Abstract
Various virus infections cause dysfunctional hemostasis and in some instances lead to the development of viral hemorrhagic fever syndrome. How do diverse viruses induce the expression of tissue factor on vascular cells? We hypothesize that a direct stimulation of pattern recognition receptors (PRR) by viral nucleic acids may be the key. Double-stranded RNA (dsRNA) is produced by many viruses and is recognized by various PRR, including Toll-like receptor-3 (TLR3). We have investigated whether poly I:C, a model for viral dsRNA, can influence cellular hemostasis. Poly I:C could up-regulate tissue factor and down-regulate thrombomodulin expression on endothelial cells but not on monocytes. The response to poly I:C was diminished upon small interfering RNA (siRNA)–mediated inhibition of TLR3, but not other PRR. In vivo, application of poly I:C induced similar changes in the aortic endothelium of mice as determined by enface microscopy. D-dimer, a circulating marker for enhanced coagulation and fibrinolysis, and tissue fibrin deposition was elevated. All the hemostasis-related responses to poly I:C, but not cytokine secretion, were blunted in TLR3−/− mice. Hence, the activation of TLR3 can induce the procoagulant state in the endothelium, and this could be relevant for understanding the mechanisms of viral stimulation of hemostasis.
Introduction
Infection with diverse viruses leads to a systemic dysfunction in coagulation and fibrinolysis1-4 and in extreme cases to the development of viral hemorrhagic fever (VHF) syndrome. Viruses causing VHF include, but are not limited to, Marburg virus, Ebola virus, Lassa virus, and Dengue virus. VHF is a severe disease that leads to multiple organ failure, hemorrhage, capillary leakage, thrombocytopenia, hypotension, and shock and is associated with high levels of fatalities.1,2,5 Most VHF causing pathogens have a RNA genome,5-8 whereby endothelial cells (EC),9 monocytes, and dendritic cells are common targets for the replication of VHF-causing viruses.10-12 Central to their pathogenesis is the uncontrolled viral replication, cytopathic effects, and immune dysregulation.13 Ebola virus infection is associated with an elevation of tissue factor (TF) expression on monocytes as well as circulating microparticles of monocytic origin that express TF.14 Endothelium is a secondary target in the case of Ebola virus,10 and although there is no cytolysis after infection,15 endothelial damage by viral glycoproteins contributes to the shock syndrome.16 Many of the changes associated with VHF are likely to be due to the concomitant increase in inflammatory mediators such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α).10,14,17-19 In a primate model of Ebola virus infection, plasma levels of activated protein C (APC) were decreased, whereas tissue plasminogen activator (tPA), D-dimer, and fibrin deposition were increased.14 Inhibition of the TF pathway with an TF-factor VIIa blocker reduced the pathology related to the Ebola virus.20
Other viruses that induce a procoagulant state through infection of EC and/or monocytes include, herpes simplex virus,3,21 cytomegalovirus,22 measles virus,23 and influenza virus.24 Direct up-regulation of TF in EC has been demonstrated in the case of influenza, adenovirus, cytomegalovirus,24 African swine fever virus,25 and the highly pathogenic avian influenza virus (H5N1).26
TF is not constitutively expressed in quiescent EC but is up-regulated by proinflammatory factors such as TNF-α and lipopolysaccharide (LPS).27 Over and above its function in hemostasis, it is also a key proinflammatory agent.28 In contrast, thrombomodulin (TM) is constitutively expressed on EC and is an important member of the intrinsic anticoagulant pathway as well as being an anti-inflammatory agent.29 The inflammation-regulatory properties by both these pathways are executed, among others, via factor Xa and APC that in turn activate protease activated receptors (PAR).28,29 Hence, through these and other mechanisms, the coagulation system also influences inflammation.30
The host response to virus infection is partly regulated by receptors that recognize viral nucleic acids as infection markers. Different pattern recognition receptors (PRR) such as those of the Toll-like receptor (TLR) family, protein kinase R (PKR), or the RNA helicases family members, such as retinoic acid inducible gene-I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5), are involved in this response.31-35 Most viruses are thought to produce double-stranded RNA (dsRNA) as a replication intermediate in their life cycle,36,37 some of which are retained in the cells or released after cell lysis.38,39 The effects of viral dsRNA in numerous in vitro and in vivo test systems can be recapitulated by synthetic poly I:C.32,36,40-43
We hypothesize that the recognition of viral nucleic acid by the host vascular cells via PRR could trigger a change from an anticoagulant to a procoagulant state. Such a mechanism would provide a unifying pathway for the effects of myriad virus infections on hemostasis. We provide evidence from in vitro and in vivo experiments that TLR3 activation can induce TF in EC. These novel observations open up new avenues for understanding the mechanisms of viral modulation of hemostasis.
Methods
Materials
Poly I:C and poly dI:dC were from GE Healthcare (Munich, Germany) and had less than 0.03 EU/mL endotoxin (QCL-1000 kit; Bio Whittaker, Walkersville, MD). Human recombinant TNF-α, IL-1β, as well as neutralizing antibodies to these cytokines were purchased from R&D Systems (Wiesbaden-Nordenstadt, Germany). LPS (Escherichia coli 0127:B8) was from Sigma-Aldrich (Munich, Germany). TLR3-antibody was from eBioscience (Kranenburg, Germany) as well as kindly provided by Dr Misako Matsumoto (Hokkaido University, Japan).
Tissue factor and thrombomodulin activity and plasma clotting time
Cell-surface functional TF activity was determined by measuring the generation of activated factor X (FXa; Haemachrom Diagnostica, Essen, Germany) using chromogenic substrate N-α-benzyloxycarbonyl-D-arginyl-glycyl-L-arginine-p-nitroaniline (s-2765; Chromogenix, Molandal, Sweden). After stimulation of cells with test substances for the indicated times, the cells were washed with N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)–buffered saline (HBS) and incubated for 60 minutes at 37°C with 5 nM factor VIIa, 150 nM FX in HBS with 3 mM CaCl2, 200 μM chromogenic substrate s-2765. Generation of FXa was quantified by measuring the increase in absorbance (405 nm) with time (due to substrate cleavage) in a temperature-regulated multiwell reader (BioTek Instruments, Winooski, VT). Maximal reaction velocity was measured and is expressed relative to control values obtained with untreated cells.
Thrombomodulin activity was assessed by measuring the capacity of the cells to generate activated protein C (APC). Cells were processed as above except that they were incubated with 160 nM protein C, 0.4 U/mL α-thrombin (National Institutes of Health), in HBS with 3 mM CaCl2 at 37°C. After 1 hour, thrombin was neutralized by the addition of 0.5 mg/mL hirudin and then incubated with 200 μM solution of the chromogenic substrate L-pyroglutamyl-L-prolyl-L-arginine p-nitroanilide hydrochloride (s-2366) at 37°C. The rate of conversion of the substrate by APC was determined as described for FXa generation.
To measure the procoagulant status of cells, plasma recalcification clotting time was determined. Cells were processed as above and then lysed with 15 mM n-octyl-β-D-glycopyranosidase in 0.1 M Imidazol buffer. Thereafter, pooled citrated human plasma was added, and clotting was started by addition of 7 mM CaCl2, and turbidity of diluted plasma was measured as absorbance at 405 nm with time. A change in 0.05 absorbance units was used an index of clotting time (in seconds). TF activity was blocked with the monoclonal antibody (mAb) VIC7, kindly provided by W. Ruf (Scripps, La Jolla, CA).
In vivo experiments in mice
TLR3−/− mice, generated as described,44 were generously provided by Dr S. Akira (Osaka University, Osaka, Japan). These were backcrossed with C57BL6 mice 6 times, giving rise to an incipient congenic strain. Wild-type (WT) C57BL6 mice kept under identical conditions were used as controls. Eight- to 10-week-old mice from both sexes were used (approximately half males and half females). Phosphate-buffered saline (PBS) and 250 μg/mouse poly I:C or 50 μg/mouse LPS (n = 8-20) were applied through the tail vein in a volume of 100 μL. At the indicated times, blood was withdrawn from the retro-orbital vein into ethylenediaminetetraacetic acid (EDTA) capillaries for the determination of D-dimer (Roche, Indianapolis, IN), TNF-α, interferon-β (IFN-β), IL-6 (eBioscience), and fibrinogen (Immunology Consultants Lab-ICL, Newberg, OR). For TAT analysis (Dade-Behring, Marburg, Germany), 200 μL 3.2% sodium citrate were administered in the vena cava 20 to 30 seconds before drawing the blood.45 Blood cell counts were performed on a KX-21 Hematology analyzer (Sysmex, Norderstedt, Germany). After killing, the mice were perfused with 4% (wt/vol) paraformaldehyde, and the liver, kidney, and aorta were removed and placed in paraformaldehyde at 4°C for 12 hours. Immunofluorescence microscopy was used to determine fibrin deposition in tissue as well as TF and TM expression in aorta by enface analysis. All animal experiments have been approved by the Regierungs Präsidium Giessen (institutional review boards) of all participating institutions.
Enface fluorescence microscopy
Aortas were cleaned of fat and adventitia, opened longitudinally to expose the lumen, and divided into 3 sections. Free aldehyde groups were quenched with 0.1 M glycin, and the sections were permeabilized and blocked as described above. TF and TM were detected with primary antibodies and visualized with rhodamin-conjugated secondary antibodies (Dianova, Hamburg, Germany). Rabbit anti–mouse tissue factor and anti–rat thrombomodulin were from American Diagnostica (Pfungstadt, Germany). Fluorescein isothiocyanate (FITC)–labeled lectin from Bandeiraea simplicifolia-1 (BS-1; Sigma-Aldrich) was used to label ECs. As negative control, primary antibodies were replaced by rabbit immunoglobulin G (IgG; Dianova). Sections were embedded in Vectashield mounting medium with nuclear 4′-6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingham, CA) and covered with a coverglass.
Aortas at regular intervals were evaluated from 4-6 mice per group. Confocal microscopy was performed with a spectral laser-scanning microscope using a plan-Apo 40× objective (NA 1.4 oil; Leica Microsystems, Heidelberg, Germany) with an image analysis system from Visitron (München, Germany). Images were captured in 0.2-μm–thick sections.
Immunofluorescence analysis of fibrin(ogen) deposition
Organs were fixed in 4% paraformaldehyde and embedded in paraffin, and 7-μm sections were cut. After deparaffinization, sections were pretreated by proteinase K (QIAGEN, Valencia, CA) for 10 minutes, permeabilized with 0.1% (wt/vol) Triton X-100, and blocked as described above. Rabbit anti–human fibrinogen (10 μg/mL; Dako, Glostrup, Denmark) was used as the primary antibody as described.
Reverse transcription–polymerase chain reaction
Total RNA was extracted using GenElute mammalian total RNA miniprep kit (Sigma-Aldrich), and reverse transcription was performed using random hexamer primer and Moloney murine leukemia virus (M-MuLV) reverse transcriptase (Applied Biosystems, Foster City, CA) from equal amounts of total RNA. Quantification of total RNA was performed by photometer (GeneQuant; GE Healthcare). Primer and the product sizes for PCRs were as follows: TF (forward primer 5′-ATG TGA AGC AGA CGT ACT TGG C-3′, reverse primer 5′-CTG TCT GTA CTC TTC CGG TTA ACT GTT C-3′, 427 bp); TM (forward primer 5′-CAT GTG CGA GAC CGG CTA CCG GCT GGC GG-3′, reverse primer 5′-TGG TAC TCG CAG TTG GCT CTG AAG-3′, 208 bp); glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward primer 5′-CCA CAT CGC TCA GAC ACC AT-3′, reverse primer 5′-CGC AAC AAT ATC CAC TTT ACC AGA G-3′, 113 bp); β-actin (forward primer 5′-ATT GCC GAC AGG ATG CAG AA-3′, reverse primer 5′-GCT GAT CCA CAT CTG CTG GAA-3′, 150 bp); IL-6 (forward primer 5′-ATG AAC TCC TTC TCC ACA AGC GC-3′, reverse primer 5′-GAA GAG CCC TCA GGC TGG ACT-3′, 628 bp); IFN-β (forward primer 5′-GAT TCA TCT AGC ACT GGC TGG-3′, reverse primer 5′-CTT CAG GTA ATG CAG AAT CC-3′, 186 bp); TLR3 (forward primer 5′-TCA CTT GCT CAT TCT CCC TT-3′, reverse primer 5′-GAC CTC TCC ATT CCT GGC-3′, 157 bp); RIG-I (forward primer 5′-CAG TAT ATT CAG GCT GAG-3′, reverse primer 5′-GGC CAG TTT TCC TTG TC-3′, 389 bp); MDA5 (forward primer 5′-AGT TTG GCA GAA GGA AGT GTC-3′, reverse primer 5′-GGA GTT TTC AAG GAT TTGAGC-3′, 480 bp); PKR (forward primer 5′-GCC TTT TCA TCC AAA TGG AAT TC-3′, reverse primer 5′-GAA ATC TGT TCTG GGC TCA TG-3′, 301 bp); tissue factor pathway inhibitor-α (TFPI-α) (forward primer 5′-TGA AGA TGG TCC GAA TGG TT-3′, reverse primer 5′-TGT TTT CAT TTC CCC CAC AT-3′, 241 bp); urokinase plasminogen activator (uPA) (forward primer 5′-CGG GGG GGC TCT GTC ACC TAC-3′, reverse primer 5′-CGG CCC CAG CTC ACA ATT CC-3′, 592 bp); tissue plasminogen activator (tPA) (forward primer 5′-GCT ACG TCT TTA AGG CGG GGA A-3′, reverse primer 5′-GTG GGC AGA GAG AAT CC-3′, 502 bp); plasminogen activator inhibitor-1 (PAI-1) (forward primer 5′-GGG AAA GGA GCC GTG GAC CAG-3′, reverse primer 5′-GGG GCA GCC TGG TCA TGT TG-3′, 355 bp); IL-1β (forward primer 5′-GAC ACA TGG GAT AAC GAG GC-3′, reverse primer 5′-ACG CAG GAC AGG TAC AGA TT-3′, 247 bp); TNF-α (forward primer 5′-CAG AGG GAA GAG TTC CCC AG-3′, reverse primer 5′-CCT TGG TCT GGT AGG AGA CG-3′, 324 bp); von Willebrand factor (VWF) (forward primer 5′-TCC TGA AGC AGA CAT ACC A-3′, reverse primer 5′-CAG GTG TCG TAA ATG CAG A-3′, 318 bp); endothelial nitric oxide synthase (eNOS) (forward primer 5′-GTT TTC CAT CAG GAG ATG GTC A-3′, reverse primer 5′-TCC TCT TCC GCC GCC AA-3′, 516 bp). Primers were synthesized by Thermo Electron (Oberhausen, Germany). Through preliminary experiments, the number of cycles for each primer pair was optimized so that the amplification in the linear range was obtained.
Small interfering RNA (siRNA)
Human umbilical vein endothelial cells (HUVECs) were transiently transfected, with small interfering RNA (siRNA) TLR3 (no. SI02655156), PKR (no. SI02655156), MDA5 (no. SI02655156), RIG-I (no. SI02655156), and GAPDH (no. SI02653266) (QIAGEN). Transfection was performed using Oligofectamine (Invitrogen, Carlsbad, CA) for 4 hours, and knockdown of respective gene was analyzed 48 hours after transfection by reverse transcription–polymerase chain reaction (RT-PCR).
Transfection of nucleic acid ligands
Nucleic acid ligands were complexed with Lipofectamine 2000 (Invitrogen) for 20 minutes and then added to the cells for 4 to 5 hours.
Cell culture
HUVECs were isolated using collagenase and cultivated on rat tail collagen-coated dishes (5 mg/mL) with endothelial basal medium (PromoCell, Heidelberg, Germany), containing 1 μg/mL hydrocortisone, 10 ng/mL epidermal growth factor (EGF), 10 ng/mL basic fibroblast growth factor (bFGF), and 5% (vol/vol) fetal calf serum (FCS). Cells from approximately 25 donors were used at passage 2 to 4. Cells were transferred to medium containing serum only for 24 hours before the experiments, and the stimulations were all performed in serum-free medium. Mononuclear cells were isolated from whole blood anticoagulated by EDTA, by gradient centrifugation method using Biocoll Separating Solution (Biochrom, Gründau, Germany), cultivated in RPMI with 10% (vol/vol) FCS. After overnight culture, the plates were washed to remove nonadherent cells and used for experiments. Staining with CD14 and Diffquick (Bio-Rad Laboratories, Hercules, CA) showed that more than 90% of the cells were monocytes.
Western blot analysis
Cells were lysed in sodium dodecyl sulfate (SDS) containing buffer and separation of polypeptides was performed by SDS–polyacrylamide gel electrophoresis (SDS-PAGE) using 10% acrylamide gel under nonreducing conditions. Electrotransfer of separated protein from the gel onto the blotting polyvinylidene fluoride (PVDF) membrane (Hybond-P PVDF Membrane; GE Healthcare) was carried out in blotting buffer. Labeling of the transferred protein by primary antibody for 16 hours at 4°C in 5% (wt/vol) skim milk in 0.1% (vol/vol) TBS–Tween 20. Secondary horseradish peroxidase (HRP)–conjugated antibody against primary antibody for 1 hour at room temperature. Detection of the HRP signal was performed by Amersham ECL Plus Western Blotting Detection Reagents (GE Healthcare). mAb VIC7 was used to detect TF, and mAbs 24FM and 3E2 were used to detect TM (kindly provided by Stago, Paris, France).
Replications and statistical analysis
All in vitro experiments were repeated al least 3 times, and similar results were obtained. Statistical significance was analyzed by analysis of variance (ANOVA) followed by various after tests as described in the figure legends. P less than .05 was considered significant.
Results
Effect of poly I:C on the expression and activity of TF and TM in EC
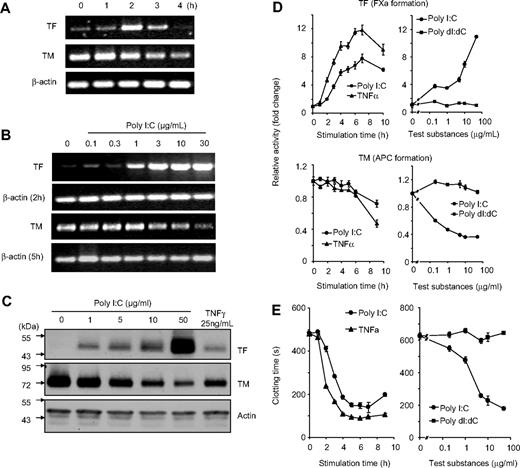
The effect of poly I:C on the regulation of cellular hemostasis factors such as TF, TM, and clotting time was investigated on EC. Stimulation of EC with poly I:C induced a dose- and time-dependent increase in TF expression and down-regulation of TM at the mRNA level as determined by RT-PCR (Figure 1A,B). Poly I:C also increased the expression of IFN-β, IL-1β, TNF-α, and IL-6 mRNA. Under these assay conditions, there were no changes in the mRNA levels for other hemostasis-related genes such as TFPI, tPA, uPA, eNOS, and PAI-1 and VWF (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Western blot analysis indicated that there were concomitant changes in the expression of TF and TM at the protein level (Figure 1C). On a functional level, TF activity was measured by an enzymatic assay through the generation of factor Xa, and TM activity was determined by measuring the generation of activated protein C (APC). TF activity was increased and TM activity was decreased by poly I:C in a dose- and time-dependent manner (Figure 1D). Finally, poly I:C stimulation led to a decrease in plasma clotting time in a dose- and time-dependent manner (Figure 1E). The maximal effect of poly I:C was obtained between 10 to 100 μg/mL and was similar to TNF-α stimulation, whereas the deoxyribose analog, poly dI:dC, had no effect (Figure 1D,E and data not shown). The effects of poly I:C on TF activity, TM activity, and clotting time were observed maximally between 5 to 16 hours (Figure 1). The effect of poly I:C on TF activity and the procoagulant activity of EC was blocked with a TF antibody (Figure S2).
Effect of poly I:C on TF/TM mRNA, protein, and activity level in EC as a function of time and concentration. (A) EC were stimulated for the indicated times with 10 μg/mL poly I:C and mRNA levels for TF, TM, and β-actin were determined by RT-PCR. The size of the 3 PCR products was 427, 208, and 170 bp, respectively, and 34, 30, and 29 cycles were used. (B) EC were stimulated with various concentrations of poly I:C, and mRNA levels for TF (after 2 hours), TM (5 hours), and β-actin (2 and 5 hours) were determined by RT-PCR. (C) EC were stimulated with various concentrations of poly I:C or 25 ng/mL TNF-α for 16 hours, and the protein levels for TF, TM, and actin were determined by Western blot analysis using the respective antibodies. (D) Poly I:C (10 μg/mL) or 25 ng/mL TNF-α were used to stimulate EC for the indicated times, and the activities of TF and TM were determined using chromogenic substrate assays for FXa generation and APC generation, respectively. Various concentrations of poly I:C or poly dI:dC were used to stimulate EC for 16 hours, and the activities of TF and TM were determined. Results are presented as relative enzyme activity; mean plus SEM of triplicate wells. (E) Poly I:C (10 μg/mL) or 25 ng/mL TNF-α were used to stimulate EC for the indicated times, and the clotting time was determined. Various concentrations of poly I:C or poly dI:dC were used to stimulate EC for 16 hours, and then plasma clotting time was measured (in seconds). Results are expressed as mean plus SEM of triplicate wells.
Effect of poly I:C on TF/TM mRNA, protein, and activity level in EC as a function of time and concentration. (A) EC were stimulated for the indicated times with 10 μg/mL poly I:C and mRNA levels for TF, TM, and β-actin were determined by RT-PCR. The size of the 3 PCR products was 427, 208, and 170 bp, respectively, and 34, 30, and 29 cycles were used. (B) EC were stimulated with various concentrations of poly I:C, and mRNA levels for TF (after 2 hours), TM (5 hours), and β-actin (2 and 5 hours) were determined by RT-PCR. (C) EC were stimulated with various concentrations of poly I:C or 25 ng/mL TNF-α for 16 hours, and the protein levels for TF, TM, and actin were determined by Western blot analysis using the respective antibodies. (D) Poly I:C (10 μg/mL) or 25 ng/mL TNF-α were used to stimulate EC for the indicated times, and the activities of TF and TM were determined using chromogenic substrate assays for FXa generation and APC generation, respectively. Various concentrations of poly I:C or poly dI:dC were used to stimulate EC for 16 hours, and the activities of TF and TM were determined. Results are presented as relative enzyme activity; mean plus SEM of triplicate wells. (E) Poly I:C (10 μg/mL) or 25 ng/mL TNF-α were used to stimulate EC for the indicated times, and the clotting time was determined. Various concentrations of poly I:C or poly dI:dC were used to stimulate EC for 16 hours, and then plasma clotting time was measured (in seconds). Results are expressed as mean plus SEM of triplicate wells.
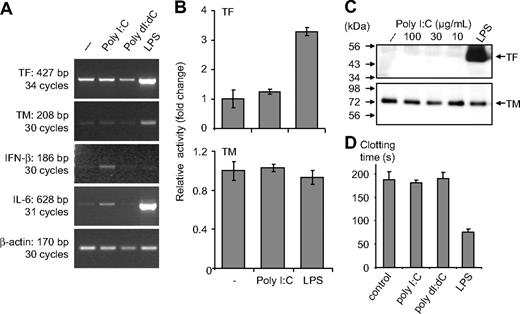
In monocytes, poly I:C-mediated expression of IL-6 and IFN-β mRNA was evident, but there were no changes in TF or TM mRNA levels (Figure 2A). No modulation of these proteins or their activity was observed after stimulation with poly I:C, whereas LPS induced a strong up-regulation of TF at the protein and the activity level (Figure 2B,C). Similarly, no effect of poly I:C was observed on TF activity when it was added to cells as a complex with liposomes to facilitate its entry into the cells (Figure S3). In monocytes, poly I:C did not influence the plasma clotting times, but a strong procoagulant effect of LPS was observed (Figure 2D). Vascular smooth muscle cells (VSMCs) constitutively express TF and TM, and no modulation of these proteins was observed after their stimulation with poly I:C (Figure S4). Hence, poly I:C can induce TF and repress TM expression on EC in a cell-specific manner and alters the hemostatic balance in favor of a prothrombotic state.
Effect of poly I:C on clotting time, TF as well as TM at the activity, protein, and mRNA level in monocytes. (A) Poly I:C or poly dI:dC (50 μg/mL) and 100 ng/mL LPS were used to stimulate monocytes for 2 hours, and then mRNA was isolated for RT-PCR analysis. The expression levels of TF, TM, IFN-β, IL-6 and β-actin were determined by RT-PCR. (B) Monocytes were stimulated with 50 μg/mL poly I:C and 100 ng/mL LPS for 5 hours, and thereafter TF and TM activity was measured. Results are presented as relative activity; mean plus SEM of triplicate wells. (C) Monocytes were stimulated with various concentrations of 10 to 100 μg/mL poly I:C or 100 ng/mL LPS for 5 hours, and the protein levels for TF and TM were determined by Western blot analysis using the respective antibodies. (D) Nucleic acids (10 μg/mL) were used to stimulate monocytes for 5 hours, and then cell extracts were prepared, and plasma clotting time was determined. LPS (100 ng/mL) was used as a standard activator. Results are presented as mean plus SEM of triplicate wells as clotting time (in seconds).
Effect of poly I:C on clotting time, TF as well as TM at the activity, protein, and mRNA level in monocytes. (A) Poly I:C or poly dI:dC (50 μg/mL) and 100 ng/mL LPS were used to stimulate monocytes for 2 hours, and then mRNA was isolated for RT-PCR analysis. The expression levels of TF, TM, IFN-β, IL-6 and β-actin were determined by RT-PCR. (B) Monocytes were stimulated with 50 μg/mL poly I:C and 100 ng/mL LPS for 5 hours, and thereafter TF and TM activity was measured. Results are presented as relative activity; mean plus SEM of triplicate wells. (C) Monocytes were stimulated with various concentrations of 10 to 100 μg/mL poly I:C or 100 ng/mL LPS for 5 hours, and the protein levels for TF and TM were determined by Western blot analysis using the respective antibodies. (D) Nucleic acids (10 μg/mL) were used to stimulate monocytes for 5 hours, and then cell extracts were prepared, and plasma clotting time was determined. LPS (100 ng/mL) was used as a standard activator. Results are presented as mean plus SEM of triplicate wells as clotting time (in seconds).
The mechanism of poly I:C–mediated alteration of the TF/TM balance in EC
One of the changes in EC after poly I:C stimulation is the up-regulation of cytokines such as TNF-α and IL-1.46 To determine whether these cytokines are indirectly responsible for the changes in EC, blocking antibodies to IL-1 and TNF-α were used. These antibodies reduced the effect of the exogenously added respective cytokine, but did not influence the change in TF activity induced by poly I:C (Figure S5).
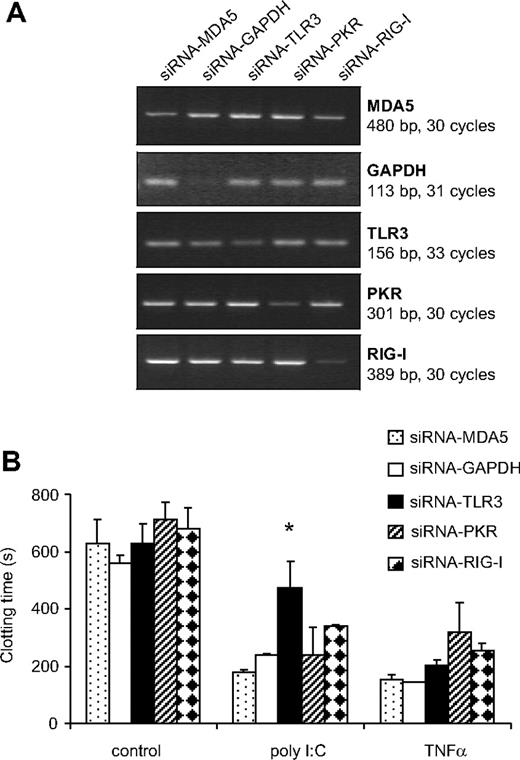
TLR3, PKR, RIG-I, as well as MDA5 mRNA expression were observed in EC, monocytes, as well as VSMCs (data not shown). To investigate the role of these PRR in mediating the effect of poly I:C, their expression was reduced using synthetic siRNA. siRNA-RIG-I reduced the expression of its respective mRNA by 93 + 3%, siRNA-PKR by 72 + 4, siRNA-MDA5 by 50 + 13, siRNA-TLR3 by 40 + 8, and siRNA-GAPDH by 68 + 6% in 3 consecutive experiments (Figure 3A). The poly I:C-mediated decrease in clotting time was reversed significantly in cells treated with TLR3-siRNA, but not with siRNA against PKR, MDA5, RIGI, or GAPDH (Figure 3B). As a control, TLR3-siRNA did not influence the decrease in clotting time mediated by TNF-α. These results indicate that TLR3 is specifically responsible for the effect of poly I:C on EC.
Effect of different siRNA to PRR on the induction of TF expression by poly I:C. (A) EC were pretreated with siRNA against TLR3, PKR, RIG-I, MDA5, as well as control siRNA against GAPDH, and 48 hours later, the amount of the respective mRNA was quantified by RT-PCR. (B) After a similar transfection with siRNA, cells were stimulated with 10 μg/mL poly I:C and 25 ng/mL TNF-α for 5 hours, and the plasma clotting time was determined. *P < .05 compared with other siRNAs in the same group using ANOVA followed by Dunnett posttest.
Effect of different siRNA to PRR on the induction of TF expression by poly I:C. (A) EC were pretreated with siRNA against TLR3, PKR, RIG-I, MDA5, as well as control siRNA against GAPDH, and 48 hours later, the amount of the respective mRNA was quantified by RT-PCR. (B) After a similar transfection with siRNA, cells were stimulated with 10 μg/mL poly I:C and 25 ng/mL TNF-α for 5 hours, and the plasma clotting time was determined. *P < .05 compared with other siRNAs in the same group using ANOVA followed by Dunnett posttest.
The role of TLR3 in mediating the effect of poly I:C on hemostasis in vivo
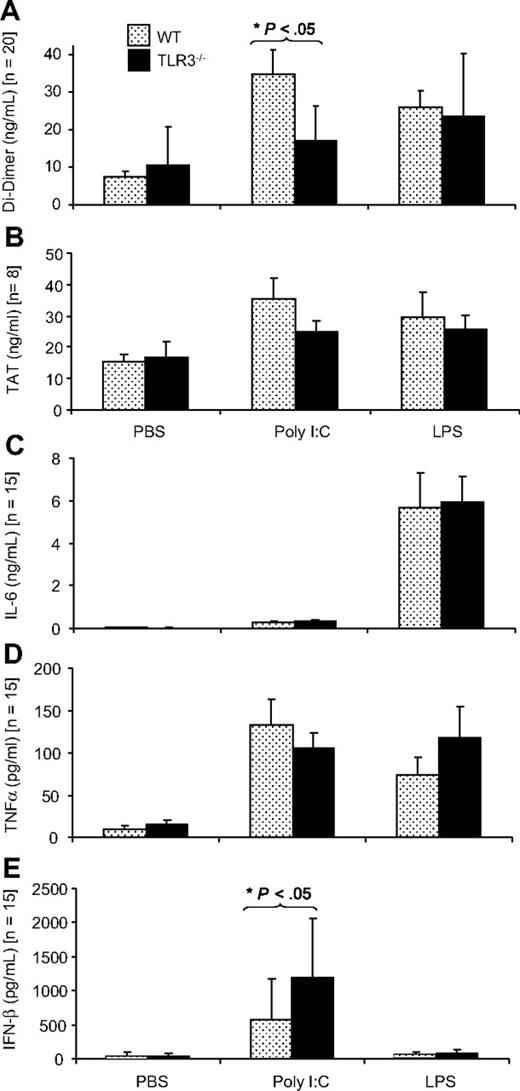
The dose of poly I:C for in vivo application was selected on the basis of previous in vivo studies that showed that poly I:C could up-regulate the expression of antiviral cytokines42 as well as influence vascular permeability.47 The effect of application of poly I:C in vivo on hemostasis-related parameters was investigated in WT mice and TLR3−/− mice. The TLR4 ligand, LPS also induces a procoagulant state in EC in vivo, and this was used as a positive control.48 In WT mice, poly I:C application led to a 4.5-fold increase in D-dimer levels indicating enhanced coagulation and fibrinolysis, whereby this increase was smaller (1.6-fold) in TLR3−/− mice, confirming an important role for TLR3 in this response (Figure 4A). Poly I:C increased TAT values, an index of the extent of coagulation, 2.3-fold in WT mice but only 1.4-fold in TLR3−/− mice, but these differences were not significant (Figure 4B). Platelet counts were decreased after poly I:C treatment, but the change was not significant. Erythrocytes were unchanged and leukocyte counts were significantly decreased in the poly I:C group, as described previously,49 but there was no difference between WT and TLR3−/− mice (Figure S6). Poly I:C–induced expression of IL-6 and TNF-α (Figure 4C,D) was similar in WT and TLR3−/− mice indicating that this was independent of TLR3. Poly I:C–mediated IFN-β induction was significantly higher in TLR3−/− mice compared with WT mice (Figure 4E).
Comparison of the effect of poly I:C and LPS on plasma D-dimer, TAT, IL-6, TNF-α, and IFN-β in WT and TLR3−/− mice in vivo. WT or TLR3−/− mice were given 250 μg poly I:C or 50 μg LPS or the equivalent volume of PBS (IV), and after 12 hours, plasma was collected, and the levels of D-dimer (A), TAT (B), IL-6 (C), TNF-α (D), and IFN-β (E) were determined by enzyme-linked immunosorbent assay (ELISA). There were 8 to 20 mice in each group, and the results are presented as mean plus SEM. Statistical significance was determined using 2-way ANOVA followed by Bonferroni posttest. *P < .05.
Comparison of the effect of poly I:C and LPS on plasma D-dimer, TAT, IL-6, TNF-α, and IFN-β in WT and TLR3−/− mice in vivo. WT or TLR3−/− mice were given 250 μg poly I:C or 50 μg LPS or the equivalent volume of PBS (IV), and after 12 hours, plasma was collected, and the levels of D-dimer (A), TAT (B), IL-6 (C), TNF-α (D), and IFN-β (E) were determined by enzyme-linked immunosorbent assay (ELISA). There were 8 to 20 mice in each group, and the results are presented as mean plus SEM. Statistical significance was determined using 2-way ANOVA followed by Bonferroni posttest. *P < .05.
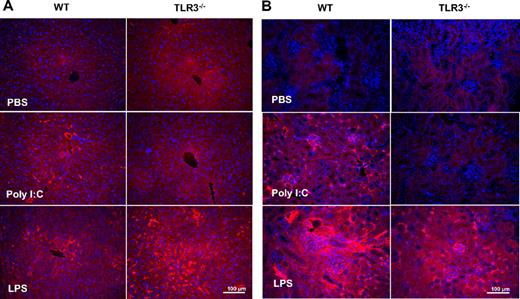
Fibrin deposition in liver and kidney was observed after poly I:C treatment in WT but not in TLR3−/− mice (Figure 5A,B). There was no change in circulating fibrinogen levels (data not shown). The effect of LPS on circulating D-dimer, IL-6, and TNF-α, as well as fibrin deposition was identical in WT and TLR3−/− mice.
Comparison of the effect of poly I:C and LPS on fibrin(ogen) deposition in WT and TLR3−/−mice in vivo. Mice were treated with poly I:C, LPS, or PBS, and the liver (A) and kidneys (B) were removed for fibrin(ogen) analysis. Sections were stained with an anti–fibrin(ogen) rabbit polyclonal antibody, and the visualization was done with rhodamine-coupled secondary antibody. From each treatment group, 3 mice were analyzed. Slides were counterstained with DAPI to show the nuclei.
Comparison of the effect of poly I:C and LPS on fibrin(ogen) deposition in WT and TLR3−/−mice in vivo. Mice were treated with poly I:C, LPS, or PBS, and the liver (A) and kidneys (B) were removed for fibrin(ogen) analysis. Sections were stained with an anti–fibrin(ogen) rabbit polyclonal antibody, and the visualization was done with rhodamine-coupled secondary antibody. From each treatment group, 3 mice were analyzed. Slides were counterstained with DAPI to show the nuclei.
The effect of poly I:C on TF/TM expression in EC in vivo was determined by enface immunofluorescence microscopy. In WT mice, poly I:C up-regulated TF on aortic EC (Figure 6A). The effect of poly I:C was comparable with that of LPS (Figure 6A) that is a known modulator of TF on EC in vivo.48 TM immunostaining was reduced after LPS or poly I:C treatment (Figure 6B). In TLR3−/− mice, these changes in TF and TM were not observed with poly I:C, but the response to LPS was identical in both strains of mice. As a control, BS-1 lectin was used as a marker for EC in each treatment group in both strains of mice, and no differences in the staining were observed. Hence, the in vitro observations on the alteration of TF/TM balance on EC via TLR3 were corroborated by in vivo data.
Comparison of the effect of poly I:C and LPS on TF and TM expression in aortic endothelium of WT and TLR3−/−mice in vivo. Mice were treated with poly I:C, LPS, or PBS, and the aortas were removed and processed for enface microscopy. Aortic endothelium from 4 to 6 mice per treatment group was analyzed for TF and TM expression by immunfluorescence analysis using rhodamine-coupled secondary antibody. As a control, each section was also stained with the endothelial marker, BS-1, that was directly labeled with FITC.
Comparison of the effect of poly I:C and LPS on TF and TM expression in aortic endothelium of WT and TLR3−/−mice in vivo. Mice were treated with poly I:C, LPS, or PBS, and the aortas were removed and processed for enface microscopy. Aortic endothelium from 4 to 6 mice per treatment group was analyzed for TF and TM expression by immunfluorescence analysis using rhodamine-coupled secondary antibody. As a control, each section was also stained with the endothelial marker, BS-1, that was directly labeled with FITC.
Discussion
Many viral infections, particularly those causing viral hemorrhagic fever, profoundly influence the hemostasis system in favor of a procoagulant state. In evolutionary terms, the coagulation process is part of the innate immune response that leads to local restriction and trapping of the infectious agent and protects the rest of the organism.50,51 Viruses that influence the hemostasis system such as Ebola virus,11,15,52 dengue fever virus,53 nipah virus,54 herpes simplex virus,55 or classical swine fever virus56 can infect EC. The resulting induction of TF expression on EC has been attributed to either viral glycoproteins, the generally toxic insult to the cells by viruses, or via the secondary release of cytokines and other mediators.1-4 In this report we propose a new pathomechanism based on the direct activation of TLR3 that can induce the procoagulant state in EC. The procoagulant activity was due to the induction of TF and repression of TM at the mRNA, protein, and activity levels. Another consequence of the increased FXa generation and the decrease in APC is that these changes are strongly proinflammatory. Indeed, it has been shown that the inhibition of TF/ factor VIIa is effective in reducing the hemostasis-related as well as inflammation-related pathogenicity of Ebola virus infection in a primate model.20
It is known that poly I:C increases the expression of cytokines46 and adhesion molecules41 on EC illustrating its proinflammatory nature. Poly I:C application in vivo also influences body temperature, locomotor activity, and blood pressure.40 We found that poly I:C increased circulating D-dimer levels indicating that both coagulation and fibrinolysis were stimulated. Fibrin deposition in kidney and liver was also elevated, again confirming the endogenous activation of coagulation. Moreover, poly I:C also induced endothelial TF and repressed TM expression in vivo thereby causing endothelial dysfunction. The effects of poly I:C were comparable with those of LPS that induces a strong disseminated intravascular coagulation (DIC)–like response via TLR4. In the TLR3−/− mice, the poly I:C–mediated changes were diminished, indicating that this was the major responsible receptor, whereas the response to LPS was not influenced. It should be noted that the TLR3−/− mice, an incipient congenic strain backcrossed with C57BL6 mice 6 times, were compared with WT-C57BL6 mice, and a small mismatch in the genetic background could influence the responses investigated.
Many of the potential nucleic acid PRR, such as TLR3, RIG-I, MDA5, and PKR, are expressed by EC. siRNA-mediated knockdown of these receptors indicated that TLR3 was the predominant receptor responsible for mediating the effects of poly I:C on EC. Whereas knockdown of RIG-I was complete, some expression of the other genes was noted after siRNA application. From the 8 different siRNA against TLR3 tested, the maximal inhibition obtained did not exceed 40% (n = 5 experiments). Use of other siRNA strategies and a more accurate mRNA quantification with quantitative PCR may provide better results. Despite the low knockdown of TLR3 expression, there was a significant reversal of the effect of poly I:C that was not observed for the unrelated agonist, TNF-α. Previous studies showed that TLR3 also mediates permeability changes in EC in response to poly I:C,47 but the cytokine response to poly I:C stimulation in immune cells is likely to be mediated by MDA532,42 and not TLR3. Monocytes and VSMCs also express TLR3 and the other potential nucleic acid receptors, but there was no effect of poly I:C on the procoagulant balance in these cells. We also observed that mouse lung-derived endothelial cells did not exhibit higher TF expression after stimulation with poly I:C, indicating that there may also be species-specific differences as described for human and mouse dendritic cells in their response to poly I:C.46 Whether this is an in vitro artifact and how this observation is related to the responses in vivo needs to be further investigated. In a similar manner, it was recently demonstrated that human dendritic cells and macrophages secreted the chemokine inducible protein-10 (IP-10) but not IL-6 and TNF-α in response to poly I:C, whereas EC showed a strong induction of IL-6 and TNF-α.46 This cell specificity may relate to a specific TLR3-related signal transduction process in HUVECs that is not common to the other cells. Thus, TLR3 is the major receptor for poly I:C in HUVECs, as has been demonstrated for other cell types.32,36,40-43 It is known that TLR3 expression in HUVECs is on the cell surface as well as internal cellular compartments.46 Naked poly I:C also binds to cells and is internalized via scavenger receptors and is also likely to have an intracellular mode of action.57
TF expression on EC is only observed in conditions of stimulation with strong proinflammatory factors such as TNF-α and IL-6.58 Poly I:C can induce the production of these and other cytokines that in turn may up-regulate TF expression on EC.46 In the in vitro experiments, a direct blockade of endogenously produced TNF-α and IL-1 did not modify the poly I:C response. In the in vivo experiments, there was a small induction in IL-6 and a robust increase in TNF-α, and these changes were identical in WT as well as TLR3−/− mice. Because the changes in the coagulation-related parameters in these mice are quite different, it is unlikely that the effect is indirectly mediated via cytokine induction. Furthermore, this result clearly illustrates that the hemostasis response to poly I:C is dependent on TLR3, but the cytokine response is independent of it.
The extension of these findings to a situation of a virus infection needs further considerations (eg, the permissiveness of a particular vascular bed–specific EC for the virus infection).4,9 Superimposed on this is the ability of viruses to inhibit the host immune response.59,60 The structural determinants of viral nucleic acids that activate the innate immune system are currently under intense investigation, and there is evidence for sequence-specific effects, double-stranded character, or nucleotide modifications.31-35 It remains to be established which viruses directly produce dsRNA in EC and activate TLR3. An activation of host cells by viral nucleic acids through a simultaneous recognition by various pattern recognition receptors is likely. Viruses that cause hemorrhagic fever belong to the group of negative strand RNA viruses that seem to produce very little dsRNA.36 Furthermore, although monocytes did not respond to poly I:C it should be noted that they are infected by viruses in the course of VHF and express TF and produce cytokines that propagate the VHF syndrome.1
The shift to a hypercoagulation state in certain virus infections could be due to a procoagulant cytokine status and the direct stimulation of TF and repression of TM via TLR3. This not only influences cellular hemostasis but is also likely to promote a proinflammatory state via stimulation of PARs. These observations could be relevant for the understanding of altered hemostasis associated with viral infections.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Baerbel Fuehler and Susanne Tannert-Otto for their excellent assistance. We also thank Dr Elke Mühlberger (University of Marburg) for reading the manuscript and her critical comments.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Bonn, Germany) to S.M.K. (SFB 547/ C14) and from the University of Giessen (Anschubfinanzierung) to A.S.
Authorship
Contribution: A.S. performed most of the in vitro and in vivo experiments with the help of T.S.W., B.F., and S.T.O., analyzed and interpreted the data, and edited the manuscript; K.H. did all the immunofluorescence analysis; D.S. and J.M.D. performed the dissection of aortas for enface microscopy; S.B., T.K., and K.T.P. provided reagents and edited the manuscript; and S.M.K. planned the experiments, analyzed and interpreted the data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sandip M. Kanse, Institute for Biochemistry, Justus-Liebig-University Giessen, Friedrichstrasse 24, 35392 Giessen, Germany; e-mail: sandip.kanse@biochemie.med.uni-giessen.de.