Abstract
The survival of patients with Waldenstrom macroglobulinemia (WM) varies enormously. The development of prognostic models in WM has been fraught by limited follow-up in current studies. Here, we update the outcome of a prospective WM trial with a median follow-up of 10 years for live patients. Of the 59 previously untreated patients who initially were observed, only 12 patients (21%) required therapy at a median follow-up of 100 months. Multivariate analysis among the 183 patients requiring therapy reaffirmed age 70 years or greater, previous nonprotocol therapy, and β-2 microglobulin (B2M) of 3 mg/dL or greater as prognostic factors. Importantly, increased serum lactate dehydrogenase (LDH) was identified as an additional independent variable, which improved risk assessment beyond the recent WM international prognostic scoring system (ISSWM). By using age, previous therapy, B2M, and LDH, we identified 3 risk groups with 8-year survival estimates of 55%, 33%, and 5% (P < .001). These data provide novel insights into factors predicting long-term outcome in WM. This trial has been registered with www.cancer.gov under ID 4852904.
Introduction
Waldenstrom macroglobulinemia (WM) is a rare plasma cell dyscrasia.1 The underlying histopathology is a lymphoplasmacytic lymphoma infiltrating the bone marrow, lymph nodes, and visceral organs such as spleen and liver. Clinical symptom manifestations result from tumor mass effects (cytopenia) or are mediated by immunoglobulin M (IgM; hyperviscosity or autoimmune phenomena).1,2 The median survival of patients with WM varies widely and averages 7 to 10 years, depending on the tumor burden at presentation and its aggressiveness.2 The Southwest Oncology Group (SWOG) directed the first US cooperative group-wide effort to enroll WM patients into a formal prospective trial (S9003) with the objectives of developing an outcome-relevant clinical staging system in the context of uniform therapy with the purine analogue fludarabine for both untreated and previously treated patients.3,4
Several studies have tried to identify variables at presentation that predict outcome in WM; however, most studies are limited by retrospective nature and/or limited follow-up.5-10 Recently, a collaborative international effort developed a WM prognostic index (International Staging System Waldenstrom Macroglobulinemia [ISSWM]) based on a combined dataset with a median follow-up of 5 years.11 This index identified 3 distinct risk groups based on age, hemoglobin, platelet count, serum monoclonal protein, and β-2 microglobulin (B2M). Here, we updated the data from SWOG S9003 trial, with a median follow-up of 10 years, both in the context of the original prognostic variables and to assess variables that might have emerged with longer follow-up.
Methods
Patients were required to have a WM diagnosis applying previously published diagnostic criteria.3 Patients who received previous chemotherapy and/or radiotherapy were eligible as long as 4 weeks had lapsed since this treatment ended and there were no residual toxicities. All patients signed an informed consent in accordance with the Declaration of Helsinki, and the study was approved by the institutional review boards of all participating institutions. The protocol design and treatment details have been reported previously.3 After the initial enrollment registration, a second registration was performed for treatment assignment, which was either immediately after the first registration in case a patient's symptoms dictated the need for such prompt intervention, or followed after an observation interval. Response and relapse definitions also have been published previously.3 Kaplan-Meier methods were used to generate survival distribution graphs and comparisons were made via the log-rank test. Stepwise selection and Cox proportional hazards regression modeling were applied for the multivariate analyses. We calculated estimated R2 values with the methods of O'Quigley and Wu, using Schoenfield residuals.12
Results and discussion
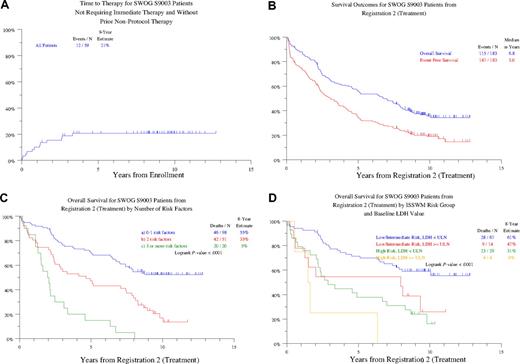
With a median follow-up of 116 months, the 10-year estimates of overall survival (OS) and event-free survival (EFS) from first registration for all 231 patients enrolled were 41% and 35%, respectively. Of the 59 previously untreated patients who did not require immediate therapy, 12 (21%) were reregistered to treatment at a median follow-up of 100 months (Figure 1A). On multivariate analysis, the only variable at baseline predictive of requirement for therapy was hemoglobin less than 11.5 g/dL. Among the 183 patients who received fludarabine, 10-year estimates of OS and EFS from second registration were 36% and 19%, respectively (Figure 1B). Univariately significant negative parameters for OS included advanced age, previous therapy, diagnosis at least 1 year before enrollment, as well as increased levels of B2M, lactate dehydrogenase (LDH), and serum M-component, along with lower IgM levels (Table 1). Multivariate analysis identified age 70 years or older, previous therapy, and increased levels of B2M and LDH as being adversely and independently associated with OS (see Table 1, multivariate 1). Age, LDH, and B2M remained as independent variables when previous therapy was not included (Table 1, multivariate 2). EFS duration was shorter in case of older age (age ≥ 70 years), lower hemoglobin concentration (< 12 g/dL), and increased levels of B2M (≥ 3 mg/L; data not shown). On multivariate analysis, only B2M remained as a significant variable (data not shown).
Survival analyses. (A) Time to treatment registration in 59 previously untreated patients who did not require therapy at initial registration. At 9 years, only 21% required initiation of therapy. (B) Survival outcomes from registration to treatment. Median durations of overall and event-free survival are 6.8 years and 3.0 years, respectively; 4 patients who were ineligible for the treatment step are excluded. (C) Overall survival according to the number of risk factors present at treatment registration. Risk factors analyzed were as follows: age 70 years or older, previous nonprotocol therapy, B2M 3 mg/L or greater and LDH more than or equal to the upper limit of normal. Three distinct groups with marked different outcomes could be distinguished. The 98 patients with no more than 1 risk factor had an 8-year survival estimate of 55%, compared with 33% among the 51 with 2 risk factors (P < .001), whereas the 20 patients with more than 2 risk factors had an 8-year survival estimate of only 5% (P < .003). (D) Overall survival according to the ISSWM-defined risk groups and serum LDH levels. ISSWM risk groups were defined as noted. Low risk: age younger than 65 years and, at most, one of the following: hemoglobin less than 11.5 g/dL, platelets less than 100 × 109/L, B2M greater than 3 mg/L, M-protein component greater than 7.0 g/dL; high risk: at least 3 of the following: age older than 65 years, hemoglobin less than 11.5 g/dL, platelets less than 100 × 109/L, B2M greater than 3 mg/L, M-protein component greater than 7.0 g/dL; intermediate risk: patients with 2 of the following: age older than 65 years, Hb less than 11.5 g/dL, platelets less than 100 × 109/L, B2M greater than 3 mg/L, M-protein component greater than 7.0 g/dL. Patients older than 65 years of age not fulfilling high-risk criteria were classified as intermediate risk. Survival was analyzed for cohorts with low-intermediate ISSWM risk (“not high”) or high ISSWM risk (“high”) on the basis of either normal or elevated serum LDH. P values: not high risk: low versus high LDH: P = .044; high risk: low versus high LDH: P = .108.
Survival analyses. (A) Time to treatment registration in 59 previously untreated patients who did not require therapy at initial registration. At 9 years, only 21% required initiation of therapy. (B) Survival outcomes from registration to treatment. Median durations of overall and event-free survival are 6.8 years and 3.0 years, respectively; 4 patients who were ineligible for the treatment step are excluded. (C) Overall survival according to the number of risk factors present at treatment registration. Risk factors analyzed were as follows: age 70 years or older, previous nonprotocol therapy, B2M 3 mg/L or greater and LDH more than or equal to the upper limit of normal. Three distinct groups with marked different outcomes could be distinguished. The 98 patients with no more than 1 risk factor had an 8-year survival estimate of 55%, compared with 33% among the 51 with 2 risk factors (P < .001), whereas the 20 patients with more than 2 risk factors had an 8-year survival estimate of only 5% (P < .003). (D) Overall survival according to the ISSWM-defined risk groups and serum LDH levels. ISSWM risk groups were defined as noted. Low risk: age younger than 65 years and, at most, one of the following: hemoglobin less than 11.5 g/dL, platelets less than 100 × 109/L, B2M greater than 3 mg/L, M-protein component greater than 7.0 g/dL; high risk: at least 3 of the following: age older than 65 years, hemoglobin less than 11.5 g/dL, platelets less than 100 × 109/L, B2M greater than 3 mg/L, M-protein component greater than 7.0 g/dL; intermediate risk: patients with 2 of the following: age older than 65 years, Hb less than 11.5 g/dL, platelets less than 100 × 109/L, B2M greater than 3 mg/L, M-protein component greater than 7.0 g/dL. Patients older than 65 years of age not fulfilling high-risk criteria were classified as intermediate risk. Survival was analyzed for cohorts with low-intermediate ISSWM risk (“not high”) or high ISSWM risk (“high”) on the basis of either normal or elevated serum LDH. P values: not high risk: low versus high LDH: P = .044; high risk: low versus high LDH: P = .108.
Univariate and multivariate analyses of overall survival for 183 patients treated with fludarabine
| Analysis . | n/N (%) . | HR (95% CI) . | P . | Cumulative R2, % . |
|---|---|---|---|---|
| Univariate | ||||
| Age > 70 y | 60/183 (33) | 2.28 (1.57-3.30) | < .001 | |
| Previous treatment | 64/183 (35) | 1.61 (1.11-2.33) | .012 | |
| Disease duration > 1 y | 72/183 (39) | 1.62 (1.12-2.34) | .010 | |
| B2M > 3 mg/L | 106/180 (59) | 1.56 (1.06-2.29) | .025 | |
| IgM < 4 g/dL | 75/183 (41) | 1.90 (1.09-2.28) | .015 | |
| LDH > ULN | 25/183 (14) | 1.69 (1.03-2.77) | .037 | |
| M-component < 2.2 g/dL | 32/116 (28) | 1.77 (1.05-3.00) | .034 | |
| ISSWM high risk* | 33/114 (29) | 2.64 (1.60-4.37) | < .001 | |
| Multivariate 1† | ||||
| Age > 70 y | 58/180 (32) | 2.29 (1.57-3.35) | < .001 | 11.702 |
| B2M > 3 mg/L | 106/180 (59) | 1.80 (1.21-2.67) | .004 | 15.594 |
| Previous treatment | 62/180 (34) | 1.73 (1.18-2.56) | .005 | 17.615 |
| LDH > ULN | 25/180 (14) | 1.77 (1.08-2.92) | .024 | 20.131 |
| Multivariate 2‡ | ||||
| Age > 70 y | 58/180 (32) | 2.32 (1.59-3.38) | < .001 | 11.702 |
| B2M > 3 mg/L | 106/180 (59) | 1.61 (1.09-2.37) | .016 | 15.594 |
| LDH > ULN | 25/180 (14) | 1.80 (1.09-2.95) | .020 | 17.571 |
| Multivariate 3§ | ||||
| ISSWM high risk | 33/114 (29) | 2.84 (1.71-4.72) | < .001 | 15.492 |
| LDH > ULN | 18/114 (16) | 2.25 (1.21-4.19) | .010 | 20.916 |
| Analysis . | n/N (%) . | HR (95% CI) . | P . | Cumulative R2, % . |
|---|---|---|---|---|
| Univariate | ||||
| Age > 70 y | 60/183 (33) | 2.28 (1.57-3.30) | < .001 | |
| Previous treatment | 64/183 (35) | 1.61 (1.11-2.33) | .012 | |
| Disease duration > 1 y | 72/183 (39) | 1.62 (1.12-2.34) | .010 | |
| B2M > 3 mg/L | 106/180 (59) | 1.56 (1.06-2.29) | .025 | |
| IgM < 4 g/dL | 75/183 (41) | 1.90 (1.09-2.28) | .015 | |
| LDH > ULN | 25/183 (14) | 1.69 (1.03-2.77) | .037 | |
| M-component < 2.2 g/dL | 32/116 (28) | 1.77 (1.05-3.00) | .034 | |
| ISSWM high risk* | 33/114 (29) | 2.64 (1.60-4.37) | < .001 | |
| Multivariate 1† | ||||
| Age > 70 y | 58/180 (32) | 2.29 (1.57-3.35) | < .001 | 11.702 |
| B2M > 3 mg/L | 106/180 (59) | 1.80 (1.21-2.67) | .004 | 15.594 |
| Previous treatment | 62/180 (34) | 1.73 (1.18-2.56) | .005 | 17.615 |
| LDH > ULN | 25/180 (14) | 1.77 (1.08-2.92) | .024 | 20.131 |
| Multivariate 2‡ | ||||
| Age > 70 y | 58/180 (32) | 2.32 (1.59-3.38) | < .001 | 11.702 |
| B2M > 3 mg/L | 106/180 (59) | 1.61 (1.09-2.37) | .016 | 15.594 |
| LDH > ULN | 25/180 (14) | 1.80 (1.09-2.95) | .020 | 17.571 |
| Multivariate 3§ | ||||
| ISSWM high risk | 33/114 (29) | 2.84 (1.71-4.72) | < .001 | 15.492 |
| LDH > ULN | 18/114 (16) | 2.25 (1.21-4.19) | .010 | 20.916 |
HR indicates hazard ratio; CI, confidence interval; B2M, β-2 microglobulin; IgM, immunoglobulin M; LDH, lactate dehydrogenase; M-component, M-protein component; ULN, upper limit of normal; and ISSWM, Waldenstrom macroglobulinemia international prognostic scoring system.
ISSWM high-risk category includes patients with at least 3 of the following: age greater than 65 years, Hb less than 11.5 g/dL, platelets less than 100 × 109/L, B2M greater than 3 mg/L, M-protein component greater than 7.0 g/dL (measured by densitometry).
Variables considered for multivariate 1 analysis included age greater than 70 years, previous treatment, disease duration greater than 1 year, CRP greater than 1 mg/L, Hb less than 12.0 g/dL, albumin less than 3.5 g/dL, B2M greater than 3 mg/L, IgM less than 4.0 g/dL, platelets less than 150 × 109/L, and LDH greater than ULN.
Multivariate 2 analysis included the same variables as multivariate 1, except previous treatment.
Variables considered for multivariate 3 analysis included ISSWM high risk, disease duration greater than 1 year, CRP greater than 1 mg/L, albumin less than 3.5 g/dL, and LDH greater than ULN.
With a median follow-up of nearly 8 years, these data demonstrate that a subset of patients with asymptomatic macroglobulinemia may not require therapy for prolonged periods and therefore represent smoldering macroglobulinemia. These data also demonstrate the capacity of purine analogues to induce durable remissions in WM. Although purine analogues currently are used mostly in combination with other agents, it is notable that 20% of patients achieved 10-year EFS with just a single agent, fludarabine, with baseline B2M as the dominant predictor of EFS. Although reaffirming the majority of the previously reported prognostic factors in patients requiring therapy, this update identified, in addition, serum LDH as an independent adverse predictor, whereas hemoglobin and IgM level were no longer independent predictors in the multivariate analysis.
Age and B2M also were included in the recently developed ISSWM, which did not analyze the impact of serum LDH.11 Therefore, we analyzed whether serum LDH retained independent predictive value when ISSWM-defined risk was included as a variable. This analysis confirmed that LDH remains as an adverse predictor independent of ISSWM risk (see Table 1, multivariate 3). The corresponding R2 values show that the inclusion of LDH in the model explains an additional 5% of the model's variability. By using age, previous nonprotocol therapy, B2M, and LDH as variables, we could identify 3 risk groups with distinctly different survival outcomes. Thus, the 98 patients with no more than one adverse variable enjoyed an 8-year survival estimate of 55% compared with 33% among the 51 with 2 risk factors and 5% for the 20 patients exhibiting more than 2 risk features (P < .001; Figure 1C). Serum LDH retained its impact on survival in the ISSWM-defined low/intermediate-risk group: those with low LDH enjoyed an 8-year survival estimate of 61%, which was significantly greater than the 47% observed among those with high LDH (P = .04; Figure 1D).
In summary, our data provide novel insights into long-term outcome in WM in the context of the largest prospective experience to date in these patients. Among initially asymptomatic patients not requiring immediate therapy, almost 80% remained in a smoldering phase for a projected 10-year time interval, supporting the current recommendation to observe such patients without therapy. Longer follow-up of this trial also identified baseline serum LDH as a major prognostic variable in WM. These data support the development of a prognostic model based on both tumor mass (B2M) and tumor aggressiveness/metabolic activity (LDH), similar to the observations in other lymphomas and in multiple myeloma.13-16 These data applied both for patients who received purine analogue as either front-line or second-line therapy (data not shown). The detection of increased serum LDH seems to confer adverse outcome in patients with ISSWM-defined low/intermediate risk. Although these studies were conducted in the era preceding the incorporation of rituximab and other active agents such as proteasome inhibitors into WM therapy,17 the mature data generated in this trial provide an important framework against which long-term outcomes with newer agents have to be measured. In this regard, it is notable that serum LDH has remained as an important prognostic factor in lymphoma patients treated with rituximab-containing regimens.15 These data also illustrate the importance of long-term follow-up to identify and refine predictors of outcome, particularly in patients with low-grade tumors such as WM.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This investigation was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services (Bethesda, MD): CA32102, CA38926, CA37981, CA20319, CA22433, CA58416, CA46441, CA13612, CA35261, CA12644, CA76462, CA35192, CA45450, CA4919, CA35090, CA58686, CA45807, CA45377, CA46282, CA28862, CA42777, CA58861, CA35199, CA35261, CA76447, CA67663, CA46113, CA45560, CA63850, CA14028, CA35431, CA35176, CA63844, CA27057, CA12212, CA52420, CA35117, CA52623, CA58658, (ECOG) CA21115, and CA13650. M.V.D. and B.B. were supported in part by funds from the National Institutes of Health.
National Institutes of Health
Authorship
Contribution: M.V.D. and B.B. coordinated the study, performed data analysis/interpretation, and wrote the paper; A.H., J.S., and J.C. performed statistical analysis and analyzed/interpreted data; and M.A.G. and S.R. contributed to patient accrual and analyzed and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Southwest Oncology Group appears in the “Appendix” (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Correspondence: Madhav V. Dhodapkar, MD, Yale Univer-sity, 333 Cedar Street, Box 208021, New Haven, CT 06510; e-mail: madhav.dhodapkar@yale.edu or Group Chair's Office, Southwest Oncology Group, 24 Frank Lloyd Wright Drive, PO Box 483, Ann Arbor, MI 48106-0483; e-mail: arlauska@med.umich.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal