Abstract
Tissue factor (TF) initiates coagulation, regulates hemostasis, and plays a critical role in mediating arterial thrombosis. TF is up-regulated in vascular smooth muscle cells (VSMCs) in atherosclerosis and arterial injury. To examine the biologic role of VSMC-derived TF, we crossed TFflox/flox mice with SM22αCre+/− mice. TF mRNA and activity were decreased in the aortic media of TF-deficient mice by 96% and 94.8%, respectively. There were no differences in TF activity measured in plasma or concentrated microparticles. TF-deficient mice were generated with the expected frequency, showed no evidence of bleeding or increased mortality, and had similar activated partial thromboplastin and tail vein bleeding times. Thrombus-mediated flow reduction in response to ferric chloride injury of the carotid arteries was significantly attenuated in VSMC-specific TF-deficient. Stable occlusion was seen in 11 of 12 wild-type mice, but in only 6 of 16 VSMC-specific TF-deficient mice (P = .001). These data suggest that VSMC-derived TF is critical in a macrovascular model of arterial thrombosis. This mouse model should be valuable in determining the contribution of VSMC-derived TF in other TF-mediated phenomena, such as restenosis.
Introduction
Tissue factor (TF) is a glycoprotein that initiates coagulation, regulates hemostasis, and plays a critical role in mediating arterial thrombosis.1,2 TF is expressed at low levels in normal intima and media, is rapidly induced after arterial injury in medial vascular smooth muscle cells (VSMCs), and subsequently accumulates in the VSMCs of the resulting neointima.3 TF is also abundant in atherosclerotic plaques, particularly in macrophages, intimal VSMCs, and the lipid-rich necrotic core.4 It has generally been thought that arterial thrombosis occurs when TF induced by injury to the arterial surface or exposed by plaque rupture comes into contact with blood coagulant factors.
Recent studies have demonstrated that TF is present in the circulation,5-8 mostly in microparticles (MPs). Although the cellular sources of circulating TF have not been precisely defined, current data suggest that platelets,9-12 monocyte/macrophages,13,14 and endothelial cells15-17 are important contributors. Although some studies have suggested that circulating TF contributes to thrombus generation,18,19 others have suggested that the levels of TF in human blood are too low to be clinically important.20,21 Various studies have found increased levels of circulating TF in patients with acute coronary syndromes,5 diffuse intravascular coagulopathy,8 endotoxemia,13 and cancer.7 However, a recent study nested in the European Prospective Investigation into Cancer and Nutrition found that high levels of circulating TF were not independently predictive of cardiovascular events in apparently healthy individuals.22
Studies using animal models of thrombosis have attempted to address the role of circulating TF. Total TF deletion in mice results in intrauterine lethality.23-25 However, TF null embryos can be rescued with a minigene containing the human TF promoter and cDNA. These mice (referred to as low TF mice) express 1% of the TF activity of wild-type mice26 and have decreased thrombosis in response to carotid arterial injury.27 Transplanting bone marrow from low TF mice into wild-type mice decreased the size of thrombus induced by laser injury to cremaster arterioles, compared with that in mice receiving wild-type marrow,19 suggesting an important role for bone marrow–derived circulating TF in thrombus formation. In contrast, a similar bone marrow transplantation strategy, using Rose Bengal injury of the carotid artery, suggested that thrombosis of large arteries was not dependent on bone marrow–derived TF and, therefore, was presumably arterial wall dependent.27
To provide further insights into the role of arterial wall and circulating TF in mediating arterial thrombosis and to examine specifically the role of VSMCs in this process, we generated VSMC-specific TF-deficient mice. These mice had a greater than 95% decrease in aortic medial TF mRNA and activity, but had similar levels of circulating TF activity as control littermates. VSMC-specific TF deficiency was associated with marked impairment in thrombosis after ferric chloride (FeCl3) injury of the carotid artery. These data suggest that VSMC-derived medial TF is critical for thrombosis in large arteries.
Methods
Generation of VSMC-specific TF-deficient mice
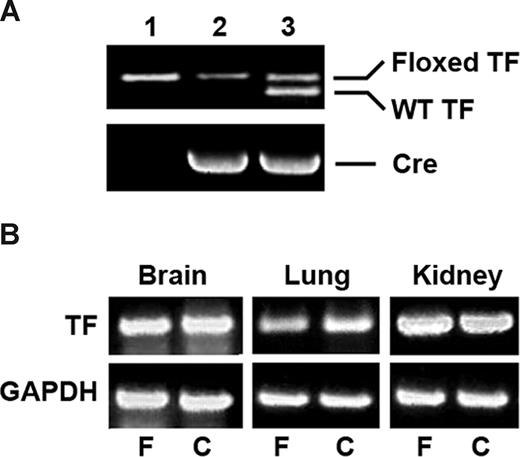
The generation of TFflox/flox mice is described in a separate manuscript.28 Mice were housed in the vivarium at the University of Rochester. Procedures and animal care were approved by the IACUC at the University of Rochester and were in accordance with the “Guide for the Care and Use of Laboratory Animals.” Transgenic mice expressing Cre recombinase under the control of mouse SM22α promoter29 were kindly provided by Dr Joseph Miano (University of Rochester). TFflox/flox mice were crossed with SM22αCre+/− mice to generate TFflox/flox/SM22αCre+/− mice (VSMC-specific TF-deficient mice). Mice were genotyped by polymerase chain reaction (PCR) using primers spanning the mouse TF promoter from nt −1097 to −539 (forward primer 5′- ATGAGGAGCTGTGTTAAAGGGTCGCAGAA-3′ and reverse primer 5′-TGCAGTAAATGCACGTGTCTGCCAT-3′) to determine the presence of the 5′ loxP site (Figure 1A). Mice were also genotyped for the Cre recombinase.29 For all studies, control TFflox/flox mice were derived from TFflox/flox/SM22αCre+/− littermates. The mice were on the C57Bl/6 background.
Analysis of VSMC-specific TF-deficient mice. (A) Genomic DNA was isolated from the tails of TFflox/flox mice (lane 1), TFflox/flox/SM22αCre+/− mice (lane 2); and TFflox/+/SM22αCre+/− mice (lane 3). PCR was performed with primers that identify the wild-type and floxed TF alleles and the Cre recombinase. (B) mRNA was isolated from brain, lung, and kidney of TFflox/flox (F) and TFflox/flox/SM22αCre+/− (C) mice and analyzed by RT-PCR for TF. GAPDH was used as an internal control for equal loading.
Analysis of VSMC-specific TF-deficient mice. (A) Genomic DNA was isolated from the tails of TFflox/flox mice (lane 1), TFflox/flox/SM22αCre+/− mice (lane 2); and TFflox/+/SM22αCre+/− mice (lane 3). PCR was performed with primers that identify the wild-type and floxed TF alleles and the Cre recombinase. (B) mRNA was isolated from brain, lung, and kidney of TFflox/flox (F) and TFflox/flox/SM22αCre+/− (C) mice and analyzed by RT-PCR for TF. GAPDH was used as an internal control for equal loading.
Thrombosis model
FeCl3 injury of the carotid artery was performed as described.30 Mice, of both sexes, 12 to 16 weeks of age, were placed on a surface preheated to 37°C and then anesthetized with 2% halothane and maintained under anesthesia with 1.5% halothane. Body temperature was monitored with a rectal probe and maintained at 37°C (±1°C). The left carotid artery was dissected. A transonic flow meter (Model 0.5VB; Transonic Systems, Ithaca, NY) was placed into the space created by the dissection and the carotid artery placed in the probe. The probe was adjusted to assure baseline flow of at least 1 mL/second, measured for at least 1 minute. Seven percent FeCl3 was applied to filter paper (1 × 1 mm), and the filter paper placed under and above the carotid artery. After 3 minutes, the filter paper was removed, the carotid artery was washed with phosphate-buffered saline (PBS), and the flow was measured. Four data points were collected per second, and the time to achieve a stable occlusion was calculated. An occlusion was determined to be stable when the flow was reduced by at least 75% of baseline for at least 3 minutes, and where the flow during that 3-minute period did not change by more than 0.16% of baseline per second.
Tail vein bleeding time
Tail vein bleeding time was performed as described.31 A transverse incision was made over the tail at a position where the diameter of the tail is 2.25 mm to 2.5 mm. The tail was incised enough to lacerate the tail vein, and immersed in saline (37°C) in a hand-held tube, which was gently rotated to prevent the shed blood from obscuring the incision site. Bleeding time was recorded as the time at which bleeding stopped. Mice were also observed for 6 hours and examined on the next morning for signs of delayed bleeding.
Tissue sampling and RNA extraction
Mice were killed, and the aortas perfused with 5 mL PBS from the left ventricle. Aortas were dissected from the aortic arch to the abdominal aorta and digested with 175 U/mL collagenase type II for 10 minutes at 37°C. After digestion, the endothelium was scraped off, and the adventitia was stripped with forceps. The resulting medial and adventitial preps were frozen immediately in liquid nitrogen and then stored at −80°C. Heart, kidney, lung, and brain were also frozen and stored as above. Total RNA was extracted using an RNeasy Micro kit (QIAGEN, Valencia, CA) according to the manufacturer's protocol.
Reverse transcription–PCR
Reverse transcription (RT)–PCR was performed using the cMaster RTplus PCR system (Eppendorf, Westbury, NY) according to the manufacturer's protocol. The 20-μL reaction mixture contained 200 ng of total RNA, 200 nM forward primer, and 200 nM reverse primer. The primers span mouse TF cDNA (NM_010171) from nt 406 to 945 (forward primer 5′- CGAGACACAAACCTTGGACAGC -3′ and reverse primer 5′- CGCTTCAGCCTTTCCTCTATGC -3′). The mixture was heated to 52°C for 30 minutes and denatured at 94°C for 2 minutes. This was followed by 36 cycles consisting of 30 seconds at 94°C, 30 seconds at 56°C, and 44 seconds at 72°C and 1 cycle of final extension at 72°C. Equal loading was determined using mouse GAPDH primers. RT-PCR products were examined on 1% agarose gels.
Real-time RT-PCR
First-strand cDNA was synthesized from 200 ng of total RNA extracted from aortic media using the Superscript first-strand synthesis system (Invitrogen, Carlsbad, CA). The reaction mixture was 10 μL and contained 2 μL of cDNA, 900 nM forward primer, 900 nM reverse primer, 1X ABI Taqman Master Mix, and DNAse-free water. PCR conditions were 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C, 60 seconds at 60°C. The mouse TF primers spanned nucleotides 301 to 800 of the full-length cDNA (NM_010171). Equal loading was determined using mouse GAPDH primers (Applied Biosystems, Foster City, CA).
Measurement of TF activity
TF activity was measured using a 2-stage chromogenic assay as described.3 Tissues were isolated as described above. Before assay, samples were ground and sonicated in 15 mM n-Octyl-B-D-glucopyranoside lysis buffer at 20 kHz for 20 seconds. Aliquots (media, 10 μg of protein; adventitia, 1 μg; heart, 200 μg) were transferred to a 96-well plate. Forty microliters of coagulation human factor VIIa and human factor X were added sequentially to a final concentration of 1 nM and 150 nM, respectively. Forty-microliter aliquots were then taken at 4, 8, and 12 minutes and placed in a well containing 100 μL bicine buffer, pH 8.5, 1 g/L bovine serum albumin (BSA), and 25 mM ethylenediaminetetraacetic acid (EDTA). Twenty-five microliters of 2 mM Spectrozyme factor Xa was added, and change in absorption at 405 nm at 37°C was measured in an enzyme-linked immunosorbent assay (ELISA) plate reader. TF activity was expressed as pM Xa/min/mg of protein.
Measurement of plasma TF activity
Blood (500 μL) was collected from the inferior vena cava (IVC) with a plastic syringe and disposable needles (26G[1/2]). Blood was immediately drawn out into tubes and mixed with 0.1 volume of 3.8% sodium citrate. The samples were centrifuged at 1500g for 25 minutes and then at 15 000g for 2 minutes at room temperature. The resulting platelet-poor plasma (PPP) was frozen and stored at −80°C until assays were performed. Twenty microliters PPP was incubated in 96-well microtiter plates with 60 μL Tris-buffered saline (TBS) containing 1 mg/mL BSA and corn trypsin inhibitor (final concentration 80 μg/mL) for 10 minutes at room temperature. TF activity was then measured using the 2-stage chromogenic assay.
Measurement of circulating microparticle TF activity
At the time of kill, approximately 500 μL blood was collected via retro-orbital eye bleeding using micro-hematocrit tubes with heparin and mixed with 0.1 volume of 3.8% sodium citrate. PPP was prepared as above, and microparticles were generated from the total sample as described.27 PPP was diluted 1:3 with 20 mM HEPES, 1 mM EDTA, pH 7.2, and ultracentrifuged at 200 000g for 1.5 hours. The pelleted microparticles were resuspended in [1/2] initial volume of 10 mM HEPES, 136 mM NaCl, pH 7.4. Three 100-μg aliquots of the microparticle suspension were analyzed for TF activity by 2-stage chromogenic assay.
Activated partial thromboplastin time
Activated partial thromboplastin time (aPTT) was assessed using COAG-A-MATE XM following the manufacturer's protocol (Organon Teknika, Durham, NC). One hundred microliters aPTT reagent containing rabbit brain phospholipids and micronized silica was added to 100 μL plasma. The mixture was incubated at 37°C for 5 minutes. One hundred microliters of 0.025 M CaCl2 (37°C) was added to activate the reaction.
Immunohistochemistry
Mouse aortas and hearts were harvested and fixed in 10% neutral formalin, processed, and embedded in paraffin as previously described.3 Sections were cut at 5 μm, and then stained in hematoxylin and eosin. For evaluation of fibrosis, cross-sections (10/animal) were made through the heart at a level midway through the left ventricle and stained with Masson trichrome (Biological Stain Commission, Rochester, NY and J. T. Baker, Phillipsburg NJ). Hemosiderin was detected by staining with Prussian blue (Perls Ferric Iron; J. T. Baker).32 To stain arterial thrombi, the carotid arteries were excised, gently perfused ex vivo with PBS, frozen immediately in liquid nitrogen, and then stored at −80°C. Frozen sections were cut at 5 μm; slides were fixed in cold acetone (for CD41 and fibrin) or formalin (for TF) and quenched in 3% hydrogen peroxide. Sections were stained for platelets (anti–mouse CD41; no. 553847; BD Pharmingen, Franklin Lakes, NJ), fibrin (anti–human fibrin fragment E; no. CL20065A; Cedarlane, Burlington, NC) and TF (anti-sTF;33 ). The anti–human fibrin antibody has previously been shown to identify mouse fibrin.34 Slides were blocked using normal rabbit serum, and the primary antibody applied for 1 hour at room temperature. Immunodetection was performed using biotinylated rabbit anti–rat mouse adsorbed (for CD41), biotinylated rabbit anti–sheep (for fibrin), or biotinylated goat anti–rabbit secondaries, peroxidase-labeled streptavidin, and visualized using Nova Red stubstrate (Vector Laboratories, Burlingame, CA). Slides were counterstained with hematoxylin and coverslipped using Cytoseal 60 (Richard Allan Scientific, Kalamazoo, MI). Negative controls included omitting the primary antibody and substituting the primary antibody with an irrelevant antibody of the same isotype.
All tissues were imaged using an Olympus (Central Valley, PA) BX-41 microscope. Images were captured using an 18.2 Color Mosaic camera and Spot Software Version 4.6 from Diagnostic Instruments (Sterling Heights, MI). Images were edited on a Macintosh G4 Powerbook computer (Apple, Cupertino, CA) using Photoshop 9.0 software (Adobe Systems, San Jose, CA).
Statistics
All results are reported as mean plus or minus SEM unless noted otherwise. Comparison of circulating TF activity in 3 groups was analyzed using 1-way analysis of variance. Two-sample t tests were used to analyze differences in TF mRNA and TF activity of aortic media, plasma TF levels, aPTT, tail vein bleeding time, and carotid artery blood flow. Time to stable occlusion after FeCl3 injury was plotted using a Kaplan-Meier curve and subjected to log-rank analysis using Prism 4 software (GraphPad, San Diego, CA).
Results
TFflox/flox mice were crossed with SM22αCre+/− mice to generate TFflox/flox/SM22αCre+/− mice (Figure 1A). The litter sizes were normal, with an average of 8 per litter. TFflox/flox/SM22αCre+/− mice were generated at a frequency of 51%, close to the expected frequency of 50%. TFflox/flox/SM22αCre+/− mice appeared grossly normal and exhibited normal weight gain. No unexplained deaths were observed over a period of 12 months. By RT-PCR, there were no differences in TF expression in the brain, lung, or kidney (Figure 1B). However, as discussed below, there were profound decreases in TF expression in arterial media and in the myocardium.
TFflox/flox/SM22αCre+/− mice have markedly reduced VSMC TF mRNA and activity
TF mRNA was substantially reduced in the aortic media of TFflox/flox/SM22αCre+/− mice (Figure 2A). By real time RT-PCR (Figure 2B), this reduction was 96% (TFflox/flox mice: ΔCt = 8.70 ± 0.65, n = 3; TFflox/flox/SM22αCre+/− mice: ΔCt = 14.11 ± 1.53, n = 5; P < .001; ΔCt = CtTF - CtGAPDH; This corresponds to normalized TF mRNA levels of 1 ± 0.223 and 0.04 ± 0.026 for TFflox/flox and TFflox/flox/SM22αCre+/− mice, respectively; P = .025). In addition, TF activity (Figure 2C) in the aortic media of TFflox/flox/SM22αCre+/− mice was reduced by 94.8% (TFflox/flox mice: 994.1 ± 387.3 pM Xa/min/mg of protein, n = 4; TFflox/flox/SM22αCre+/− mice: 51.8 ± 15.6, n = 6; P = .017). These data indicate that TF was efficiently deleted from the arterial VSMC in TFflox/flox/SM22αCre+/− mice. Histologic examination demonstrated no abnormalities of the carotid arteries, coronary arteries, or aortas (data not shown).
Analysis of TF mRNA and enzymatic activity in mouse aortas. RNA was harvested from the aortic media of TFflox/flox mice (Flox/flox; n = 3) and VSMC-specific TF-deficient mice (Flox/flox/Cre; n = 5) and analyzed for TF mRNA by (A) RT-PCR and (B) real time RT-PCR. For real time RT-PCR, TF mRNA levels in Flox/flox/Cre mice are expressed relative to those of Flox/flox mice, defined arbitrarily as 1.0. (C) TF activity was measured in extracts of aortic media of Flox/floxCre (n = 6) and Flox/flox (n = 4) mice by 2-stage colorimetric Xa generation assay and is expressed as pM factor Xa/min. *P < .05.
Analysis of TF mRNA and enzymatic activity in mouse aortas. RNA was harvested from the aortic media of TFflox/flox mice (Flox/flox; n = 3) and VSMC-specific TF-deficient mice (Flox/flox/Cre; n = 5) and analyzed for TF mRNA by (A) RT-PCR and (B) real time RT-PCR. For real time RT-PCR, TF mRNA levels in Flox/flox/Cre mice are expressed relative to those of Flox/flox mice, defined arbitrarily as 1.0. (C) TF activity was measured in extracts of aortic media of Flox/floxCre (n = 6) and Flox/flox (n = 4) mice by 2-stage colorimetric Xa generation assay and is expressed as pM factor Xa/min. *P < .05.
TFflox/flox/SM22αCre+/− mice exhibit early cardiac fibrosis in association with markedly reduced cardiac TF mRNA and activity
The Sm22α promoter used in this study has also been shown to be expressed in the heart and in some adventitial fibroblasts.29 Like in the aorta, TF mRNA (TFflox/flox mice: ΔCt = 7.07 ± 0.17, n = 3; TFflox/flox/SM22αCre+/− mice: ΔCt = 10.21 ± 0.42, n = 3; P = .002, corresponding to an 88.6% reduction in mRNA) and activity (TFflox/flox mice: 382.8 ± 22.8 pM Xa/min/mg of protein, n = 4; TFflox/flox/SM22αCre+/− mice: 7.3 ± 1.8, n = 9; P < .001) were markedly reduced in the heart. Although these animals appeared normal and in particular did not display signs of heart failure, histologic examination revealed evidence of cardiac fibrosis similar to that previously described for low-TF mice35 (Figure 3B,D,E). This was associated with interstitial bleeding, as evidenced by the presence of red blood cells (Figure 3F) and hemosiderin (Figure 3E,H). Cardiac fibrosis was seen in 12 of 13 TFflox/flox/SM22αCre+/− mice examined, and began as early as 10 weeks (TFflox/flox/SM22αCre+/− mice: 2/3 at 10 weeks, 6 of 6 at 7 months, and 4 of 4 at 1 year; TFflox/flox mice: 0 of 3 at 10 weeks, 0 of 3 at 7 months, and 1 of 4 at 1 year).
Evaluation of cardiac fibrosis and bleeding in TF-deficient mice. (A-F) Masson trichrome staining of 10-week hearts from TFflox/flox (A,C) and TFflox/flox/SM22αCre+/− (B,D-F) mice. (A,B) Low-power (1.25×/0.04 PlanAPO) cross sections taken midway through the left ventricle. (C,D) Higher-power (40×/0.30 UPlanFL) sections from the same hearts. (E,F) Further magnifications (40×/0.30 UPlanFL) from panel B showing hemosiderin (arrows) and active hemorrhage (red blood cells). Fibrosis appears as blue. (G,H) Prussian blue (Perls Ferric Iron) staining (40×/0.30 UPlanFL) of TFflox/flox (G) and TFflox/flox/SM22αCre+/− (H) mice.
Evaluation of cardiac fibrosis and bleeding in TF-deficient mice. (A-F) Masson trichrome staining of 10-week hearts from TFflox/flox (A,C) and TFflox/flox/SM22αCre+/− (B,D-F) mice. (A,B) Low-power (1.25×/0.04 PlanAPO) cross sections taken midway through the left ventricle. (C,D) Higher-power (40×/0.30 UPlanFL) sections from the same hearts. (E,F) Further magnifications (40×/0.30 UPlanFL) from panel B showing hemosiderin (arrows) and active hemorrhage (red blood cells). Fibrosis appears as blue. (G,H) Prussian blue (Perls Ferric Iron) staining (40×/0.30 UPlanFL) of TFflox/flox (G) and TFflox/flox/SM22αCre+/− (H) mice.
TF activity was also reduced in aortic adventitia (TFflox/flox mice: 283.2 ± 46.3 nM Xa/min/mg of protein, n = 5; TFflox/flox/SM22αCre+/− mice: 97.4 ± 52.4, n = 4). However, levels remained several orders of magnitude higher than those measured in wild-type aortic media or in the heart.
VSMC-specific TF-deficient mice have no difference in circulating TF activity
There were no significant differences in TF activity (Figure 4A) in PPP from TFflox/flox mice and TFflox/flox/SM22αCre+/− mice (TFflox/flox mice: 9.52 ± 3.39 pM Xa/min/mg of protein, n = 4; TFflox/flox/SM22αCre+/− mice: 12.13 ± 3.75 pM Xa/min/mg of protein, n = 4; P = .446). To further confirm this, TF activity was measured in microparticles generated by high-speed ultracentrifugation of PPP (Figure 4B). No significant differences in microparticle TF activity were noted (C57BL/6 mice: 34.73 ± 10.4 pM Xa/min/mg of protein, n = 5; TFflox/flox mice: 40.23 ± 31.67 pM Xa/min/mg of protein, n = 4; TFflox/flox/SM22αCre+/− mice: 30.7 ± 18.4 pM Xa/min/mg of protein, n = 6; P = .76). These data suggest that neither VSMC- nor cardiac-derived TF is a significant source of circulating TF activity.
Analysis of circulating TF activity. TF enzymatic activity was measured in (A) PPP collected from Flox/flox (n = 4) and Flox/flox/Cre (n = 4) mice; and (B) circulating MPs isolated from PPP of C57BL/6 (n = 5), Flox/flox (n = 4), and Flox/flox/Cre (n = 6) mice. Blood was collected at the time of kill in animals not subjected to FeCl3 injury. TF activity was measured by 2-stage colorimetric Xa generation assay and is expressed as pM factor Xa/min. P = NS for all groups.
Analysis of circulating TF activity. TF enzymatic activity was measured in (A) PPP collected from Flox/flox (n = 4) and Flox/flox/Cre (n = 4) mice; and (B) circulating MPs isolated from PPP of C57BL/6 (n = 5), Flox/flox (n = 4), and Flox/flox/Cre (n = 6) mice. Blood was collected at the time of kill in animals not subjected to FeCl3 injury. TF activity was measured by 2-stage colorimetric Xa generation assay and is expressed as pM factor Xa/min. P = NS for all groups.
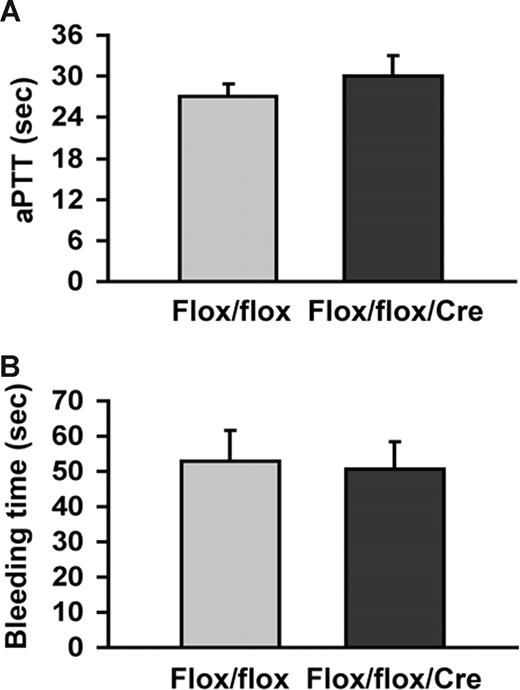
As shown in Figure 5A, there was no significant difference in aPTT (TFflox/flox mice: 27.1 ± 3.1 seconds, n = 3; TFflox/flox/SM22αCre+/− mice: 30.1 ± 5.8 seconds, n = 4; P = .418). In addition, TFflox/flox and TFflox/flox/SM22αCre+/− mice had similar bleeding times (Figure 5B: TFflox/flox mice: 52.8 ± 17.4 seconds, n = 5; TFflox/flox/SM22αCre+/− mice: 50.7 ± 18.8 seconds, n = 6; P = .86).
Analysis of aPTT and tail vein bleeding time. (A) aPTT was measured in Flox/flox (n = 3) and Flox/flox/Cre (n = 4) mice from blood collected at the time of kill in animals not subjected to FeCl3 injury. (B) Tail vein bleeding times were performed in Flox/flox (n = 5) and Flox/flox/Cre mice (n = 5). P = NS for all groups.
Analysis of aPTT and tail vein bleeding time. (A) aPTT was measured in Flox/flox (n = 3) and Flox/flox/Cre (n = 4) mice from blood collected at the time of kill in animals not subjected to FeCl3 injury. (B) Tail vein bleeding times were performed in Flox/flox (n = 5) and Flox/flox/Cre mice (n = 5). P = NS for all groups.
Arterial thrombosis is markedly impaired in VSMC-specific TF-deficient mice
We used FeCl3 injury of the carotid artery to examine the role of VSMC-specific TF in mediating arterial thrombosis. As shown in Figure 6A, in TFflox/flox mice, FeCl3 injury resulted in a marked decrease in flow, attributable to the development of mural thrombus.36 Flow reduction was significantly attenuated (P < .05 for all times from 4 to 15 minutes) in the TFflox/flox/SM22αCre+/− mice (Figure 6B). A stable occlusion (defined as a decrease in flow of ≥ 75% from baseline for ≥3 minutes, and where the flow during that 3-minute period did not change by more than 0.16% of baseline per second) was seen in 11 of 12 TFflox/flox mice. In contrast, only 6 of 16 TFflox/flox/SM22αCre+/− mice developed a stable occlusion (P = .001 by log rank analysis; hazard ratio for occlusion = 0.2262). Flow reduction was similar in male (n = 6 TFflox/flox/SM22αCre+/−; n = 7 TFflox/flox) and female (n = 10 TFflox/flox/SM22αCre+/−; n = 5 TFflox/flox) mice. For those animals that did occlude, the mean time of occlusion was 318 seconds for TFflox/flox mice and 432 seconds for TFflox/flox/SM22αCre+/− mice.
Analysis of carotid arterial thrombosis in mice. Representative flow tracings recorded in response to FeCl3 injuryto carotid arteries of (A) Flox/flox and (B) Flox/flox/Cre mice. Time of stable occlusion is shown by an arrow. (C) Graphic representation of flow (mL/min) as a function of time for Flox/flox and Flox/floxCre mice. P < .05 for all times from 4 minutes to 15 minutes. (D) Time to stable occlusion (stable occlusion is defined as patency ≤ 25% for ≥ 3 minutes) is plotted as a Kaplan-Meier Curve for the same mice. By log rank analysis, P = .001. There were no significant differences between males and females in their flow rates or propensity to occlude in either group.
Analysis of carotid arterial thrombosis in mice. Representative flow tracings recorded in response to FeCl3 injuryto carotid arteries of (A) Flox/flox and (B) Flox/flox/Cre mice. Time of stable occlusion is shown by an arrow. (C) Graphic representation of flow (mL/min) as a function of time for Flox/flox and Flox/floxCre mice. P < .05 for all times from 4 minutes to 15 minutes. (D) Time to stable occlusion (stable occlusion is defined as patency ≤ 25% for ≥ 3 minutes) is plotted as a Kaplan-Meier Curve for the same mice. By log rank analysis, P = .001. There were no significant differences between males and females in their flow rates or propensity to occlude in either group.
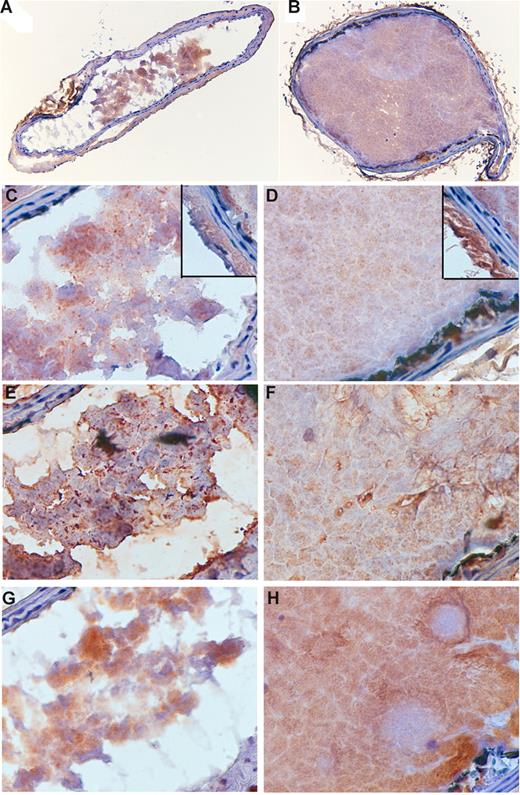
To provide further insight into the difference between thrombi formed in wild-type and SMC-deficient mice, sections were stained for TF, fibrin, and platelets (Figure 7). Although thrombi were smaller and nonocclusive in TFflox/flox/SM22αCre+/− mice, the intensity of staining for all 3 antigens was similar. Of particular note, all thrombi stained diffusely for TF and fibrin.
Immunohistochemical analysis of carotid arterial thrombi. Sections of thrombi produced in Flox/flox/Cre (A,C,E,G) and Flox/flox (B,D,F,H) mice (representative of 3 animals each) were stained for TF (A-D), fibrin (E,F), and platelets (G,H). A,B = 10×/0.30 UPlanFL; C-H = 60×/0.80 Ach. Insets in panels C and D show TF staining of adventitia and media.
Immunohistochemical analysis of carotid arterial thrombi. Sections of thrombi produced in Flox/flox/Cre (A,C,E,G) and Flox/flox (B,D,F,H) mice (representative of 3 animals each) were stained for TF (A-D), fibrin (E,F), and platelets (G,H). A,B = 10×/0.30 UPlanFL; C-H = 60×/0.80 Ach. Insets in panels C and D show TF staining of adventitia and media.
Discussion
Thrombosis is a major cause of morbidity and mortality. Studies using inhibitors of TF activity, including antibodies to TF,37,38 active site-inactivated factor VIIa,39 and TF pathway inhibitor (TFPI)40,41 have provided evidence that TF plays a critical role in mediating arterial thrombosis. However, the relative contributions of arterial wall and circulating TF to thrombosis remain to be determined. We now report that mice deficient in VSMC-derived TF have markedly reduced arterial thrombosis after FeCl3 injury.
The functional significance of blood-borne TF has been controversial. Giesen and colleagues18 demonstrated that thrombi staining heavily for TF form on “naive” pig aortic media or collagen-coated slides perfused ex vivo with human blood. The addition of active site-inhibited factor VIIa substantially reduced thrombus mass and abolished fibrin formation, suggesting that circulating TF mediates thrombus formation. Experiments by Mann and colleagues,20,21 however, suggested that the level of TF activity in blood from healthy people is too low to trigger coagulation.
By intravital microscopy, Furie and colleagues42 demonstrated that circulating TF accumulates in the developing thrombus and contributes to fibrin formation in a microvascular thrombosis model involving laser injury of cremaster arterioles. Transplanting bone marrow from low-TF mice into wild-type mice markedly reduced thrombus size, suggesting that bone marrow–derived TF contributes to thrombus propagation.19 In contrast, Fay and colleagues27 found that transplanting low-TF bone marrow into wild-type mice did not reduce large arterial thrombosis induced in mouse carotid arteries by Rose Bengal, suggesting that sources of TF other than the bone marrow (eg, the arterial wall) are more important in mediating thrombosis.
The present study also used a large arterial model of injury and found that deleting TF from arterial VSMCs was sufficient to markedly reduce thrombosis, directly implicating arterial wall TF as the major contributor to thrombosis in this animal model of large arterial injury. Importantly, no significant differences were found in circulating TF activity, whether measured directly from plasma or from concentrated microparticles. This result indicates that circulating TF is not sufficient to produce stable thrombi, but does not rule out the possibility that it contributes to thrombus propagation. In fact, thrombi from TFflox/flox/SM22αCre+/− mice, although smaller and nonocclusive, stained equally well for TF and fibrin, supporting the idea that circulating TF may be involved in thrombus propagation. This is consistent with a recent report showing that TF is present in pathologic thrombi but not in hemostatic plugs.43 These data also suggest that VSMC-derived microparticles are not an important source of circulating TF. This is consistent with recent data showing that VSMCs are not a source of TF-positive microparticles in human blood.44
Deletion of the TF gene in mice results in embryonic lethality between embryonic days 9.5 and 11.5 due to hemorrhage and abnormal development of yolk sac vasculature.23-25 Despite a reduction in VSMC TF activity by approximately 95%, VSMC-specific TF-deficient mice were born from normal litters with the expected frequency and appeared grossly normal. These mice had no abnormalities in tail vein bleeding time, and no unexpected deaths were observed during more than 12 months of observation. This suggests that VSMC-derived TF is not required for maintaining hemostasis during development.
As noted above, SM22αCre has been previously shown to be expressed in cardiac myocytes29 and, thus, the TFflox/flox/SM22αCre+/− mice also had a marked decrease in cardiac TF (≈2% of the procoagulant activity of TFflox/flox littermates). These animals showed evidence of intracardiac hemorrhage and cardiac fibrosis as early as 10 weeks of age. This is similar to our findings with the low TF mice, which had a global 99% reduction in TF activity, substantial cardiac fibrosis, and evidence of hemorrhage.35 Whereas the low-TF mice had a shortened life span and early deaths, the TFflox/flox/SM22αCre+/− mice had no unexpected deaths and appeared to thrive as late as 1 year of age. The current study is in contrast to a recent report of using the cardiac myosin light chain 2v promoter to generate a cardiac-specific TF deletion.28 These mice, which had a 69% reduction in TF activity did not have evidence of cardiac fibrosis at 6 months. Three of 12 mice displayed mild levels of hemosiderin and fibrosis at 9 months, and more substantial fibrosis could be evinced by treating with isoproterenol. Put into context with the 2 earlier studies, the current study would support the concept that the role of TF in maintaining normal cardiac structure is concentration dependent and, whereas approximately 30% TF activity is sufficient to prevent intracardiac hemorrhage under physiologic conditions, 1% to 5% activity is below the necessary threshold. A second possibility, however, is that VSMC TF activity, either alone or in concert with cardiac TF, is critical to prevent intracardiac bleeding. In that regard, the low-TF and TFflox/flox/SM22αCre+/− mice had profound deficiencies in VSMC TF activity, whereas the cardiac-specific TF deletion did not. Additional mice may be necessary to distinguish between these 2 possibilities.
This study suggests that although VSMC-derived TF plays no obvious role in hemostasis, it plays an important role in arterial thrombosis. In general, immunohistochemical studies have shown minimal TF staining in the medial wall or in medial VSMCs of normal arteries (reviewed in Taubman et al45 ). In contrast, TF activity can be measured in normal arterial media, as demonstrated in this report, and significant levels are exposed on the normal arterial surface by gentle endothelial denudation.3 This discrepancy, in part, reflects the extremely low catalytic constant for the TF:VIIa interaction, making it far more sensitive than typical antibody:antigen interactions. This study suggests that the relatively small amount of VSMC-derived TF present in normal arterial media is sufficient to generate occlusive thrombi when exposed to the circulation.
The FeCl3 model causes significant injury to the endothelium and to the arterial media and would be expected to expose medial TF to the circulation.36 In this regard, the model is similar to the Rose Bengal model described above in which low TF mice had a marked decrease in thrombosis, and arterial wall TF appeared to be critical to thrombus formation.19 In contrast to FeCl3 or Rose Bengal, the laser injury model used by Furie and coworkers produces a small injury that largely leaves the endothelial surface largely intact and does not expose medial VSMC to the circulation. Not surprisingly, circulating TF contributes to thrombosis initiated by this laser injury model.27 The exposure to the circulation of medial TF by FeCl3 or Rose Bengal may mimic the exposure of arterial TF caused by endovascular injury, such as angioplasty or stent insertion, or by plaque rupture. It is important to note, however, that the present study was performed on intrinsically normal arteries, which express low levels of TF, whereas human atherosclerotic arteries contain macrophages and activated endothelial cells, rich sources of TF. In addition, the FeCl3 injury model is artificial, and it remains to be determined to what extent it is representative of human arterial thrombosis. Therefore, one cannot draw specific conclusions about the role of SMC-derived TF in mediating thrombogenesis associated with plaque rupture or in response to interventions in atherosclerotic arteries.
Adventitial fibroblasts and medial SMC share many properties and some cells may share similar origins. It is thus not surprising that the TFflox/flox/SM22αCre+/− mice displayed a significant reduction in adventitial TF activity, although not nearly as pronounced as that seen in the media. It is unlikely that this reduction contributed to the findings in this report. For one, levels of TF activity in normal adventitia are extremely high and remained extremely high in the TFflox/flox/SM22αCre+/− mice (>2 orders of magnitude) relative to those seen in normal media. Such levels are likely to produce maximum activation of the coagulation cascade and were sufficient to allow for normal hemostasis after tail vein injury. In addition, the media remains intact and the adventitia is not exposed to the circulation after FeCl3 injury.
In arterial injury models, TF is markedly induced in medial VSMCs and subsequently accumulates in the VSMCs of the developing intima (reviewed in Taubman et al45 ). TF is also abundant in the intimal and medial VSMCs of atherosclerotic plaques (reviewed in Taubman et al45 ). Studies using inhibitors of TF activity or TF antibodies have provided evidence that TF plays a key role in mediating intimal hyperplasia and luminal narrowing in response to arterial injury (reviewed in Taubman et al45 ). The current study suggests that VSMC-derived TF plays a key role in mediating arterial thrombosis. The VSMC-TF deficient mouse should be valuable in determining the role of VSMC-derived TF in mediating other pathologic processes, such as intimal hyperplasia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by a grant from the National Heart, Lung, and Blood Institute, R01 HL73364 (M.B.T.).
National Institutes of Health
Authorship
Contribution: L.W. wrote the paper and performed experiments; C.M. performed experiments; R.F.S. wrote the paper and performed data analysis; M.R. performed experiments; N.M. provided key reagents and helped in study design; and M.B.T. wrote the paper, performed data analysis, and helped in study design.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark B. Taubman, MD, 601 Elmwood Avenue, Box MED, Rochester, NY 14642; e-mail: mark_taubman@urmc.rochester.edu.