To the editor:
Mutations of FoxP3 result in the disturbance of FoxP3 expression and lack of functional CD4+CD25high regulatory T cells in humans, causing immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. The only curative approach for IPEX syndrome, which is fatal within the first year of life in many cases, is allogeneic hematopoietic stem cell transplantation (HSCT). We monitored lineage-specific chimerism in a FoxP3-deficient patient after nonmyeloablative HSCT and graft rejection over 6 years. The patient lost donor chimerism in T cells, B cells, NK cells, monocytes, and granulocytes 1 year after transplantation, but remained clinically healthy without immunosuppression. To elucidate the immunologic basis of his continuing remission, we performed detailed lymphocyte subset analyses and detected FoxP3-expressing CD4+CD25high T cells, comprising 1% to 2% of the CD4+ T cells (∼ 10/μL), which were more than 90% donor-derived. The patient is disease-free and shows no signs of autoimmunity, suggesting that stable, selective engraftment of regulatory T cells is possible and associated with cure from IPEX syndrome.
HSCT may be a curative approach for IPEX syndrome.1,–3 However, the required levels of donor chimerism and conditioning intensity are unknown. Myeloablative conditioning is associated with substantial transplantation-related mortality,2,,–5 whereas nonmyeloablative conditioning carries an increased risk of rejection because of dysregulated effector T-cell function.6
Here we report a boy with IPEX syndrome who underwent peripheral blood HSCT at the age of 11 months from an unrelated 10/10-matched donor (2 × 107/kg CD34+, 109/kg CD3+ cells) after nonmyeloablative conditioning according to the local protocol (fludarabine 6 × 30 mg/m2; melphalan 140 mg/m2; alemtuzumab 5 × 0.2 mg/kg). The post-HSCT course was uncomplicated with complete engraftment on day +12. All IPEX-linked symptoms resolved until day +30. Acute graft-versus-host disease (skin, II°) was treated with steroids until day +56; cyclosporin A was tapered 1 year after transplantation. At this time, in vitro responses to vaccine antigens and phytohemagglutinin were normal. Six years posttransplantation the patient remains insulin-dependent due to irreversible islet cell damage but has no active autoimmunity. The clinically healthy boy shows no increased frequency or severity of infections (Table S1; available on the Blood website; see the Supplemental Materials link at the top of the online article). Chimerism was determined in peripheral blood cell subsets regularly (described elsewhere6 ). Approval was obtained from the St Anna Children's Hospital (Vienna, Austria) Institutional Review Board for these studies. Informed consent was obtained in accordance with the Declaration of Helsinki.
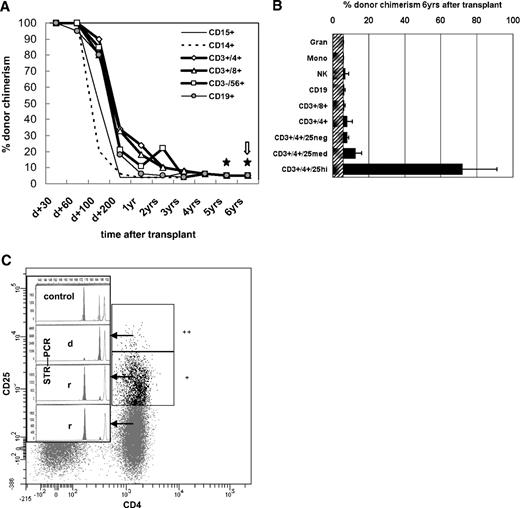
After 100% donor cell engraftment, there was a continuous decline throughout the first year to an overall and subset-specific donor chimerism of 5% to 10% (± 6%; Figure 1A) without any clinical symptoms of relapse. Five-, 5.5-, and 6-year posttransplantation chimerism was determined additionally in the CD4+CD25high, CD4+CD25med, and CD4+CD25neg T-cell subsets (Figure 1B). In the CD4+CD25high subset, comprising 1% to 2% of CD4+ cells (∼ 10 cells/μL), donor chimerism was 53% to 91% (± 6%) at separate occasions within 6 months, whereas all other CD4+ T-cell subsets were of recipient origin (Figure 1C). FoxP3 protein expression of fresh CD4+ patient cells was restricted to the CD25high (predominantly donor-derived) CD127low compartment (Figure S1), in line with the inability of recipient-type cells to express FoxP3 due to a translational start-site mutation. FoxP3mRNA was detectable in full blood and CD4+CD25med and CD4+CD25high cells (not shown; for methods, see Seidel et al7 ).
Lineage- and sublineage-specific chimerism after nonmyeloablative HSCT in IPEX syndrome. Flow cytometric cell sorting of leukocyte lineages and lineage-specific chimerism analyses were performed regularly after hematopoietic stem cell transplantation as described elsewhere.6 Gating strategy: Syto41/CD45RO fluorescein isothiocyanate (FITC), CD3 peridinin chlorophyll protein (PerCP), CD4 allophycocyanin (APC), CD25 phycoerythrin (PE), analyzed and sorted on a FACSAria (Becton Dickinson, Sunnyvale, CA). Acquisition and data analysis was performed with FACSDiva (Becton Dickinson) software. Monoclonal antibodies (mAb) used were CD3 (UCHT1), CD4 (MT310), CD8 (DK25), CD14 (TÜK4), CD15 (C3D1), CD19 (HD37), CD45 (T29/33; all from Dako, Glostrup, Denmark); CD3 (SK7), CD14 (MoP9), CD33 (P67.6), CD38 (HB-7), CD45 (2D1), CD56 (NCAM16.2; all from Becton Dickinson); and CD19 (J4.119) and CD45RA (2H4; both from Coulter, Krefeld, Germany). The purity of the sorted blood cell populations shown in panel A was ≥ 98% as confirmed by rerunning the stained, sorted samples. (A) The proportion of donor cells (shown as percent donor chimerism, y-axis) is shown over time after transplantation (x-axis). ★ indicates 2 of 3 occasions within the 6th year after HSCT that included more in-depth sublineage chimerism analyses as shown in panels B and C.  indicates the occasion when the latest bone marrow aspiration was performed with practically identical chimerism results as shown for simultaneously analyzed peripheral blood except that CD34+ cells, which were not detectable in the periphery, were of 8% to 14% donor origin (not shown). (B) This diagram shows more detailed sublineage chimerism analyses including the CD4+CD25neg, CD4+CD25med, and CD4+CD25high T cells as indicated, performed at the latest 3 visits at 5, 5.5, and 6 years after transplantation (mean + error bars = range). (C) The plot is representative of 2 specimens from 2 independent outpatient visits at 5.5 and 6 years after HSCT, which were analyzed for lineage-specific and subpopulation-specific chimerism by fluorescence-activated cell sorting and short tandem repeat–polymerase chain reaction (STR-PCR). It shows the CD3/CD4-gated fractions of CD25neg/CD25med/CD25high cells with their STR-PCR peaks (insert; r, recipient; d, donor; “control” showing both individual and the common STR-marker peaks). CD4+ T cells comprised 58% of all T cells, CD4+CD25high were approximately 1% of all CD3+CD4+ cells (shown as “++”), CD4+CD25med were 16% of CD3+CD4+ (marked “+”). As indicated visually by the donor peak (“d”) in the insert, CD4+CD25high cells were 91% (± 6%) donor-derived, whereas CD25med were 5% (± 6%), and CD4+CD25neg 3% (± 6%) donor-derived and thus > 89% to 91% recipient (“r”). STR-PCR chimerism results of granulocytes, monocytes, NK and B cells were all < 6% donor-derived (ie, complete recipient chimerism; not shown). Alleles were quantified by capillary electrophoresis and fluorescence-based quantification using the ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
indicates the occasion when the latest bone marrow aspiration was performed with practically identical chimerism results as shown for simultaneously analyzed peripheral blood except that CD34+ cells, which were not detectable in the periphery, were of 8% to 14% donor origin (not shown). (B) This diagram shows more detailed sublineage chimerism analyses including the CD4+CD25neg, CD4+CD25med, and CD4+CD25high T cells as indicated, performed at the latest 3 visits at 5, 5.5, and 6 years after transplantation (mean + error bars = range). (C) The plot is representative of 2 specimens from 2 independent outpatient visits at 5.5 and 6 years after HSCT, which were analyzed for lineage-specific and subpopulation-specific chimerism by fluorescence-activated cell sorting and short tandem repeat–polymerase chain reaction (STR-PCR). It shows the CD3/CD4-gated fractions of CD25neg/CD25med/CD25high cells with their STR-PCR peaks (insert; r, recipient; d, donor; “control” showing both individual and the common STR-marker peaks). CD4+ T cells comprised 58% of all T cells, CD4+CD25high were approximately 1% of all CD3+CD4+ cells (shown as “++”), CD4+CD25med were 16% of CD3+CD4+ (marked “+”). As indicated visually by the donor peak (“d”) in the insert, CD4+CD25high cells were 91% (± 6%) donor-derived, whereas CD25med were 5% (± 6%), and CD4+CD25neg 3% (± 6%) donor-derived and thus > 89% to 91% recipient (“r”). STR-PCR chimerism results of granulocytes, monocytes, NK and B cells were all < 6% donor-derived (ie, complete recipient chimerism; not shown). Alleles were quantified by capillary electrophoresis and fluorescence-based quantification using the ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
Lineage- and sublineage-specific chimerism after nonmyeloablative HSCT in IPEX syndrome. Flow cytometric cell sorting of leukocyte lineages and lineage-specific chimerism analyses were performed regularly after hematopoietic stem cell transplantation as described elsewhere.6 Gating strategy: Syto41/CD45RO fluorescein isothiocyanate (FITC), CD3 peridinin chlorophyll protein (PerCP), CD4 allophycocyanin (APC), CD25 phycoerythrin (PE), analyzed and sorted on a FACSAria (Becton Dickinson, Sunnyvale, CA). Acquisition and data analysis was performed with FACSDiva (Becton Dickinson) software. Monoclonal antibodies (mAb) used were CD3 (UCHT1), CD4 (MT310), CD8 (DK25), CD14 (TÜK4), CD15 (C3D1), CD19 (HD37), CD45 (T29/33; all from Dako, Glostrup, Denmark); CD3 (SK7), CD14 (MoP9), CD33 (P67.6), CD38 (HB-7), CD45 (2D1), CD56 (NCAM16.2; all from Becton Dickinson); and CD19 (J4.119) and CD45RA (2H4; both from Coulter, Krefeld, Germany). The purity of the sorted blood cell populations shown in panel A was ≥ 98% as confirmed by rerunning the stained, sorted samples. (A) The proportion of donor cells (shown as percent donor chimerism, y-axis) is shown over time after transplantation (x-axis). ★ indicates 2 of 3 occasions within the 6th year after HSCT that included more in-depth sublineage chimerism analyses as shown in panels B and C.  indicates the occasion when the latest bone marrow aspiration was performed with practically identical chimerism results as shown for simultaneously analyzed peripheral blood except that CD34+ cells, which were not detectable in the periphery, were of 8% to 14% donor origin (not shown). (B) This diagram shows more detailed sublineage chimerism analyses including the CD4+CD25neg, CD4+CD25med, and CD4+CD25high T cells as indicated, performed at the latest 3 visits at 5, 5.5, and 6 years after transplantation (mean + error bars = range). (C) The plot is representative of 2 specimens from 2 independent outpatient visits at 5.5 and 6 years after HSCT, which were analyzed for lineage-specific and subpopulation-specific chimerism by fluorescence-activated cell sorting and short tandem repeat–polymerase chain reaction (STR-PCR). It shows the CD3/CD4-gated fractions of CD25neg/CD25med/CD25high cells with their STR-PCR peaks (insert; r, recipient; d, donor; “control” showing both individual and the common STR-marker peaks). CD4+ T cells comprised 58% of all T cells, CD4+CD25high were approximately 1% of all CD3+CD4+ cells (shown as “++”), CD4+CD25med were 16% of CD3+CD4+ (marked “+”). As indicated visually by the donor peak (“d”) in the insert, CD4+CD25high cells were 91% (± 6%) donor-derived, whereas CD25med were 5% (± 6%), and CD4+CD25neg 3% (± 6%) donor-derived and thus > 89% to 91% recipient (“r”). STR-PCR chimerism results of granulocytes, monocytes, NK and B cells were all < 6% donor-derived (ie, complete recipient chimerism; not shown). Alleles were quantified by capillary electrophoresis and fluorescence-based quantification using the ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
indicates the occasion when the latest bone marrow aspiration was performed with practically identical chimerism results as shown for simultaneously analyzed peripheral blood except that CD34+ cells, which were not detectable in the periphery, were of 8% to 14% donor origin (not shown). (B) This diagram shows more detailed sublineage chimerism analyses including the CD4+CD25neg, CD4+CD25med, and CD4+CD25high T cells as indicated, performed at the latest 3 visits at 5, 5.5, and 6 years after transplantation (mean + error bars = range). (C) The plot is representative of 2 specimens from 2 independent outpatient visits at 5.5 and 6 years after HSCT, which were analyzed for lineage-specific and subpopulation-specific chimerism by fluorescence-activated cell sorting and short tandem repeat–polymerase chain reaction (STR-PCR). It shows the CD3/CD4-gated fractions of CD25neg/CD25med/CD25high cells with their STR-PCR peaks (insert; r, recipient; d, donor; “control” showing both individual and the common STR-marker peaks). CD4+ T cells comprised 58% of all T cells, CD4+CD25high were approximately 1% of all CD3+CD4+ cells (shown as “++”), CD4+CD25med were 16% of CD3+CD4+ (marked “+”). As indicated visually by the donor peak (“d”) in the insert, CD4+CD25high cells were 91% (± 6%) donor-derived, whereas CD25med were 5% (± 6%), and CD4+CD25neg 3% (± 6%) donor-derived and thus > 89% to 91% recipient (“r”). STR-PCR chimerism results of granulocytes, monocytes, NK and B cells were all < 6% donor-derived (ie, complete recipient chimerism; not shown). Alleles were quantified by capillary electrophoresis and fluorescence-based quantification using the ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA).
In this patient, FoxP3 expression and levels of CD4+CD25highCD127lowFoxP3-expressing cells are detectable at a low-normal range after HSCT. That the patient has no signs of autoimmunity 6 years after HSCT suggests that the donor-derived CD4+CD25highCD127lowFoxP3-expressing T cells have regulatory function in vivo, although we cannot exclude that the highly immunosuppressive conditioning regimen for HSCT per se caused persistent remission. Despite lacking in vitro analyses for regulatory T (Treg) function, which were not feasible because of the low cell frequency in this patient, we speculate that selective long-term engraftment of active donor Treg cells occurred and has been responsible for the continuing disease-free state of the patient.
Although these descriptive observations might lack mechanistic insight, they suggest that (1) severe IPEX syndrome may be cured with nonmyeloablative HSCT; (2) long-term remission is achievable with a minimal donor cell population restricted to approximately 10/μL CD4+CD25+CD127lowFoxP3+ cells; and (3), because remission of immune dysregulation is observed after HSCT without FoxP3 reconstitution in other lymphocyte subsets, Treg-independent FoxP3 function within T-effector cells might be less relevant than hypothesized in vivo.4,8,–10
Authorship
The online version of this letter contains a data supplement.
Acknowledgments: We thank the patient and his family for their cooperation and trust, as well as for giving their written informed consent for analyses and publication of his clinical and immunological parameters in this article. Furthermore, we are indebted to Eleonora Gambineri and Troy Torgerson for stimulatory communication about the patient's course of clinical and laboratory parameters, and, not least, Michaela Nesslböck and Dieter Printz for their excellent technical assistance.
Contribution: M.G.S., A.H., and S.M.-M. designed the study and wrote the paper; M.G.S. performed mRNA analyses and prepared all figures; G.F., T.L., and B.J. performed and evaluated flow cytometry, chimerism analyses, and in vitro cell preparation; and A.L., A.H., R.B., C.P., M.G.S., S.M.-M., and H.G. were substantially involved in transplantation-associated and immunological clinical management as well as diagnostic monitoring during the observation period of this patient.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Susanne Matthes-Martin, Assoc Prof, Department of Stem Cell Transplantation, St Anna Children's Hospital, Kinderspitalgasse 6, A-1090 Wien, Austria; e-mail: Susanne.Matthes@stanna.at.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal