and phosphatase inhibitor cocktails (Sigma-Aldrich). Cell debris were removed by centrifugation, and the total cell homogenates were stored at −80°C until used for Western blot or array hybridization.
Samples were run using Nu-PAGE 4% to 12% gels (Invitrogen) and blotted onto nitrocellulose membranes. Blocked membranes were incubated overnight with monoclonal antibodies (mAbs) against human phospho-ERK1/2 (p44/42 MAP kinase; Thr202/Tyr204) and phospho-p38-mitogen–activated protein kinases (MAPKs; Thr180/Tyr182) from Cell Signaling Technology (Danvers, MA) at 4°C. After washing, the membranes were incubated with a secondary conjugated horseradish peroxidase antibody for 1 hour at room temperature and then revealed with SuperSignal West Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL). Membranes were stripped and incubated again to detect the nonphosphorylated proteins p38-MAPK and ERK1/2 (Cell Signaling Technology).
Invitrogen Mercator Phospho array kit (Biosource, Invitrogen) was used to determine phosphorylated amounts of the 8 proteins included, following the manufacturer's instructions. Data extracted from the scanned arrays were background-corrected as proposed by Zhu et al.25
Flow cytometry
Cells were detached with trypsin-free commercial ethylenediaminetetraacetic acid/PBS solution (Versene; Invitrogen). Cells were stained with the following mAbs: αV-phycoerythrin (PE; P2W7, Santa Cruz Biotechnology, Santa Cruz, CA), β5 and β3-fluorescein isothiocyanate (FITC), CD4-allophycocyanin, CCR5-PE, CXCR4-PE, and CD14-FITC (BD Biosciences, San Jose, CA) and analyzed in a FACSCalibur or LSR II flow cytometer (BD Biosciences). MDMs were previously incubated for 20 minutes with human immunoglobulin G at 1 mg/mL.
Immunofluorescence
Cells were grown, differentiated, and/or treated with S36578 at indicated concentrations on coverslips (multiwell chambered coverslips, Invitrogen) before fixation with formaldehyde 1%. Fixed cells were rinsed with PBS, blocked, and permeabilized with 0.01% Triton X-100, 5% FCS in PBS for 1 hour. Afterward, cells were incubated overnight at 4°C with mouse monoclonal anti-integrin αV P2W7 antibody (1:50; Santa Cruz Biotechnology) diluted in 0.5% FCS in PBS. Primary antibody was detected by incubation with an antimouse secondary biotin-conjugated antibody at room temperature for 1 hour (1:200; BD Biosciences) followed by incubation with streptavidin-FITC (1:200; Southern Biotechnology, Birmingham, AL), and cultures were counterstained for F-actin with rhodamine-conjugated phalloidin (1 pg/mL; Sigma-Aldrich) for 30 minutes. Cells were then rinsed and mounted with Prolong Gold antifade reagent (Invitrogen). Cell preparations were viewed and acquired with a Leica SP2 Confocal Microscope and software (Leica Microsystems, Wetzlar, Germany) using a oil plan apochromatic 63× objective (1.32 numeric aperture) for a final magnification of 630×.
Quantitative RT-polymerase chain reaction (qRT-PCR)
Total cellular RNA was purified from transfected cells by RNeasy mini kit (QIAGEN, Dorking, United Kingdom) as recommended by the manufacturer, including the DNAse I treatment step. Relative levels of αV and β5 or HIV-1-Tat mRNA were measured by 2-step quantitative RT-PCR and normalized by comparative Ct method to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression. In all experiments, a sample treated in the absence of RT enzyme was used as a negative control. For human genes, primers and DNA probes (assay-on-demand) were purchased from Applied Biosystems (Foster City, CA), selecting assays whose probe spans an exon junction to avoid detecting genomic DNA. For HIV-1 Tat mRNA, the following primers and probe were used: forward: AACAGTCAGACTCATCAAGCTTCTCT; reverse: TTCTTCCACCTCTCTCTCCG TCT and FAM labeled probe: TCAAAGCAACCCACCTC. Reactions were analyzed on an ABI PRISM 7000 (Applied Biosystems).
For quantitative analysis of viral DNA, DNA-extraction kit (QIAGEN) was used. Quantitative amplification of LTR for viral entry detection has been previously described.26 Alu-LTR preamplification followed by the LTR quantitative amplification was performed to detect integrated HIV-1.27 To normalize HIV copy values per cell, amplification of cellular RNaseP gene was performed (Applied Biosystems). DNA extracted from 8E5 cells (harboring one copy of integrated HIV-1) was used to build a standard curve.
Transfections
For siRNA treatment, 100 nM of siRNA was mixed with Lipofectamine 2000 reagent (Invitrogen), and 2 × 105 HeLa-P4R5-MAGI cells were seeded in 24-well plates in the absence of serum, using OPTIMEM medium (Invitrogen). After 24 hours, medium with serum was added and phenotype assessed 24 hours later.
siRNA custom sequence for siAlphaV#1 was AAGUUACUUCGGCUUCGCCGU, whereas siAlphaV#2, siBeta5, and siNontargeting control were commercially available pools of 4 different siRNAs from Dharmacon RNA Technologies (Lafayette, CO).
For Tat-transactivation assays or NF-κB activation assays, plasmids (100 ng of pcTat28 or 60 ng of pNF-κB/control-luciferase reporter [Clontech, Mountain View, CA]) were transfected into 1.5 × 105 preseeded HeLa-P4R5-MAGI cells using Lipofectamine 2000 reagent following the manufacturer's instructions. To avoid transfection differences between wells, 5 hours later, cells were detached, pooled, and reseeded in the absence or presence of the indicated treatments.
Virus stocks
HIV-1 × 4 tropic strains NL4-3 and 92UG021 were grown in MT-4 lymphoid cells. The macrophage tropic X4 HIV-1 92UG024 (provided by O. Keppler, Heidelberg, Germany) was grown in MT-4 cells and MDMs. The R5 HIV-1 strain BaL was grown in stimulated PBMCs. NL4-3 virus pseudotyped with vesicular stomatitis virus (VSV) envelope was produced in 293T cells by cotransfection of 9 μg HIV-1 NL4-3 expression plasmid lacking functional envelope (pNL4-3.Luc.R-E-, National Institutes of Health AIDS Reagent Program) together with 9 μg of VSV expression plasmid (Clontech) using the CalPhos transfection system (Clontech). Supernatants containing virus were harvested 72 hours after transfection, filtered, and stored at −80°C until used. Cell-free viral stock was estimated using an enzyme-linked immunoassay for HIV-p24 antigen detection (INNOTEST HIV p24-antigen, Innogenetics, Gent, Belgium) and titrated to obtain a similar β-galactosidase fold induction in HeLa-P4R5-MAGI cells (see “β-galactosidase detection assay”).
HIV infection and replication
HeLa-P4R5-MAGI cells (2.5 × 104) were seeded in 24-well plates and infected. siRNA-treated cells were infected 48 hours after transfection. After the required time (2 days for siRNA experiments, 5 days for S36578 experiments), the supernatant together with trypsin-detached cells were pelleted and lysed with 90 μL lysis buffer (Tris-HCl, 0.05% IGEPAL) and kept frozen until levels of viral replication were determined by β-galactosidase assay.
MDMs were infected with the R5 HIV-1 strain BaL or the X4 HIV-1 strain 92UG024 on day 3 after M-CSF stimulation, both in adhesive or nonadhesive conditions. In all the cases, compounds were added simultaneously with the virus. Three days after infection, l00 μL of culture supernatant was replaced by 100 μL M-CSF supplemented with fresh complete medium with or without the corresponding drug. HIV production was analyzed 7 days after infection by enzyme-linked immunosorbent assay HIV-p24 antigen detection in culture supernatants.
β-Galactosidase detection assay
β-Galactosidase activity in 30-μL cell extracts was quantified by a colorimetric assay as described elsewhere.29 Absorbance (405-620 nm) of noninfected samples was substracted from the rest of the samples, and values expressed as percentage of β-galactosidase activity relative to nondrug treated control.
Single-cycle infection assays
Similar to infections described in “HIV infection and replication,” cells were inoculated with viral stocks and 72 hours later cells were concentrated and luciferase activity measured using the Bright-Glo luciferase assay system (Promega, Madison, WI) on a plate-reading luminometer (Fluoroskan Ascent FL; Thermo Fisher Scientific, Waltham, MA).
Viability assays
For cell viability, MDMs were treated with the same drugs at the same concentrations as for acute infection experiments. Measurement of cell cytotoxicity was performed by a methyl tetrazolium-based colorimetric assay (MTT method) as described before.19,30
For HeLa-P4R5-MAGI cells, viability was estimated by counting total live cells by flow cytometry in the absence of viral infections. Fluorescent bead counts (Citognos, Salamanca, Spain) were used as reference for the analysis of samples.
Statistical analysis
Data are presented as mean plus or minus SD. Paired Student t test was used for comparison between 2 groups, using the GraphPad Prism software (GraphPad, San Diego, CA). P values less than .05 were considered significant.
Results
αV integrin function modulates HIV-1 infection in HeLa-P4R5-MAGI cells
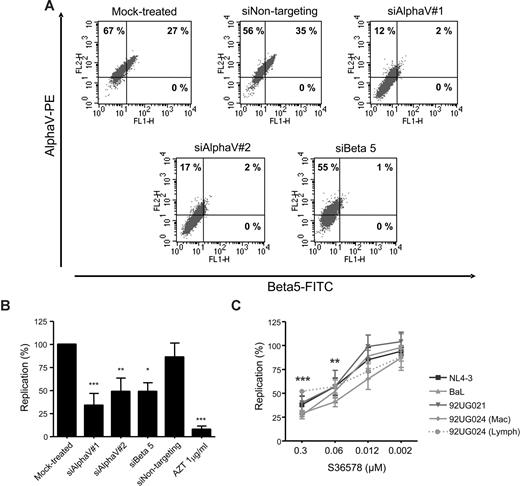
HIV-1 reporter HeLa cells are useful for the identification and study of cellular cofactors affecting HIV-1 replication.29,31 HeLa cells showed high levels of αV integrin and a partial expression of β5 integrin as seen by flow cytometry (Figure 1A), whereas other β integrin subunits known to be coupled with αV (β3 and β6) were undetectable (data not shown). Treatment with 2 different siRNAs targeting αV expression (siAlphaV#1 and siAlphaV#2) efficiently inhibited αV surface expression (14% and 19% αV expression, respectively; Figure 1A) compared with mock-treated cells (94%) or cells transfected with a control siRNA (siNontargeting, 91%). Interestingly, we observed that inhibition of integrin αV surface expression was linked to a complete down-regulation of integrin β5. Conversely, transfection of an siRNA targeting β5 not only completely abolished β5 expression (1% β5 expression) but also partially inhibited αV expression (56% αV expression). As a control, we did not observe changes in HIV-1 receptor/coreceptor expression (CD4, CXCR4, or CCR5; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article; and data not shown). In addition, quantitative PCR analysis confirmed specific inhibition of αV and β5 mRNAs by their corresponding siRNAs (Figure S2). These data suggest that, as previously shown for αVβ1,32 formation of integrin heterodimers would be dependent on the availability of both subunits forming the functional integrin.
Blocking integrin αV function inhibited HIV-1 replication in HeLa-P4R5-MAGI cells. (A) RNAi mediated inhibition of αV and β5 integrins. Flow cytometry dot plots of αV (vertical axis) and β5 (horizontal axis) of mock-treated cells or treated with different siRNAs (siAlphaV#1, siAlphaV#2, siBeta5, and siNontargeting as a control) 48 hours after transfection. One representative experiment is shown. (B) Inhibition of HIV-1 replication in αV-silenced and β5-silenced HeLa cells. Percentage of HIV-1 NL4-3 replication compared with the mock control is shown. Data are mean plus or minus SD of at least 3 independent experiments. (C) S36578 inhibition of viral replication of different HIV strains. Percentage of replication (mean ± SD of at least 3 independent experiments) at different S36578 concentrations for each of the viruses used is shown. *P < .05; **P < .005; ***P < .001.
Blocking integrin αV function inhibited HIV-1 replication in HeLa-P4R5-MAGI cells. (A) RNAi mediated inhibition of αV and β5 integrins. Flow cytometry dot plots of αV (vertical axis) and β5 (horizontal axis) of mock-treated cells or treated with different siRNAs (siAlphaV#1, siAlphaV#2, siBeta5, and siNontargeting as a control) 48 hours after transfection. One representative experiment is shown. (B) Inhibition of HIV-1 replication in αV-silenced and β5-silenced HeLa cells. Percentage of HIV-1 NL4-3 replication compared with the mock control is shown. Data are mean plus or minus SD of at least 3 independent experiments. (C) S36578 inhibition of viral replication of different HIV strains. Percentage of replication (mean ± SD of at least 3 independent experiments) at different S36578 concentrations for each of the viruses used is shown. *P < .05; **P < .005; ***P < .001.
Acute infection of siRNA-treated cells with NL4-3 HIV-1 strain showed that αV or β5 down-regulation inhibited HIV-1 replication (34% ± 13% for siAlphaV#1, 49% ± 14% for siAlphaV#2, and 49% ± 10% for siBeta5) compared with mock-treated cells or cells treated with a control siRNA (86% ± 15%; Figure 1B). Treatment with AZT, an HIV-1 RT inhibitor, potently blocked HIV-1 replication (8% ± 4%). siRNA treatment of HeLa cells did not change cellular proliferation or viability as measured by flow cytometry (Figure S4A). At the end of the infection experiments, αV expression was found to be partially recovered, mainly because of the transient nature of siRNA expression (Figure S3).
Results obtained from RNAi experiments were confirmed using a small heterocyclic nonpeptide RGD mimetic (S36578) selective for αVβ3 and αVβ5 integrins.21,22 Treatment of HeLa-P4R5-MAGI cells with different concentrations of S36578 showed a dose-dependent inhibition of several HIV-1 strains (Figure 1C), including 92UG024, 92UG021, and BaL HIV-1 isolates. The X4-tropic strain NL4-3 and R5-tropic strain BaL showed similar inhibition patterns, with maximum levels of inhibition at 0.3 μM of 38% plus or minus 9% for NL4-3 and 27% plus or minus 4% for BaL. At the same time, viral stocks of the HIV-1 92UG024 obtained in lymphoid cells (αV-negative MT-4 cell line) or macrophages (αV-positive) were compared in order to discard that the effect of S36578 was the result of an interaction with αV present in the virus membrane. Importantly, treatment of HeLa cells with S36578 did not change cellular proliferation or viability as measured by flow cytometry (Figure S4B).
S36578 did not block HIV-1 replication and was not cytotoxic in nonadhesive lymphoid MT-4 cells or stimulated PBMCs with undetectable levels of αV integrin expression (data not shown), suggesting the specificity of the effect observed in αV-positive cells.
Drug-mediated blockade of αV inhibits HIV-1 replication in MDMs
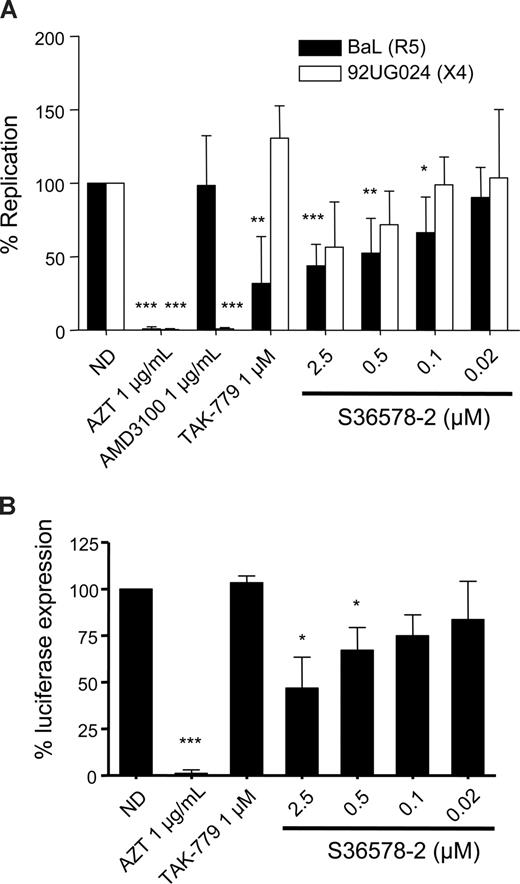
Previous experiments with an αV-specific antibody had suggested a role of αV integrins in HIV-1 infection of MDMs. Infection of MDMs with the R5-tropic strain BaL and the X4-tropic strain 92UG024 was inhibited by S36578 (Figure 2A). The maximum levels of inhibition achieved at 2.5 μM were of 43% plus or minus 14% for BaL and 56% plus or minus 30% for 92UG024 macrophage-tropic X4 strain. CCR5 antagonist TAK-779 and CXCR4 antagonist AMD3100,23 respectively, inhibited R5- and X4-tropic virus, whereas AZT inhibited both of them, compared with nontreated MDMs. Treatment with S36578 did not affect MDM viability as measured by a previously validated MTT colorimetric assay19 (Figure S4C).
S36578 inhibited HIV-1 replication in MDMs. (A) S36578 integrin antagonist inhibited HIV-1 replication with both R5 and X4 tropic HIV-1 strains. Percentage of replication measured as p24 production 7 days after infection for BaL and 92UG024 is shown. Data are the mean plus or minus SD of at least 3 independent experiments. (B) S36578 anti-HIV activity in VSV-pseudotyped HIV. Percentage of luciferase expression 3 days after infection with a VSV-G–pseudotyped HIV is represented. Data are the mean plus or minus SD of at least 3 independent experiments. ND indicates no drug. *P < .05; **P < .005; ***P < .001.
S36578 inhibited HIV-1 replication in MDMs. (A) S36578 integrin antagonist inhibited HIV-1 replication with both R5 and X4 tropic HIV-1 strains. Percentage of replication measured as p24 production 7 days after infection for BaL and 92UG024 is shown. Data are the mean plus or minus SD of at least 3 independent experiments. (B) S36578 anti-HIV activity in VSV-pseudotyped HIV. Percentage of luciferase expression 3 days after infection with a VSV-G–pseudotyped HIV is represented. Data are the mean plus or minus SD of at least 3 independent experiments. ND indicates no drug. *P < .05; **P < .005; ***P < .001.
A modified NL4-3 virus, carrying a luciferase reporter gene, lacking native Envelope protein, and pseudotyped with the vesicular stomatitis virus envelope (VSV-G), was also inhibited by S36578, with a maximum inhibition of 47% plus or minus 16% at 2.5 μM (Figure 2B) and the control RT inhibitor AZT, suggesting that αV blockade was not affecting HIV-1 receptor/coreceptor-dependent entry. As expected, the HIV-1 entry inhibitor TAK-779 did not affect luciferase expression with VSV-G–pseudotyped HIV.
Other compounds, derived from the S36578 series,21 were also tested for their capacity to inhibit HIV-1 replication in MDMs. A correlation between their anti-HIV activity and the capacity to inhibit integrin binding in cell adhesion assays in vitro was observed, confirming the αV-dependent specificity of the anti-HIV effect (data not shown).
Seeding of MDMs in vitronectin-coated plates did not prevent and sometimes enhanced the infection/replication of HIV-1 BaL (Figure S7), suggesting that this native ligand of αVβ3 and αVβ5 integrins does not have anti-HIV activity. High concentrations of soluble vitronectin partially reduced HIV-1 replication and did not show antagonist effect when combined with S36578 (Figure S9).
αV-mediated attachment influences HIV infection
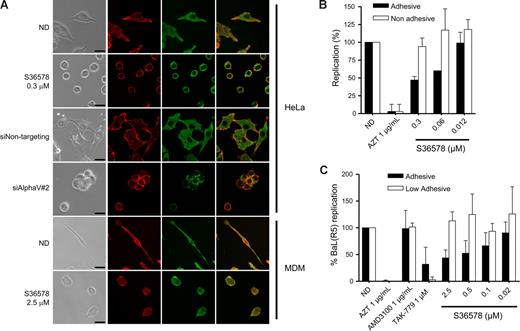
RNA-mediated down-regulation of αVβ5 or treatment with S36578 induced a loss of cell adhesion (data not shown) and a change in cell morphology (Figure 3A) in the absence of cell death. Confocal microscopy suggested a change in integrin αV arrangement, indicating that adhesion of HeLa cells is highly dependent on αV functionality. Treatment of MDMs with S36578 also induced changes in cellular shape because of the αV blockade (Figure 3A). However, MDMs treated with S36578 remained attached to the plate, suggesting that there are other integrins or other adhesion molecules, different from αV, mediating MDM attachment. Of note, treatment with S36578 did not induce a down-regulation of integrin αV expression as seen by immunofluorescence staining (Figure 3A) or flow cytometry (data not shown).
Impairment of integrin αV function by RNAi or S36578 mediated a change in cell shape and adhesion. (A) Immunofluorescence of HeLa cells and MDMs either nontreated or treated with siRNAs (siNontargeting and siAlphaV#2) or S36578. Cells were immunostained for integrin αV (green) and counterstained for filamentous actin with phalloidin (red). A change in cell shape and adhesion was observed as a consequence of the impairment of αV function. Bar represents 25 μm. (B) Antiviral activity of S36578 comparing adhesive versus nonadhesive conditions in HeLa cells. Percentage of replication compared with the untreated control of cells cultured in adhesive (■) or nonadhesive conditions (□) is represented. Data are mean plus or minus SD of 2 independent experiments. (C) Antiviral activity of S36578 comparing adhesive versus low adhesive conditions in MDMs. Percentage of replication compared with the untreated control of cells cultured in adhesive (■) or low adhesive conditions (□) is represented. Data are mean plus or minus SD of at least 3 independent experiments. ND indicates no drug; MDM, monocyte-derived macrophages.
Impairment of integrin αV function by RNAi or S36578 mediated a change in cell shape and adhesion. (A) Immunofluorescence of HeLa cells and MDMs either nontreated or treated with siRNAs (siNontargeting and siAlphaV#2) or S36578. Cells were immunostained for integrin αV (green) and counterstained for filamentous actin with phalloidin (red). A change in cell shape and adhesion was observed as a consequence of the impairment of αV function. Bar represents 25 μm. (B) Antiviral activity of S36578 comparing adhesive versus nonadhesive conditions in HeLa cells. Percentage of replication compared with the untreated control of cells cultured in adhesive (■) or nonadhesive conditions (□) is represented. Data are mean plus or minus SD of 2 independent experiments. (C) Antiviral activity of S36578 comparing adhesive versus low adhesive conditions in MDMs. Percentage of replication compared with the untreated control of cells cultured in adhesive (■) or low adhesive conditions (□) is represented. Data are mean plus or minus SD of at least 3 independent experiments. ND indicates no drug; MDM, monocyte-derived macrophages.
To further evaluate the influence of αV-mediated adhesion in HIV-1 replication, we performed experiments under nonadhesive conditions (for HeLa cells) or low-adhesive conditions for MDMs, situations in which cell attachment and subsequently the function of adhesion molecules are minimized.33,34 Treatment of HeLa cells cultured in nonadhesive conditions with S36578 did not inhibit HIV-1 replication compared with nontreated cells (Figures 3B, S5B), whereas controls (AZT, TAK-779) retained their anti-HIV activity. Similarly, S36578 did not inhibit HIV-1 replication in MDMs cultured in low-adhesive plates (Figures 3C, S6A). As previously shown,35-37 HIV-1 replication levels were lower when experiments were performed under nonadhesive or low-adhesive conditions compared with the standard conditions (Figures S5B, S6A). In case of HeLa cells, cellular growth kinetics were affected when cultured in suspension (Figure S5A). Under these conditions, and contrary to the observations in S36578 or siRNA treatments, expression levels of cell surface receptors, including integrin αV, were decreased in both HeLa cells and MDMs (Figure S7). However, these data suggest that αV-mediated attachment may be one of the cellular factors contributing to higher HIV-1 replication levels under adhesive conditions. Thus, blocking integrin αV function may lead to a change in integrin-mediated signaling that could be responsible for the observed anti-HIV activity.
Blockade of αV-mediated attachment inhibits HIV transcription
HeLa cells treated with αV-targeting siRNA or S36578 were infected with NL4-3 for 8 and 24 hours. Analysis by qRT-PCR at 8 hours did not show differences in proviral DNA (Figure S10), indicating that αV blockade did not affect viral entry or reverse transcription.26 Viral integration at 24 hours was quantified by Alu-LTR PCR followed by proviral DNA analysis.27 Again, αV inhibition did not alter HIV-1 proviral DNA integration (Figure S10). In both cases, the RT inhibitor AZT or the integrase inhibitor L-73198824 showed the expected inhibition pattern. These data, together with results obtained from single-cycle infections with VSV-pseudotyped HIV (Figure 2B), suggested viral transcription as the key step involved in inhibition of HIV-1 replication mediated by αV blockade.
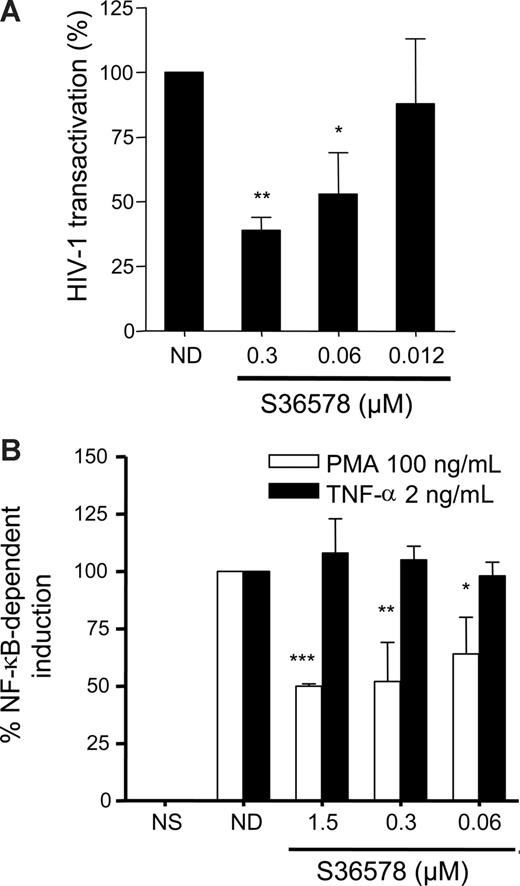
An HIV-1 Tat transactivation assay29 was performed in HeLa cells in the presence or absence of S36578. In this assay, β-galactosidase expression (under the control of the HIV-1 LTR promoter) is monitored when an HIV-1 Tat-expressing plasmid driven by cytomegalovirus (CMV) promoter (pcTat) is expressed. S36578 blocked Tat-induced LTR activation (Figure 4A), supporting the idea that blocking αV affects HIV-1 transcription. S36578 did not modify Tat transcription from the pcTat plasmid, measured by qRT-PCR (data not shown), nor did it inhibit green fluorescent protein expression of a CMV promoter green fluorescent protein-expression control plasmid measured by flow cytometry (Figure S11). This result would also suggest a specificity for the integrin αV mediated blockade of HIV-1 transcription but not other viruses such as CMV. In addition, Tat transactivation assay performed in HeLa cells cultured under nonadhesive conditions showed no differences in promoter activation as a consequence of S36578 treatment, in agreement with HIV-1 infection assays (Figure S12).
S36578 inhibited HIV-1 transcription. (A) S36578 inhibited HIV-1 Tat-induced transcription in HeLa cells. Percentage of HIV-1 transactivation compared with the untreated control is shown, measured as β-galactosidase activity 2 days after transfection of pcTat plasmid. Data are mean plus or minus SD of at least 3 independent experiments. (B) S36578 inhibited PMA-induced but not TNF-induced NF-κB transcription. Percentage of NF-κB induction depending on TNF-α (■) or PMA (□) measured as luciferase activity is shown. Data are mean plus or minus SD of at least 3 independent experiments. *P < .05; **P < .005; ***P < .001.
S36578 inhibited HIV-1 transcription. (A) S36578 inhibited HIV-1 Tat-induced transcription in HeLa cells. Percentage of HIV-1 transactivation compared with the untreated control is shown, measured as β-galactosidase activity 2 days after transfection of pcTat plasmid. Data are mean plus or minus SD of at least 3 independent experiments. (B) S36578 inhibited PMA-induced but not TNF-induced NF-κB transcription. Percentage of NF-κB induction depending on TNF-α (■) or PMA (□) measured as luciferase activity is shown. Data are mean plus or minus SD of at least 3 independent experiments. *P < .05; **P < .005; ***P < .001.
HIV-1 transcription is dependent on NF-κB transcription factor binding to viral LTR.15,36 In addition, integrin-mediated signaling may lead to the downstream activation of cascade signaling pathways converging to NF-κB activation.1,10,38,39 Therefore, we tested whether blocking αV adhesion with S36578 was able to inhibit PMA or TNF-α-mediated activation of NF-κB. Under similar levels of stimulation of NF-κB reporter plasmid, αV blockade affected PMA-induced activation (50% ± 1% inhibition), whereas TNF-α–mediated activation was not altered (Figure 4B).
S36578 influences MAPK signaling pathways
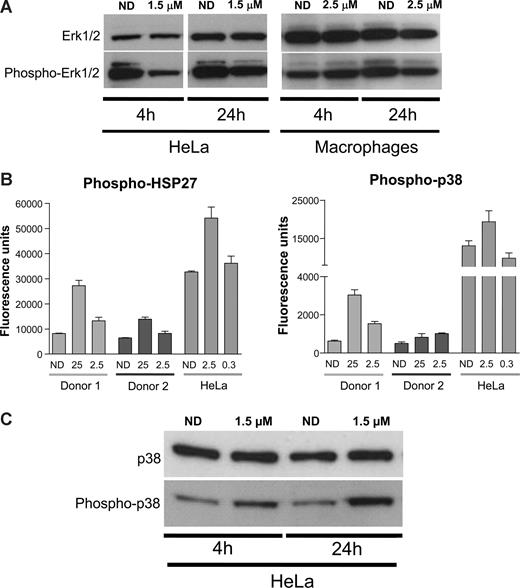
MAPKs are central mediators that propagate extracellular signals from the cell membrane to the nucleus.40 They are activated by different extracellular signals, mitogens, cytokines, but also integrin-mediated stimuli41 and, once activated, mediate several biologic responses by phosphorylating a large number of substrates.38,40 Previous reports suggested that PMA-mediated activation of NF-κB and its subsequent induction of HIV transcription could be mediated by ERK1/2 MAPK.42 In addition, αV inhibition by the racemate form of S36578 has been associated with changes in activation levels of ERK1/2.43 Therefore, Western blot analysis of HeLa and MDMs treated or not with S36578 was performed. In agreement with published data, S36578 treatment of HeLa cells decreased the levels of ERK1/2 phosphorylation after treatment (Figure 5A). In the case of MDMs, less pronounced decreased levels of ERK1/2 phosphorylation were observed at 24 hours after treatment (Figure 5A).
S36578 acts through a MAP-dependent signaling pathway. (A) Western blot of ERK1/2 in HeLa cells and MDMs treated with S36578. Treatment at 4 and 24 hours is shown. Macrophage samples of 4 and 24 hours are from different donors. (B) Increased phosphorylation of p38-MAPK and HSP27 measured with human mercator phosphoarray. MDMs from 2 different donors (Donor 1 and Donor 2) and HeLa cells were analyzed, each of them at 2 different concentrations of S36578 (25 μM and 2.5 μM for MDMs; 2.5 μM and 0.3 μM for HeLa). The bar plots represent the averaged fluorescence intensity and SD for each sample. ND indicates no drug. (C) Western blot showing an increased phosphorylation of p38-MAPK in HeLa cells as a consequence of S36578 treatment.
S36578 acts through a MAP-dependent signaling pathway. (A) Western blot of ERK1/2 in HeLa cells and MDMs treated with S36578. Treatment at 4 and 24 hours is shown. Macrophage samples of 4 and 24 hours are from different donors. (B) Increased phosphorylation of p38-MAPK and HSP27 measured with human mercator phosphoarray. MDMs from 2 different donors (Donor 1 and Donor 2) and HeLa cells were analyzed, each of them at 2 different concentrations of S36578 (25 μM and 2.5 μM for MDMs; 2.5 μM and 0.3 μM for HeLa). The bar plots represent the averaged fluorescence intensity and SD for each sample. ND indicates no drug. (C) Western blot showing an increased phosphorylation of p38-MAPK in HeLa cells as a consequence of S36578 treatment.
The involvement of the MEK/ERK pathway in HIV-1 replication in MDMs was confirmed using U0126, a specific inhibitor of MEK1/2 kinase. Treatment of MDMs with U0126 could partially inhibit HIV-1 replication in MDMs, although partial cytotoxicity was observed (Figure S13). This result is in agreement with previous reports suggesting the involvement of MEK/ERK pathway in HIV-1 replication.42
Even though we demonstrated a role of ERK1/2 activation in response to S36578, other proteins from integrin and/or MAPK-related signaling pathways may be involved in HIV modulation. We compared phosphorylation levels of 8 different proteins (FAK, Paxillin, Akt, EGFR, Src, HSP27, p38, and ATF2) in cell extracts of HeLa cells and MDMs either treated or nontreated with S36578 for 24 hours. No significant changes could be detected in any of the conditions, except for the increased phosphorylation of p38-MAPK and its downstream substrate heat-shock protein 27 (HSP27; Figure 5B and data not shown). Two different concentrations of S36578 were tested, suggesting a dose-dependent response, and results were consistent in MDMs from the 2 donors tested. To confirm these observations, Western blot of total p38 and phosphorylated p38 was performed. Increased phosphorylation of p38-MAPK after S36578 treatment was also observed for HeLa cells (Figure 5C). These results provide additional evidence of the involvement of MAP-kinase pathway in αV-mediated attachment.
Discussion
The complex interactions between monocytes and macrophages and HIV-1 are of special importance because these cells are relatively refractory to the cytophatic effects of HIV-1 and probably serve as major reservoirs for the virus and as a vehicle for disseminating HIV to various tissues.11-13 Previous identification of an mAb targeting the αV integrin subunit with anti-HIV activity, as well as the suggested involvement of αVβ3 integrin heterodimer in HIV-1 pathogenesis,20 warranted further elucidation of the role of cell adhesion receptors in HIV infection. In the present work, we have demonstrated an effect on HIV-1 transcription of integrin-mediated adhesion in monocyte-derived macrophages.
Adhesion to the substrate and differentiation state enhances HIV replication in macrophages.35-37 In addition, it has been shown that the monocyte to macrophage differentiation process implies an up-regulation of integrin expression.14,20 Here, we demonstrate that integrin αV is one of the mediators of this replication enhancement. Importantly, the impairment of integrin-mediated signaling, either by RNA inteference or using a small heterocyclic nonpeptide RGD mimetic compound (S36578) selective for αVβ3 and αVβ5 integrins, disrupts such signaling pathways, therefore compromising HIV-1 replication.
Our results suggest that blocking integrin-mediated adhesion would finally lead to an inhibition of HIV transcription mediated by NF-κB. These data are in accordance with previous results showing that HIV replication is induced by binding of the transcriptional activator NF-κB to the enhancer region of the viral LTR.36 NF-κB can be induced by several stimuli, including PMA and TNF-α.42 Interestingly, blocking αV-mediated attachment inhibits NF-κB activation when induced by PMA but not by TNF-α. It has been shown that, contrary to TNF-α and other stimuli, PMA induction may regulate NF-κB by a MAP-kinase–dependent pathway,42 suggesting the involvement of MAP-kinases in response to αV-integrin blockade.
It is known that integrin-mediated cell adhesion causes activation and nuclear translocation of MAP kinases.41,44 On the other hand, different signal transduction pathways involving calcium mobilization, phosphatidylinositol-3 kinase activation, and downstream phosphorylation of MAP kinases have been shown to control HIV transcription and posttranscription events.15 Previous results43 suggested a decreased phosphorylation of ERK1/2 MAP kinase as a consequence of the blockade of integrin-mediated signaling. Although this observation could explain on its own the inhibition of HIV-1 replication, we also observed changes in the phosphorylation of p38-MAPK and its downstream phosphorylation target HSP27.45 Interestingly, HSP27 phosphorylation has been linked to an inhibition of NF-κB activation,46 suggesting that HSP27 could also play a role in HIV-1 transcriptional inhibition when blocking integrin αV. Thus, we provide the first experimental evidence of the link between integrin-mediated adhesion and HIV-1 transcription, as a consequence of MAP-kinase pathway activation.
Cellular adhesion is mediated by several integrins. However, attachment of macrophages was not totally inhibited by S36578 at concentrations that block HIV-1 replication, suggesting that other integrins apart from αV are mediating macrophage attachment. Then, it would be of interest to address whether targeting these integrins would have a similar effect on HIV-1 replication. The α4β7 integrin has been recently shown to be involved in HIV-1 replication in lymphocytes,5 highlighting the potential relevance of integrins in modulating HIV replication.
Although little is known of the specific role of the different integrins in macrophage functions, conditional αV knockout mice models have shown that the absence of αV did not affect lymphocyte migration to lymphoid organs or in vitro activation of lymphocytes.47 On the contrary, these models indicate an important role for αV integrin on myeloid cells (macrophages, dendritic cells, and/or neutrophils) in immune regulation.48
HIV infection is accompanied by a dramatic loss of CD4+ T cells predominantly from the mucosal surfaces. Macrophage phagocytosis of apoptotic cells is a process where αV is involved,48,49 a situation building a plausible scenario in which integrin activation could contribute to HIV infection and spreading. Indeed, in vitro uptake of apoptotic cells by macrophages has been shown to enhance HIV replication.48,49 Similarly, it would be of interest to explore the in vivo role of integrin αV in macrophage migration, attachment, and infiltration to target tissues, processes in which integrin activation could be contributing to HIV-1 dissemination and spread.
Defining the cellular factors and mechanisms involved in the regulation of HIV infection in macrophages could be important for the long-term goal of eradicating viral reservoirs in infected patients. Our results show a partial inhibition of HIV-1 replication when αV integrin was blocked. This partial effect does not exclude the possibility of considering adhesion molecules and cell adhesion as a relevant step mediating HIV replication and spread. Thus, integrins may be considered an attractive and potential target for further research as they may be relevant for controlling HIV-1 and other infectious diseases.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the National Institutes of Health AIDS Reference Reagent Program for reagents.
This work was supported in part by the Spanish Ministerio de Educación y Ciencia project BFU2006-00 966 (J.A.E.), SAF2007-63 622-C02 (B.C.), the Fondo de Investigación Sanitaria PI060624, Red de Investigación en SIDA, FIPSE (36 487/05), and the European Project TRIoH (LSHB-CT-03-503480).
Authorship
Contribution: E.B. and E.P. performed experiments, analyzed data, and wrote the manuscript; J.S. performed experiments and reviewed and corrected the manuscript; B.C. reviewed and corrected the manuscript; F.P.-S. and G.C.T. provided vital reagents, analyzed data, and wrote the manuscript; and J.A.E. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: F.P.-S. and G.C.T. are employees of Institut de Recherches Servier with proprietary rights in S36578. All other authors declare no competing financial interests.
Correspondence: José A. Esté, Fundació irsiCaixa, Laboratori de Retrovirologia, Hospital Universitari Germans Trias i Pujol, Ctra. Del Canyet s/n, Badalona, Barcelona 08916, Spain; e-mail: jaeste@irsicaixa.es.
References
Author notes
*E.B. and E.P. contributed equally to this work.