Abstract
Acquired factor XIII (FXIII) deficiency due to autoantibody against FXIII is a very rare severe hemorrhagic diathesis. Antibodies directed against the A subunit of FXIII, which interfere with different functions of FXIII, have been described. Here, for the first time, we report an autoantibody against the B subunit of FXIII (FXIII-B) that caused life-threatening bleeding in a patient with systemic lupus erythematosus. FXIII activity, FXIII-A2B2 complex, and individual FXIII subunits were undetectable in the plasma, whereas platelet FXIII activity and antigen were normal. Neither FXIII activation nor its activity was inhibited by the antibody, which bound to structural epitope(s) on both free and complexed FXIII-B. The autoantibody highly accelerated the elimination of FXIII from the circulation. FXIII supplementation combined with immunosuppressive therapy, plasmapheresis, immunoglobulin, and anti-CD20 treatment resulted in the patient's recovery. FXIII levels returned to around 20% at discharge and after gradual increase the levels stabilized above 50%.
Introduction
Blood coagulation factor XIII (FXIII) is a protransglutaminase of tetrameric structure (FXIII-A2B2).1,2 Its potentially active A subunit (FXIII-A) is synthesized in cells of bone marrow origin; it is also present in platelets and monocytes/macrophages in dimeric form (FXIII-A2). The noncatalytic B subunit (FXIII-B) is in excess and it is essential for the stabilization of FXIII-A2 in plasmatic conditions. FXIII is converted into an active transglutaminase (FXIIIa) by limited proteolysis of FXIII-A and by Ca2+-induced dissociation of FXIII-B. Cross-linking of fibrin α- and γ-chains and α2-plasmin inhibitor to fibrin by FXIIIa stabilizes fibrin and protects it from prompt elimination by plasmin.3 Inherited FXIII-A deficiency is a severe bleeding diathesis with the high risk of intracranial bleeding in nonsupplemented patients.4 Only 5 cases of inherited FXIII-B deficiency with mild-to-moderate bleeding tendency have been reported.5-8 In the absence of FXIII-B, plasma FXIII activity and FXIII-A concentration were considerably decreased, whereas in platelets a normal amount of FXIII-A was measured.9 Thirty-six cases of severe FXIII deficiency due to an autoantibody against FXIII-A have been reported. In a few cases, the autoantibody was characterized and classified into subgroups according to its inhibition of FXIII activation, FXIIIa activity, or binding to fibrin.10-15 In about one third of the cases the autoantibody was associated with systemic lupus erythematosus (SLE). No report on an autoantibody directed against FXIII-B has been published so far.
Methods
A 28-year-old woman suffering from SLE with end-stage kidney disease was on hemodialysis. For preparation of cubital fistula she was admitted to a county hospital. In the proximity of the surgical wound, hematomas developed that were explored. The wounds showed no tendency of healing and remained open. A few days later extensive intramuscular hematoma appeared on the right thigh, then also on the left. There was no personal or family history of bleeding tendency and the usual screening tests for hemorrhagic diathesis were negative. The patient became increasingly anemic (hemoglobin < 50 g/L) and was transferred to our University Clinic. The study protocol was approved by the Ethics Committee of the University of Debrecen. Informed consent was obtained from the patient in accordance with the Declaration of Helsinki.
FXIII activity of plasma and platelet lysate was determined by UV kinetic method16 using REA-chrom FXIII assay kit (Reanal-ker, Budapest, Hungary). For activity measurement, washed platelet suspension was solubilized in 1% Triton X-100. FXIII-A2B2 complex antigen in plasma17 and FXIII-A antigen in plasma and platelets18 were measured by sandwich enzyme-linked immunosorbent assay (ELISA) (R-ELISA FXIII and R-ELISA FXIII-A; Reanal-ker). In platelets FXIII-A was also detected by Western blotting. Plasma FXIII-B antigen was determined by a sandwich ELISA using 2 monoclonal antibodies against different FXIII-B epitopes. The biotinylated19 capture antibody, diluted plasma, and the peroxidase-labeled detection antibody were mixed and incubated for 1 hour in streptavidin-coated microtiter plate. After extensive washing the peroxidase activity was determined.
To detect if a neutralizing inhibitor was present, the following experiments were carried out: (1) The patient plasma was incubated with equal volume of normal plasma at 37°C for 1 hour, and FXIII activity was measured. (2) The patient's plasma supplemented with various concentrations of purified plasma FXIII20 was incubated for 1 hour at 37°C and the recovery of added FXIII activity was determined. (3) Nonactivated or thrombin (20 U/mL) plus CaCl2 (10 mM)–activated plasma FXIII (21 μg/mL) was incubated for 1 hour at 37°C with 10 mg/mL patient's or normal IgG prepared on HiTrap Protein G HP affinity column (Amersham, Uppsala, Sweden); then FXIII activity was measured.
The binding of patient's IgG to purified FXIII-A2B2,20 FXIII-A2,21 and FXIII-B22 was investigated by ELISA and Western blotting. For ELISA, 10 μg/mL FXIII or a FXIII subunit was coated to a microtiter plate. After blocking, coated antigens were incubated for 2 hours with various concentrations of biotinylated IgG prepared from the patient's or normal plasma. The reaction was developed by avidin-biotinylated peroxidase complex (Vector, Burlingame, CA). For Western blotting, FXIII-A2 or FXIII-B was subjected to SDS polyacrylamide gel electrophoresis (PAGE) in reducing and nonreducing conditions. The electroblot was incubated with the patient's IgG (0.1 mg/mL). The immune reaction was developed by biotinylated goat anti–human IgG and avidin-biotinylated peroxidase complex.
Results and discussion
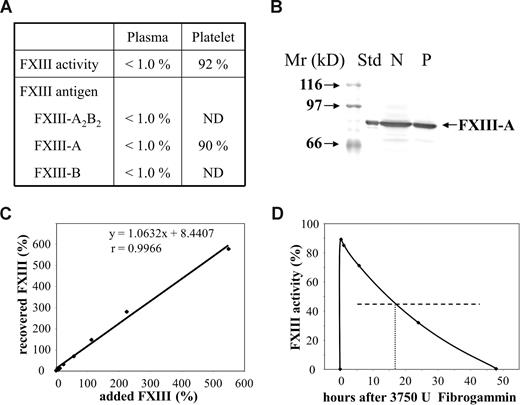
The concentration of coagulation factors of intrinsic and extrinsic pathway, α2-plasmin inhibitor, von Willebrand factor antigen, and ristocetin cofactor were in the reference interval. Plasma FXIII activity and plasma concentration of complex FXIII-A2B2 and FXIII subunit antigens were below the limit of detection, whereas FXIII activity and FXIII-A antigen in platelets were normal (Figure 1A,B). This finding unequivocally indicated severe FXIII deficiency, however the pattern of different FXIII parameters was unique. Normal FXIII activity and antigen in platelets can also be detected in inherited FXIII-B deficiency or when an autoantibody against FXIII-A is present. As opposed to our case, in FXIII-B deficiency FXIII activity and FXIII-A antigen are considerably decreased, but well detectable in the plasma. In the case of anti–FXIII-A autoantibody FXIII-B antigen in the plasma is approximately 50%. We excluded the presence of an autoantibody that would interfere with the activation of FXIII or the activity of FXIIIa in a series of experiments. A 1:1 mixture of patient's and normal plasma showed FXIII activities in the range of 40% to 60% in repeated experiments. When the patient's plasma was supplemented with an increasing amount of purified plasma FXIII, full recovery of added FXIII activity was demonstrated (Figure 1C). Finally, the patient's IgG in plasma concentration did not inhibit FXIII activation or FXIIIa activity to any significant extent.
Factor XIII levels in the patient's plasma and platelets, and the fate of factor XIII added to the patient's plasma in vitro and in vivo. (A) Factor XIII activity and antigen levels in the patient's plasma and platelet lysate. Results on plasma FXIII activity and FXIII antigens are expressed as a percentage of normal plasma content. Mean plasma FXIII activity and antigen values of more than 100 healthy individuals were considered as normal plasma FXIII activity and antigen concentrations.16,17 Platelet FXIII activity and FXIII-A antigen are expressed as a percentage of mean FXIII activity and FXIII-A antigen measured in platelet lysates from 10 healthy individuals. ND indicates not determined. (B) FXIII-A antigen in the patient's platelets as detected by Western blotting. Platelet protein (50 μg) was subjected to SDS PAGE. FXIII-A was detected by affinity purified sheep anti–FXIII-A antibody (Affinity Biologicals, Ancaster, ON), biotinylated rabbit anti–sheep IgG, and avidin-biotinylated peroxidase complex (Vector). Std indicates FXIII-A2 purified from human platelets; N, lysate of normal platelets; and P, lysate of the patient's platelets. (C) The recovery of various concentrations of purified plasma FXIII incubated with the patient's plasma in vitro. The amounts of added and recovered FXIII are expressed as a percentage of average normal plasma FXIII activity. The equation and the “r” value of the straight line demonstrate full recovery in the whole concentration range. (D) Plasma FXIII activity at various intervals following the administration of 3750 U Fibrogammin-P to the patient. The horizontal broken line represents half-maximal FXIII activity; the vertical dotted line shows the time when half of the added FXIII activity was eliminated from the circulation.
Factor XIII levels in the patient's plasma and platelets, and the fate of factor XIII added to the patient's plasma in vitro and in vivo. (A) Factor XIII activity and antigen levels in the patient's plasma and platelet lysate. Results on plasma FXIII activity and FXIII antigens are expressed as a percentage of normal plasma content. Mean plasma FXIII activity and antigen values of more than 100 healthy individuals were considered as normal plasma FXIII activity and antigen concentrations.16,17 Platelet FXIII activity and FXIII-A antigen are expressed as a percentage of mean FXIII activity and FXIII-A antigen measured in platelet lysates from 10 healthy individuals. ND indicates not determined. (B) FXIII-A antigen in the patient's platelets as detected by Western blotting. Platelet protein (50 μg) was subjected to SDS PAGE. FXIII-A was detected by affinity purified sheep anti–FXIII-A antibody (Affinity Biologicals, Ancaster, ON), biotinylated rabbit anti–sheep IgG, and avidin-biotinylated peroxidase complex (Vector). Std indicates FXIII-A2 purified from human platelets; N, lysate of normal platelets; and P, lysate of the patient's platelets. (C) The recovery of various concentrations of purified plasma FXIII incubated with the patient's plasma in vitro. The amounts of added and recovered FXIII are expressed as a percentage of average normal plasma FXIII activity. The equation and the “r” value of the straight line demonstrate full recovery in the whole concentration range. (D) Plasma FXIII activity at various intervals following the administration of 3750 U Fibrogammin-P to the patient. The horizontal broken line represents half-maximal FXIII activity; the vertical dotted line shows the time when half of the added FXIII activity was eliminated from the circulation.
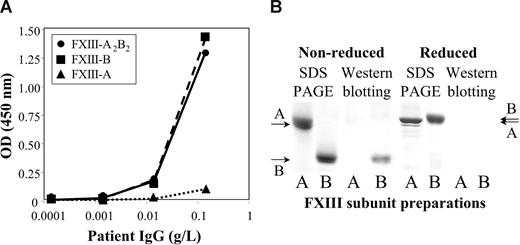
The half-life of therapeutically administered FXIII in the circulation of FXIII-deficient patients is 9 to 12 days.23-25 In our case, the half-life of FXIII was 17 hours (Figure 1D). As we got delayed access to FXIII concentrate (Fibrogammin-P; Dade-Behring, Marburg, Germany), it was first administered on day 8 (ie, when aggressive immunosuppressive therapy was well under way). Thus, the half-life of FXIII at admittance might have been even shorter. The results indicated the presence of nonneutralizing inhibitor, which bound to FXIII and highly accelerated its elimination. Figure 2A demonstrates that the autoantibody bound to both FXIII-A2B2 and FXIII-B. The binding to FXIII-A2 was minimal, and equal to the binding of normal IgG. Similarly, in Western blotting experiments under nonreducing conditions the antibody bound to FXIII-B, but not to FXIII-A (Figure 2B). The binding was lost in reducing condition. FXIII-B consists of 10 Sushi domains, each hold together by 2 disulfide bonds.1,2 As in reducing conditions, disulfide bonds are broken and the 3-dimensional structure of FXIII-B becomes compromised; the result indicates that the antibody is directed against structural epitope(s).
The binding of the patient's IgG to FXIII subunits. (A) The binding of patient's IgG to highly purified FXIII-A2B2 (●), FXIII-A2 (▲), or FXIII-B (■) coated to a microtiter plate. (B) The binding of the patient's IgG to FXIII subunits as detected by Western blotting. A and B under the individual lanes indicate FXIII-A and FXIII-B, respectively. SDS PAGE was carried out both in nonreducing and reducing conditions. The arrows on the right and left sides indicate the position of FXIII-A and FXIII-B. Note the difference in the mobility of reduced and nonreduced FXIII-B. The left 2 lanes show Coomassie blue staining of electropherograms of 2.5 μg FXIII-A2 and FXIII-B. The right lanes demonstrate the detection of 0.5 μg FXIII subunits by Western blotting. Electroblots were incubated with the patient's IgG, and the immune reaction was visualized by biotinylated goat anti–human IgG and avidin-biotinylated peroxidase complex. IgG from the serum of healthy individuals gave no reaction (not shown in the figure).
The binding of the patient's IgG to FXIII subunits. (A) The binding of patient's IgG to highly purified FXIII-A2B2 (●), FXIII-A2 (▲), or FXIII-B (■) coated to a microtiter plate. (B) The binding of the patient's IgG to FXIII subunits as detected by Western blotting. A and B under the individual lanes indicate FXIII-A and FXIII-B, respectively. SDS PAGE was carried out both in nonreducing and reducing conditions. The arrows on the right and left sides indicate the position of FXIII-A and FXIII-B. Note the difference in the mobility of reduced and nonreduced FXIII-B. The left 2 lanes show Coomassie blue staining of electropherograms of 2.5 μg FXIII-A2 and FXIII-B. The right lanes demonstrate the detection of 0.5 μg FXIII subunits by Western blotting. Electroblots were incubated with the patient's IgG, and the immune reaction was visualized by biotinylated goat anti–human IgG and avidin-biotinylated peroxidase complex. IgG from the serum of healthy individuals gave no reaction (not shown in the figure).
The therapeutic regimen included steroid, cyclophosphamide, cyclosporin, plasmapheresis, IgG preparation, anti-CD20, recombinant FVIIa, fresh frozen plasma, and red blood cell transfusion. The patient received 2 more doses of Fibrogammin-P. After day 23, the FXIII level started to rise and stabilized around 20%. She was discharged on day 35 with a hemoglobin value of 98 g/L and with healed cubital wound. Two years later she underwent renal transplantation. Since discharge, she has been exempt of bleeding symptoms with FXIII level between 50% and 83%.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The study was supported by grants OTKA K62087, OTKA-NKTH NI69238 (Hungarian National Research Fund, Budapest, Hungary), MTA 2006TKI227 (Hungarian Academy of Sciences, Budapest, Hungary), and ETT406/2006 (Hungarian Ministry of Health, Budapest, Hungary).
Authorship
Contribution: É.A. and G.H. performed the binding study and were involved in determining the laboratory parameters; Á.S. and Z. Boda collected clinical data and were involved in designing the study; A.K. participated in writing the paper and together with Z. Bereczky and É.K. performed laboratory assays; and L.M. directed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: László Muszbek, Clinical Research Center, University of Debrecen, Medical and Health Science Center, PO Box 40, H-4012 Debrecen, Hungary; e-mail: muszbek@med.unideb.hu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal