Notch signaling has been shown to regulate various aspects of vascular development. However, a specific role of the ligand Delta-like 1 (DLL1) has not been shown thus far. Here, we demonstrate that during fetal development, DLL1 is an essential Notch ligand in the vascular endothelium of large arteries to activate Notch1 and maintain arterial identity. DLL1 was detected in fetal arterial endothelial cells beginning at embryonic day 13.5. While DLL4-mediated activation has been shown to suppress vascular endothelial growth factor (VEGF) pathway components in growing capillary beds, DLL1-Notch signaling was required for VEGF receptor expression in fetal arteries. In the absence of DLL1 function, VEGF receptor 2 (VEGFR2) and its coreceptor, neuropilin-1 (NRP1), were down-regulatedin mutant arteries, which was followed by up-regulation of chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII), a repressor of arterial differentiation and Nrp1 expression in veins. Consistent with a positive modulation of the VEGF pathway by DLL1, the Nrp1 promoter contains several recombinant signal-binding protein 1 for J κ (RBPJκ)–binding sites and was responsive to Notch activity in cell culture. Our results establish DLL1 as a critical endothelial Notch ligand required for maintaining arterial identity during mouse fetal development and suggest context-dependent interrelations of the VEGFA and Notch signaling pathways.
Introduction
A functional cardiovascular system with precisely formed and connected arteries and veins is established early during mammalian embryogenesis and is essential for subsequent development and survival. First, endothelial precursors, the angioblasts, differentiate into endothelial cells and form a primitive vascular network in a process called vasculogenesis. These first vessels are simple tubes composed entirely of endothelial cells. Subsequently, the simple vascular network is remodeled into a hierarchical network of veins, arteries and capillaries in a process called angiogenesis.1 Angiogenesis is a precisely orchestrated multistep process that involves localized cell proliferation and migration, the extension of endothelial sprouts and their conversion into patent tubules, as well as the selective pruning of some vessel connections. During angiogenesis, the division into morphologically, functionally, and molecularly unique arteries and veins is established and supporting cell types, such as smooth muscle cells and pericytes, are recruited to form mature and fully functional vessels.2
Vascular endothelial growth factor (VEGF) signaling is essential for establishment of the embryonic vasculature as is indicated by absence or severe malformations of vessels in embryos lacking VEGF or its receptors (reviewed in Rossant and Howard3 ). Early during angiogenesis, ie, before development of a functional circulation, the distinction between arteries and veins is established in a process termed arteriovenous differentiation. Accordingly, certain markers and signaling molecules, such as the transmembrane protein ephrinB2 (EFNB2), are specifically expressed in the endothelium of developing arteries. Conversely, the expression of the receptor tyrosine kinase EPHB4, an interaction partner of ephrinB2, and other gene products are confined to the venous endothelium. Loss of either EphrinB2 or EPHB4 leads to very similar defects in vascular remodeling, suggesting that they mediate bidirectional signaling essential for angiogenesis and the establishment of boundaries between venous and arterial domains.4,–6
The evolutionarily conserved Notch signaling pathway plays a central role in the specification of cell fates by mediating local cell interactions in different tissues in diverse organisms.7,–9 The Notch gene of Drosophila, as well as its vertebrate homologues, encode large cell surface receptors that interact with their ligands, namely the products of the Delta and Serrate (in vertebrates called Jagged) genes, in a cell-to-cell contact-dependent fashion. Notch, Delta, and Serrate/Jagged encode transmembrane proteins with multiple EGF-like repeats in their extracellular domains.10 Upon ligand binding, the intracellular portion of Notch is proteolytically cleaved, translocates to the nucleus, and forms a complex with the transcriptional regulator recombinant signal-binding protein 1 for J κ (RBPjk), which will then activate transcription of target genes such as Hes and Hey family bHLH transcriptional regulators.11,–13
Four different Notch receptors (Notch 1-4) and 4 activating ligands, Delta-like (Dll) 1, 4, Jagged1, and Jagged2, have been identified in mice. Several receptors and ligands are expressed in the developing vasculature. Notch1, Notch4, Delta1, Delta4, and Jagged2 are specifically expressed in the arterial endothelium. Notch3 was detected in the smooth muscle cells surrounding arteries but not veins, whereas Jagged1 is expressed in both the endothelial and smooth muscle cells surrounding arteries.14,15 Notch4 and Delta4 expression appear to be vessel-specific and entirely restricted to the endothelium, including developing capillaries.16,17
Studies in zebrafish and mice have demonstrated essential functions of Notch signaling for vascular remodeling of the primitive vascular plexus and establishment of arterial fate (reviewed in Rossant and Howard,3 Alva and Iruela-Arispe,18 Shawber and Kitajewski,19 Adams and Alitalo,20 and Siekmann et al21 ). Mouse embryos deficient for Jagged1, Notch1, or Notch1/Notch4 die between embryonic day (E) 9.5 and 10.5 and display severe vascular malformations.22,23 In all these mutants, initial establishment of the vascular network appears to be largely normal, indicating essential roles in angiogenesis, but not in vasculogenesis. During zebrafish development, loss of Notch or the downstream Notch target gene gridlock (the ortholog of mouse Hey2), leads to a loss of the arterial cell marker ephrinB2 (in zebrafish: ephrinB2a) and an increase in the expression of the venous marker EphB4 in the dorsal aorta.24,25 Similarly, in early mouse embryos DLL4-mediated Notch activity is required in a dose-dependent manner for arterial differentiation and ephrinB2 expression,26,27 indicating that DLL4 is the critical endothelial Notch ligand directing normal arterial patterning in early mammalian embryos. In zebrafish embryos, Notch activity is regulated by VEGF-A suggesting that arterial specification occurs via a VEGF-A/Notch signaling cascade resulting in the up-regulation of the arterial endothelial cell marker ephrinB2.28
In adult heterozygous Dll1 mutants, ischemia-induced arteriogenesis was severely impaired,29 indicating that DLL1 regulates postnatal arteriogenesis and suggesting that DLL1 might also play a role in the vascular endothelium earlier in development. Here we show that DLL1 is specifically expressed in fetal arterial endothelial cells beginning at E13.5, is a critical regulator of arterial patterning, and, in contrast to the role of the Notch pathway in growing capillaries, positively regulates VEGF signaling. Embryos with reduced DLL1 levels or specific ablation of DLL1 in vascular endothelium rapidly lost Notch1 activation after E13.5 although DLL4 expression was maintained. Arterial endothelial and smooth muscle markers, notably VEGF receptor 2 (VEGFR2) and its coreceptor neuropilin-1 (NRP1), were down-regulated. These changes in the mutant arteries preceded the ectopic expression of venous markers such as chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII), which normally suppresses Nrp1 expression and the arterial differentiation program in the venous endothelium.30 Our results establish DLL1 as a critical endothelial Notch ligand required for maintaining arterial identity during fetal development and suggest context-dependent interrelations of the VEGF-A and Notch signaling pathways.
Methods
Immunohistochemistry
Immunostraining was performed on 10-μm sections of MeOH/DMSO (dimethyl sulfoxide)–fixed paraffin embedded embryos of the indicated age. Mutant and wild type embryos were analyzed in parallel with the indicated antibody under identical conditions. At least 3 embryos were analyzed with each marker at a given developmental stage. Slides were dehydrated, for antigen retrieval slides were boiled in 10 mM sodium citrate (pH 6.0). Blocking was performed in 1% bovine serum albumin (BSA) and sections were incubated with the primary antibody overnight. After incubation with the appropriate horseradish peroxidase (HRP)–conjugated secondary antibody, the signal was developed with a TSA-Cy3 System (Tyramide Signal Amplification; Perkin Elmer, Waltham, MA). Vascular smooth muscle cells were marked using α-smooth muscle actin antibodies and cell nuclei with Draq5 (Biostatus Limited, Leicestershire, United Kingdom). Antibodies used were: anti-Notch1 ICD Val-1744 (1:100; Cell Signaling Technology, Danvers, MA); anti-EphrinB2 (1 μg/mL), anti-EphB4 (1μg/mL), anti-Neuropilin1 (Nrp1, 5 μg/mL; all R&D Systems, Minneapolis, MN); anti-VEGF (147; 2 μg/mL), anti-VEGFR2 (Flk1, C-20; 2 μg/mL), anti-VEGFR1 (Flt1, C-17; 2 μg/mL), anti-Smoothelin (c-20; 2 μg/mL), anti-Jagged1 (H-114; 2 μg/mL; all Santa Cruz Biotechnology, Santa Cruz, CA); anti-DLL4 (15 μg/mL), anti-DLL1 (10 μg/mL; Abcam, Cambridge, MA); anti-COUPTFII (0.01 μg/mL; Perseus Proteomics, Tokyo, Japan); anti-eNOS (2 μg/mL; Affinity Bioreagents, Rockford, IL); anti-Notch1 (bTan; 1/50; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); anti–phospho-VEGFR2 (pFlk1-Tyr 951; 2 μg/mL; Santa Cruz Biotechnology); anti–phospho-VEGFR2 (pFlk1-Tyr 1175; 1/100; Cell Signaling Technologies); anti–α-smooth muscle actin-FITC (1/800; Sigma-Aldrich, St Louis, MO). Secondary antibodies used were: anti–mouse IgG-HRP, anti–rabbit IgG-HRP, anti–rat IgG-HRP (all 1/200; GE Healthcare Europe, LittleChalfont, United Kingdom); anti–goat IgG-HRP (1/100; Dianova, Hamburg, Germany).
Slides were analyzed by confocal laser-scanning microscopy using a TCS-SP2 AOBS with a PLAPO 63×/1.4 objective (Leica Microsystems; Wetzlar, Germany). For image acquisition, Leica Confocal Software version 2.5 (Leica Microsystems) was used. Pictures were processed and assembled using Photoshop and Illustrator CS (Adobe Systems, San Jose, CA).
Western blot analysis
Outflow tracts of E15.5 embryos were dissected, flash-frozen in liquid nitrogen, and minced in Ripa buffer. Equal amounts of protein from cell or pooled tissue lysates were separated by SDS-polyacrylamid gel electrophoresis, blotted onto nitrocellulose membranes (Immobilon; Millipore, Billerica, MA) and probed with primary and secondary antibodies. Except for Neuropilin1 (anti-Neuropilin1, 1 μg/mL; Abcam), the same antibodies as for immunohistochemistry were used. As a loading control, a mouse anti-actin antibody (MP Biochemicals, Solon, OH) was used.
Cre induction and blocking of VEGF and Notch activity
To generate the endothelial specific knock-out, mice were injected on 3 consecutive days intraperitoneally with 100 μL Tamoxifen (Sigma-Aldrich) at a concentration of 10 mg/mL in corn oil (Sigma-Aldrich). The monoclonal neutralizing anti–mouse VEGF antibody (N-mVEGF) was purchased from ReliaTech (Braunschweig, Germany), diluted in physiologic salt solution and injected into the tail vein of pregnant females on 2 consecutive days. For Notch inhibition experiments, N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT; 50 mg/kg; Alexis Biochemicals, San Diego, CA) was injected (10 μL in DMSO) into the tail vein. All animal studies were approved by the institutional review board at the Institute for Molecular Biology according to German rules and stipulations.
Nrp1 promoter constructs
Five kilobases of the upstream region of Neuropilin1 were amplified from a BAC clone (RP23-320F22; ImaGenes, Berlin, Germany) using the Expand High-Fidelity PCR System (Roche, Basel, Switzerland). Mutagenesis was carried out using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and confirmed by sequencing. Constructs were cloned into the luciferase reporter construct pGL4.27 (luc2P/minP/Hygo; Promega, Madison, WI).
In situ hybridization
In situ hybridization was performed by standard procedures.
Mice
Dll1lacz 31, Dll1Dll1kineo, 32Dll1loxp 33, and R-NICD34 mice were described previously.
Cell lines
CHO cells stably expressing DLL1 or DLL4 were generated by transfection of CHO cells with expression plasmids using Jetpei (BIOMOL Research Laboratories, Plymouth Meeting, PA) according to the manufacturer's instructions followed by G418 selection. HeLa cells stably expressing Notch1 were provided by A. Israel (Institut Pasteur, Paris, France).
Retina staining
Eyes were dissected at P6, P9, and P15, fixed in 4% paraformaldehyde (PFA) for 1 hour at room temperature, washed twice with phosphate-buffered saline (PBS), and enucleated. Retinas were dissected, incubated with biotinylated Griffonia simplicifolia lectin1 (Isolectin B4; Vector Laboratories, Burlingame, CA; detected with streptavidin 405 Alexa), anti-Desmin (Abcam; detected with donkey anti–rabbit 488 Alexa), and Cy3-conjugated α-smooth muscle actin and flat mounted. For DLL1 staining, eyes were fixed in 4% PFA for 5′ and dissected retinas were refixed in 100% MeOH. Mounted retinas were analyzed using the microscope Leica DMI 6000B with a HC PL Fluotar 10×/0.3 objective (Leica Microsystems). For image acquisition, LAS AF software (Leica Microsystems) was used.
Brain staining
Brains were dissected at P6, P9, and P15, fixed overnight in 4% PFA, washed in PBS, dehydrated to 100% MeOH, and incubated in 5% H2O2 in Methanol for 5 hours to destroy endogenous peroxidase. Brains were rehydrated, incubated with anti-Pecam (Pharmingen, San Diego, CA) and a biotinylated secondary antibody (Vector Laboratories), incubated in avidin-biotin-peroxidase complex (ABC)–Reagent (Vector Laboratories) and the signal was developed using DAB (diaminobenzidin; Vector Laboratories). Images were taken with a Leica M420 macroscope with Leica Apozoom 1.6 (Leica Microsystems). A FUJIK digital camera HC-300Z and the software Photograb-300Z (Fujifilm, Tokyo, Japan) were used to acquire images.
Skin staining
Heads of E17.5 embryos were fixed in 4% PFA overnight. The skin was dissected and incubated for 4 hours in blocking solution (1%BSA, 0.5% Tween-20 in PBS) incubated with the primary antibody (anti-Endomucin [gift of D. Vestweber, Max-Planck-Institute for Biomedicine, Münster, Germany]), anti–collagen typeIV [Chemicon, Billerica, MA]) O/N at 4°C, washed, incubated with the secondary antibody (donkey anti–rat Alexa 488 and donkey anti–rabbit Alexa 543; Molecular Probes, Carlsbad, CA) and flat mounted with Flouromount (Southern Biotech, Birmingham, AL). Mounted skin preparations were analyzed with a Leica DM 5000B microscope with a HC PL Fluo 10×/0.3 objective (Leica Microsystems). Images were acquired with a Leica DFC 300FX camera and Leica FireCam software (Leica Microsystems).
Ink injection
Ink (diluted 1:100 in PBS) was injected into the carotid artery of E17.5 embryos. Embryos were fixed in 4% PFA O/N, dehydrated to 100% methanol, and cleared in benzyl alcohol/benzyl benzoate (2:1). For microscope and image acquisition, see brain staining.
Morphometric and branchpoint analysis
Analysis of the vessel wall thickness and vessel lumen was carried out on hematoxylin-eosin (HE)–stained paraffin sections of E15.5 and E18.5 embryos. Images were taken with a Leica DM 5000B microscope with a HC PL Fluo 20×/0.5 objective (Leica Microsystems). Images were acquired with a Leica DFC 300FX camera and Leica FireCam software (Leica Microsystems). Wall thickness and lumen were measured using ImageJ (National Institutes of Health, Bethesda, MD). Branchpoints were counted on endomucin-stained skin preparations of E17.5 embryos using Imaris (Bitplane, Zurich, Switzerland). Microscope and image acquisition were the same as for skin stainings.
TA assay
CHO cells stably expressing DLL1 or DLL4 were transiently transfected with Jag1 expression plasmids using Jetpei (Peqlab Biotechnologie, Erlangen, Germany) following the manufacturer's instructions. HeLa cells stably expressing Notch1 were transiently transfected with a Jag1 or manic fringe Mfng expression plasmid and a RBPJκ luciferase reporter construct. Aliquots of 106 transfected HeLaN1 cells were cocultivated on 6-well plates for 24 hours with 106 CHO cells, stably expressing the respective ligands. Luciferase activity was measured using the Dual-Luciferase reporter Assay System (Promega). Firefly luciferase activity was normalized to cotransfected Renilla luciferase activity (pRL-TK; Promega).
Results
Dll1 is critical for Notch1 activation in arterial endothelial cells
Before E13.5, expression of DLL1 was not detected in blood vessels. At E13.5, DLL1 expression in vascular endothelial cells was detected in arteries, but not in capillaries or veins, and persisted in arterial vascular endothelium throughout subsequent development (Figure 1, and Figure S4, available on the Blood website; see the Supplemental Materials link at the top of the online article), suggesting a function in fetal arteries well after early Notch-regulated angiogenic processes. To analyze the function of Dll1 in the fetal vascular endothelium, we made use of a hypomorphic Dll1 allele (Dll1Dll1kineo) that significantly reduces Dll1 transcription32 when combined with a Dll1lacZ null allele.31 Heteroallelic mice (Dll1lacZ/Dll1kineo) carrying these alleles survive until birth and have, among other malformations, severe skeletal muscle defects. Muscle defects in early heteroallelic embryos were indistinguishable from muscle differentiation defects in Dll1 null mutants,32 suggesting that in Dll1lacZ/Dll1kineo embryos, Dll1 activity is essentially abolished. To detect a potential requirement of DLL1 for Notch activity in fetal blood vessels, we analyzed the aorta and other large vessels of the trunk at the level of the outflow tract (Figure 2A). We first analyzed Notch1 activation using an antibody (Val1744) specifically recognizing the activated form of Notch1, NICD. At E13.5, activated Notch1 (NICD) was detected in wild type and mutant arteries (Figure 2B a, a′, b, b′). In contrast, no NICD was detected in mutant vascular endothelium at E15.5 and E18.5 (Figure 2B d, d′, f, f ′), although Notch1 protein was present at all analyzed stages (Figure 2B g-l, g′-l′). Consistent with absence of NICD, Hey2 transcription was down-regulated in mutant vessels (Figure S2A n, n′). To address whether loss of Notch1 activation affects expression of other receptors or Notch pathway components, we analyzed their expression by in situ hybridization. Notch2, 3, and 4, and Dll4 expression was apparently unaffected, whereas Jag1 and Mfng appeared up-regulated in mutant vessels (Figure S2A c-j, l, p). Because DLL4 is critical for Notch activity in early embryonic vessels,26,27 we analyzed whether DLL4 protein expression is maintained in Dll1 mutant embryos. DLL4 protein was clearly detected in mutant vessels by antibody staining (Figure 2B n, n′, p, p′, r, r′), indicating that DLL4 is not sufficient to efficiently activate Notch1 in this context, although we cannot exclude that DLL4 might activate (an)other Notch receptor(s) expressed in fetal arteries. Collectively, these results indicate that DLL1 is a critical activator of Notch1 in fetal arterial endothelium, and suggest nonredundant functions of DLL1 and DLL4 in this context.
DLL1 expression in fetal arteries. DLL1 (red) is highly expressed in the endothelium (arrows in C′ and E′) and in the smooth muscle layer (arrowheads in C′ and E′) starting at embryonic day (E) 13.5 in arterial vessels, but not in veins (D). At E12.5, there is no detectable expression in arteries or veins (A,B). This pattern persists until E18.5 (E,F). A′ to F′ shows magnifications of panels A through F. Green indicates antibody staining for α-smooth muscle actin.
DLL1 expression in fetal arteries. DLL1 (red) is highly expressed in the endothelium (arrows in C′ and E′) and in the smooth muscle layer (arrowheads in C′ and E′) starting at embryonic day (E) 13.5 in arterial vessels, but not in veins (D). At E12.5, there is no detectable expression in arteries or veins (A,B). This pattern persists until E18.5 (E,F). A′ to F′ shows magnifications of panels A through F. Green indicates antibody staining for α-smooth muscle actin.
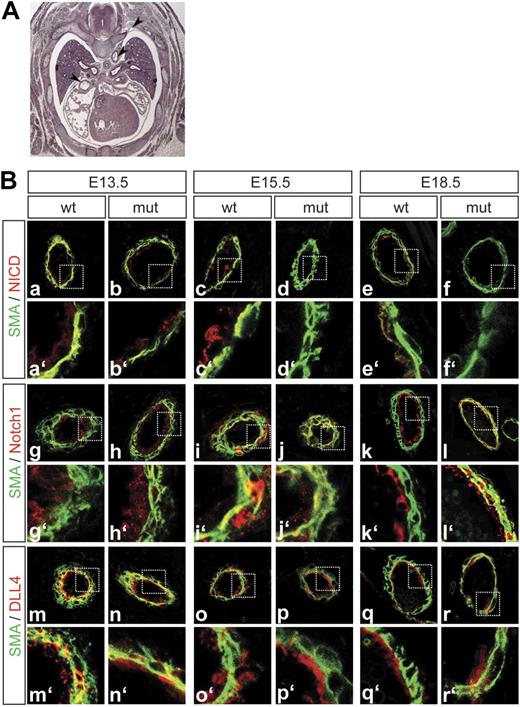
Notch1 activation is reduced in Dll1 mutants. (A) Histologic section of an E15.5 embryo indicating the level along the anterior-posterior body axis used for the analysis of the fetal vessel phenotype. (B) Antibody staining for cleaved Notch (a-f), full-length Notch1 (g-l), and DLL4 (m-r). Green: α-smooth muscle actin, red indicates antigen. Notch activation is abolished in Dll1 mutant embryos at E15.5 and E18.5 (c-f), whereas Notch1 and DLL4 are still expressed at these embryonic stages (g-r). At E13.5, mutant vessels show expression patterns of the analyzed markers similar to wild type (a,b,g,h,m,n). Although DLL4 is normally expressed it does not seem to be able to activate Notch1. a′ to r′ show magnification of the boxed areas indicated in subpanels a through r.
Notch1 activation is reduced in Dll1 mutants. (A) Histologic section of an E15.5 embryo indicating the level along the anterior-posterior body axis used for the analysis of the fetal vessel phenotype. (B) Antibody staining for cleaved Notch (a-f), full-length Notch1 (g-l), and DLL4 (m-r). Green: α-smooth muscle actin, red indicates antigen. Notch activation is abolished in Dll1 mutant embryos at E15.5 and E18.5 (c-f), whereas Notch1 and DLL4 are still expressed at these embryonic stages (g-r). At E13.5, mutant vessels show expression patterns of the analyzed markers similar to wild type (a,b,g,h,m,n). Although DLL4 is normally expressed it does not seem to be able to activate Notch1. a′ to r′ show magnification of the boxed areas indicated in subpanels a through r.
Normal levels of Notch ligands in vascular endothelial cells are crucial for embryonic angiogenesis26,27 and ischemia-induced arteriogenesis.29 Thus, loss of DLL1 might simply reduce the concentration of ligands below a threshold required for sufficient Notch activity. Therefore, we tested whether DLL1 can enhance the ability of DLL4 to activate Notch in a coculture assay in vitro. However, coexpression of DLL1 and DLL4 did not improve Notch activation in vitro (Figure S2B) arguing against a synergistic role of the 2 ligands. Likewise, coexpression of JAG1 weakly improved Notch activation by DLL1, but not DLL4, in vitro (data not shown). Because fringe glycosyltransferases can modify Notch receptors and thereby enhance or attenuate the responsiveness of Notch to different ligands,35 and Mfng expression appeared up-regulated in mutant endothelium, we tested whether MFNG differently influences the ability of DLL1 and DLL4 to activate Notch in vitro. Expression of MFNG in Notch1-expressing HeLa cells (HeLa-N1) enhanced Notch activity approximately 8-fold when these cells were cocultured with either DLL1 or DLL4 expressing CHO cells (Figure S2B) compared with HeLa-N1 cells without MFNG. When MFNG was coexpressed with DLL1 or DLL4 in CHO cells, Notch activation in HeLa-N1 cells was unaffected. Thus, up-regulation of Mfng expression has no inhibitory effect on DLL4 activity but might rather indicate the induction of a compensatory (albeit insufficient) mechanism for the restoration of Notch signaling in Dll1 mutant fetal arteries.
Dll1 signals maintain arterial gene expression
To address the consequences of abolished Dll1 mediated Notch activity in endothelial cells, we analyzed the expression of NRP1, VEGFR2, and ephrinB2 proteins specifically expressed in endothelial cells of arteries, and of smoothelin, which is restricted to arterial vessel walls. At E13.5, when NICD was still detected in mutant vessels (Figure 2B b, b′), all markers were expressed in apparently normal patterns (Figure 3B, B′,H, H′, N, N′, T, T′, Z, Z′). However, at E15.5 and E18.5, expression of all markers was severely down-regulated or abolished (Figure 3D, D′, F, F′, J, J′, L, L′, P, P ′, R, R ′,V, V ′, W, W′). COUP-TFII, a transcription factor that is normally exclusively expressed in venous endothelial cells and perivascular cells surrounding arteries and veins, and is essential for vein identity,30 was ectopically activated in the endothelium of arteries at low levels at E15.5 and was readily detected at E18.5 (arrowheads in Figure 3ZB, ZB′, ZD, ZD′). Loss of NICD and artery-specific gene expression was also found in large vessels in the head and abdomen (Figure S1). We conclude that DLL1 mediated Notch activity is required to maintain artery-specific gene expression in fetal arteries throughout the body.
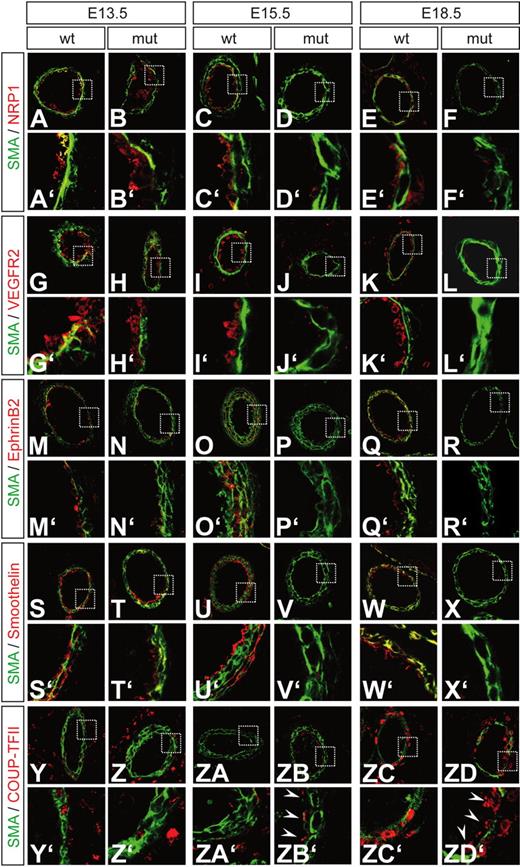
Arterial maker expression is severely reduced in Dll1 mutant embryos. At E13.5, mutant embryos still show normal expression of arterial markers comparable to wild-type embryos (A, B, G, H, M, N, S, T). At E15.5 and E18.5, the arterial markers Neuropilin1 (C-F), VEGFR2 (I-L), EphrinB2 (O-R) and Smoothelin (U-X) are severely reduced in arterial vessels. The venous marker COUP-TFII, which is normally expressed in perivascular cells surrounding arteries, is ectopically up-regulated in arterial endothelial cells (Y-ZD) at E15.5 (arrowheads in ZB′) and E18.5 (arrowheads in ZD′). Green indicates α-smooth muscle actin; red indicates antigen. A′ to ZD′ show magnifications of the boxed areas indicated in A-ZD.
Arterial maker expression is severely reduced in Dll1 mutant embryos. At E13.5, mutant embryos still show normal expression of arterial markers comparable to wild-type embryos (A, B, G, H, M, N, S, T). At E15.5 and E18.5, the arterial markers Neuropilin1 (C-F), VEGFR2 (I-L), EphrinB2 (O-R) and Smoothelin (U-X) are severely reduced in arterial vessels. The venous marker COUP-TFII, which is normally expressed in perivascular cells surrounding arteries, is ectopically up-regulated in arterial endothelial cells (Y-ZD) at E15.5 (arrowheads in ZB′) and E18.5 (arrowheads in ZD′). Green indicates α-smooth muscle actin; red indicates antigen. A′ to ZD′ show magnifications of the boxed areas indicated in A-ZD.
Dll1 function is required in endothelial cells
As the analyzed allele combination affects DLL1 function systemically, some effects might not be specific for DLL1 function in the vascular endothelium. To analyze whether the observed changes can be attributed specifically to DLL1 function in endothelial cells, we deleted DLL1 in endothelial cells using a floxed Dll1 allele33 and a transgenic mouse line, in which a tamoxifen-inducible Cre (CreERT2) was inserted into a large PAC clone of the Cadh5 (VE-Cadherin) gene resulting in endothelium-specific expression (R.H.A., unpublished data, August 5, 2006). Fetuses lacking DLL1 in the arterial endothelium showed a down-regulation of markers similar to heteroallelic mutant embryos (Figure 4B b, b′, f, f′, j, j ′, n, n′, r, r′; Figure 4C). Arguing further for a specific role of DLL1-Notch signaling in the endothelium, expression of NICD (a constitutively active form of Notch) using a mouse line carrying a Cre-inducible NICD cDNA inserted into the ROSA26 locus34 restored arterial gene expression in Dll1 mutants (Figure 4B c, c′, g, g′, k, k′, o, o′, s, s′; Figure 4C). Conversely, administration of the γ-secretase inhibitor DAPT, an inhibitor of Notch signaling, led to the down-regulation of arterial markers as in Dll1 mutants (Figure 4B d, d′, h, h′, l, l′, p, p′, t, t′; Figure 4C), confirming that DLL1 is indeed the pivotal Notch ligand controlling arterial identity during fetal development.
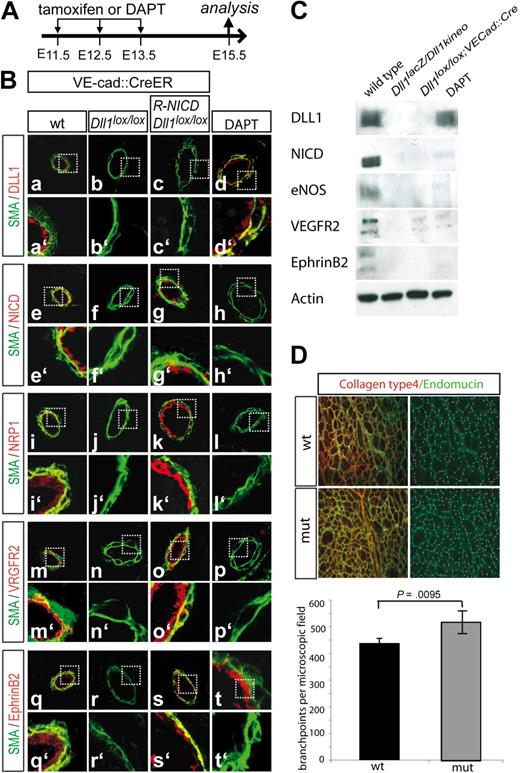
The endothelial-specific KO of Dll1 resembles the hypomorphic arterial phenotype and can be rescued by constitutive overexpression of activated Notch. (A) Regimen of Tamoxifen or DAPT application. Tamoxifen or the γ-secretase inhibitor DAPT was injected into pregnant mice on 3 consecutive days (E11.5-E13.5). Embryos were analyzed at E15.5. (B) Microphotographs of vessels stained with antibodies. Antibody staining for Dll1 shows that is successfully removed from the endothelium in tamoxifen-treated Dll1loxp; VE-Cadherin-Cre-ERT embryos (a,b). Notch activation is disturbed in these embryos (e, f) and the expression of the arterial markers Neuropilin1 (i,j), VEGFR2 (n,m) and EphrinB2 (q,r) is down-regulated. Concomitant endothelial-specific overexpression of constitutively active Notch (g) restored the expression of arterial markers (k,o,s). DLL1 expression is not affected by constitutive Notch activity (c). Administration of the γ-secretase inhibitor DAPT blocked Notch activation (h) and reduced arterial makers (l,p,t) similar to loss of DLL1-mediated Notch activation. DLL1 expression is not affected (d). Green indicates α-smooth muscle actin; red indicates antigen. a′ to t′ show magnifications of the boxed areas indicated in a through t. (C) Western blot analysis with protein lysates of the heart outflow tract confirmed the results obtained by immunohistochemistry. Actin was used as a loading control. (D) Increased capillary branching in the skin of E17.5 Dll1 hypomorphic embryos indicated by antibody staining for collagen typeIV (red) and endomucin (green). Data are mean values of 5 embryos per genotype. Bars indicate SD.
The endothelial-specific KO of Dll1 resembles the hypomorphic arterial phenotype and can be rescued by constitutive overexpression of activated Notch. (A) Regimen of Tamoxifen or DAPT application. Tamoxifen or the γ-secretase inhibitor DAPT was injected into pregnant mice on 3 consecutive days (E11.5-E13.5). Embryos were analyzed at E15.5. (B) Microphotographs of vessels stained with antibodies. Antibody staining for Dll1 shows that is successfully removed from the endothelium in tamoxifen-treated Dll1loxp; VE-Cadherin-Cre-ERT embryos (a,b). Notch activation is disturbed in these embryos (e, f) and the expression of the arterial markers Neuropilin1 (i,j), VEGFR2 (n,m) and EphrinB2 (q,r) is down-regulated. Concomitant endothelial-specific overexpression of constitutively active Notch (g) restored the expression of arterial markers (k,o,s). DLL1 expression is not affected by constitutive Notch activity (c). Administration of the γ-secretase inhibitor DAPT blocked Notch activation (h) and reduced arterial makers (l,p,t) similar to loss of DLL1-mediated Notch activation. DLL1 expression is not affected (d). Green indicates α-smooth muscle actin; red indicates antigen. a′ to t′ show magnifications of the boxed areas indicated in a through t. (C) Western blot analysis with protein lysates of the heart outflow tract confirmed the results obtained by immunohistochemistry. Actin was used as a loading control. (D) Increased capillary branching in the skin of E17.5 Dll1 hypomorphic embryos indicated by antibody staining for collagen typeIV (red) and endomucin (green). Data are mean values of 5 embryos per genotype. Bars indicate SD.
Phenotypic consequences of lost artery-specific gene expression
To address which consequences the loss of artery-specific gene expression has for the structure of these vessels and the vasculature, we first examined Dll1lacZ/Dll1kineo and endothelium-specific Dll1 mutant fetuses histologically, but detected no obvious structural defects (data not shown). Morphometric analyses did not show significant differences in the thickness of the arterial walls, but revealed a reduction of the lumens of the aorta and other large arteries in both mutants (Figure S3A). Whole mount staining for Collagen type IV and endomucin in skin preparations showed no evidence for shunts or other gross abnormalities, but revealed a statistically significant (P < .01) increase in capillary branch points (Figure 4D). Ink injections into the carotid artery of E17.5 mutant embryos also did not show major abnormalities, although main vessels in the head appeared disorganized in some mutant embryos (Figure S3B). To analyze potential defects in the vasculature further, we turned to postnatal angiogenesis. Because pups with deletion of DLL1 from the vascular endothelium during embryogenesis were not viable, we induced recombination postnatally on P1, P2, and P3, and analyzed the vasculature in the retina and brain of mutants on P6, P9, and P15, up to which point we did not observe lethality in mutant pups. Staining of retinas for Isolectin B4, Desmin, and α-smooth muscle actin revealed no obvious differences in the capillary network or in pericyte and smooth muscle cell coverage between wild-type and mutant pups (Figure S3C a-c, f-h). In addition, PECAM staining of mutant brains did not show severe abnormalities in the superficial vessel network (Figure S3C d-e, i-j). However, we cannot exclude that more severe defects develop over time in adult or aging mice.
DLL1 regulates VEGF responsiveness in arterial endothelial cells
Loss of DLL1 led to down-regulation of NRP1, which is required for efficient VEGFR2 function. COUP-TFII represses Nrp1 in veins,30 and COUP-TFII itself is repressed by the Notch target HEY2 in endothelial progenitor cells and differentiating ES cells.36 This suggested that loss of DLL1-mediated Notch activity in arteries results in up-regulation of COUP-TFII, which in turn could cause down-regulation of NRP1. However, no NRP1 protein was detected in E15.5 mutant arteries although COUP-TFII was barely expressed, arguing for a potential alternative mechanism of Nrp1 down-regulation. To clarify whether activation of COUP-TFII underlies repression of Nrp1 in Dll1 mutants, we analyzed the expression of NICD, NRP1 and COUP-TFII on serial sections of mutant arteries at E14.0 and 14.5. At E14.0, NICD and NRP1 were detected in arteries, and COUP-TFII was present in vascular smooth muscle and perivascular cells, but not in endothelial cells (Figure 5A a-c). At E14.5 NICD and NRP1 were no longer detected (Figure 5A d-f) but also COUP-TFII was still absent from arterial endothelial cells (arrowheads). This suggested that the initial down-regulation of Nrp1 is not due to repression by COUP-TFII, but independently caused by loss of activation by Notch1, which is consistent with the down-regulation of Nrp1 in Notch1 mutant embryos.37 In support of a direct link between Notch and Nrp1 transcription, the promoter region of Nrp1 contains, in addition to COUP-TFII binding sites, also 5 RBPjκ sites (Figure 5B). To test whether these sites are functional, we fused the Nrp1 promoter to a luciferase reporter gene and measured its activation by Notch in comparison to an established (RbpJ)6-luciferase reporter gene38 in HeLa cells stably expressing Notch1 after coculture with DLL1 expressing CHO cells. The Nrp1 reporter was activated to levels of approximately 40% of the efficient (RbpJ)6-luciferase reporter (Figure 5C) indicating that the Nrp1 promoter responds to Notch activation. Multiple RBPjκ sites are also present in the Nrp1 promoters of the rat, human, canine and zebrafish Nrp1 genes, although positions and spacing differ (data not shown), suggesting that Nrp1 regulation by Notch is conserved. The lower activation of the Nrp1 promoter construct compared with (RbpJ)6-luciferase might be due to the presence of COUP-TFII in HeLa cells,39 which could reduce expression from the promoter by competing with activation by NICD bound to RBPjκ. Activation of the Nrp1 promoter was severely reduced or abolished by mutations in individual or all RBPjκ sites (Figure 5D), indicating specificity. The drastic effect of mutations already in individual RBPjκ sites, in particular sites 1, 2, and 3 might be explained by the increased ratio of COUP-TFII and RBPjκ binding sites (and the balance between repressor and activator).
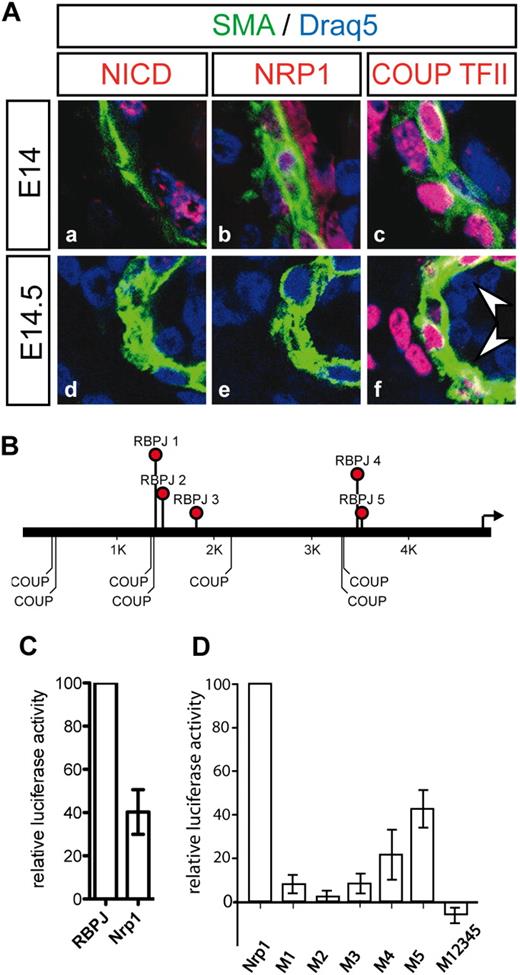
NRP1 expression depends on Notch activity. (A) Down-regulation of Neuropilin1 at E14.5 precedes up-regulation of COUP-TFII. At E14, Notch activation (a), and NRP1 and COUP-TFII expression (b,c) appeared normal in mutant embryos. At E14.5, Notch activity (d) and expression of Neuropilin1 (NRP1) was reduced (e), although COUP-TFII was not yet up-regulated in the arterial endothelium of E14.5 embryos (f). Green indicates α-smooth muscle actin; red indicates antigen. Nuclei are counterstained with Draq5 (blue). (B) Arrangement of RBPJκ and COUP-TFII binding sites in 5 kb of the Nrp1 promoter region. (C) The Neuropilin1 promoter responds to Notch1 activation in vitro. Coculture of CHO cells stably expressing Dll1 with HeLa-N1 cells activated an RBP-luc reporter (set as 100%) and less strongly the Nrp1-luc reporter. (D) Effective activation of the Nrp1 promoter fragment by Notch requires all RBPJκ binding sites. M1-M12345 refers to mutations in the binding sites indicated in panel B. Data are the results of 3 independent experiments. Bars indicate SD.
NRP1 expression depends on Notch activity. (A) Down-regulation of Neuropilin1 at E14.5 precedes up-regulation of COUP-TFII. At E14, Notch activation (a), and NRP1 and COUP-TFII expression (b,c) appeared normal in mutant embryos. At E14.5, Notch activity (d) and expression of Neuropilin1 (NRP1) was reduced (e), although COUP-TFII was not yet up-regulated in the arterial endothelium of E14.5 embryos (f). Green indicates α-smooth muscle actin; red indicates antigen. Nuclei are counterstained with Draq5 (blue). (B) Arrangement of RBPJκ and COUP-TFII binding sites in 5 kb of the Nrp1 promoter region. (C) The Neuropilin1 promoter responds to Notch1 activation in vitro. Coculture of CHO cells stably expressing Dll1 with HeLa-N1 cells activated an RBP-luc reporter (set as 100%) and less strongly the Nrp1-luc reporter. (D) Effective activation of the Nrp1 promoter fragment by Notch requires all RBPJκ binding sites. M1-M12345 refers to mutations in the binding sites indicated in panel B. Data are the results of 3 independent experiments. Bars indicate SD.
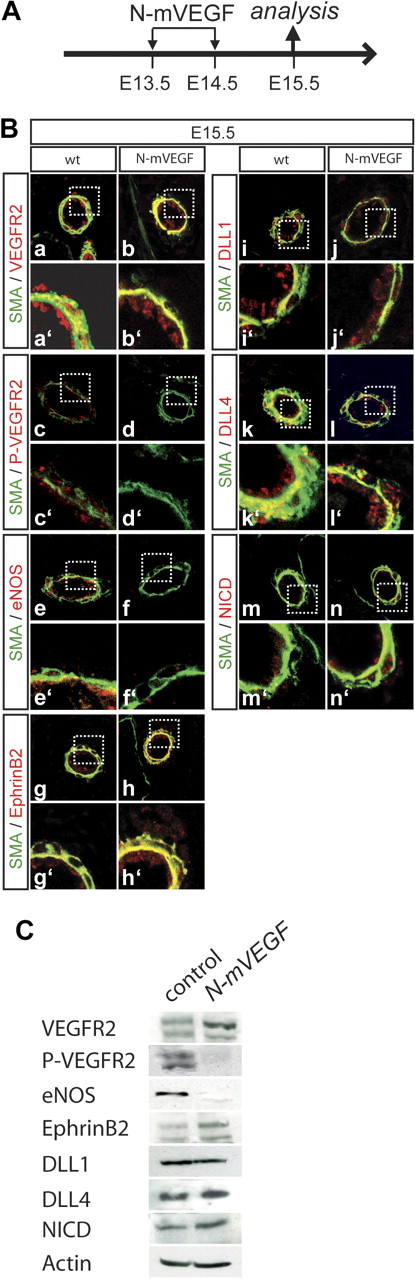
These results suggested that Notch activity directly regulates Nrp1 expression and thus efficient reception of VEGF-A signals in fetal arteries. To address whether VEGF-A activity affects Notch expression or activity in fetal arteries, we blocked VEGF-A by repeated injection of a neutralizing anti–mouse VEGF-A antibody into pregnant females. This treatment did not affect expression of VEGFR2 (Figure 6B b, b′; Figure 6C), but prevented its activation as indicated by the absence of tyrosine phosphorylated (activated) VEGFR2, and by down-regulation of eNOS, which is positively regulated by VEGF-A40 (Figure 6B d, d′, f, f′; Figure 6C). Importantly, neither expression of DLL1 (Figure 6B j, j′; Figure 6C) or DLL4 (Figure 6B l, l′; Figure 6C), nor activation of Notch1 (Figure 6B n, n′; Figure 6C) and ephrinB2 expression (Figure 6B h, h′; Figure 6C) was down-regulated. EphrinB2 expression in the absence of activated VEGFR2 also suggests a direct role for Notch signaling in regulating the expression of this arterial marker, as has recently been shown for the ventricular chamber.41
Blocking of VEGF signaling does not impair Notch signaling. (A) Regimen of antibody application. The neutralizing antibody against mouse VEGF (N-mVEGF) was injected into the tail vein on 2 consecutive days (E13.5 and E14.5), and embryos were analyzed at E15.5. (B) Analysis of VEGF-A and Notch activity. Staining for phosphorylated VEGFR2 and eNos as a downstream target of VEGFR2 confirms that VEGF-A signaling is effectively inhibited (a,b,e,f). VEGFR2 itself is still normally expressed (c,d). Notch activation (m,n), the expression of Notch ligands Dll1 and Dll4 (i,l) and of EphrinB2 (g,h) is not changed in N-mVEGF–treated embryos. Green indicates α-smooth muscle actin; red indicates antigen. a′ to n′ show magnifications. (C) Western blot analysis of markers analyzed in panel B. Actin was used as a loading control.
Blocking of VEGF signaling does not impair Notch signaling. (A) Regimen of antibody application. The neutralizing antibody against mouse VEGF (N-mVEGF) was injected into the tail vein on 2 consecutive days (E13.5 and E14.5), and embryos were analyzed at E15.5. (B) Analysis of VEGF-A and Notch activity. Staining for phosphorylated VEGFR2 and eNos as a downstream target of VEGFR2 confirms that VEGF-A signaling is effectively inhibited (a,b,e,f). VEGFR2 itself is still normally expressed (c,d). Notch activation (m,n), the expression of Notch ligands Dll1 and Dll4 (i,l) and of EphrinB2 (g,h) is not changed in N-mVEGF–treated embryos. Green indicates α-smooth muscle actin; red indicates antigen. a′ to n′ show magnifications. (C) Western blot analysis of markers analyzed in panel B. Actin was used as a loading control.
Collectively, these findings suggest that in mouse fetal arteries Notch signaling maintains arterial identity independent from the VEGF-A pathway.
Discussion
A role for Notch signaling in vascular development is firmly established. However, a specific role of the Notch ligand DLL1 in vessel development has been elusive. Here, we show that DLL1 is pivotal for activation of Notch in fetal arteries to maintain artery-specific gene expression and responsiveness to VEGFR2/NRP1-mediated VEGF signals.
DLL1 is a critical ligand for Notch activation in fetal arteries
Dll1 null mutant embryos show prominent hemorrhaging around E10.31 However, Dll1 expression in the vasculature was not detected before E13.5 (Figure S4), suggesting that the early hemorrhagic phenotype in Dll1 mutant embryos arises secondarily to the defects affecting tissue architecture and patterning, and does not reflect a specific requirement for DLL1 function in endothelial cells of early blood vessels, whereas DLL4 and JAG1 are essential for the early specification of the arterial fate, the regulation of tip cell behavior, and vascular remodeling.22,27,42,43 After E13.5, activation of Notch1, which is the essential Notch receptor in the vascular endothelium regulating embryonic and postnatal angiogenesis,23,44,45 was virtually abolished in arteries lacking DLL1. Surprisingly, NICD was not generated at detectable levels despite the presence of both DLL4 and JAG1 in the arterial endothelium, suggesting that in this context both DLL4 and JAG1 cannot activate Notch1. Consistent with our observation, endothelium-specific loss of JAG1 was shown to disrupt vascular smooth muscle cell development but did not affect endothelial Notch activation and arterial-venous differentiation.46 It has been proposed that, in E10.5 embryos, endothelial JAG1 acts by signaling to adjacent smooth muscle precursors to promote their differentiation, whereas DLL4 signals to adjacent endothelial cells to influence angiogenesis and arterial specification.46 Here, we report that signaling by DLL1 and Notch plays a critical and indispensable role in the differentiation of fetal arteries.
Loss of Notch1 activation was accompanied by down-regulation of genes specifically expressed in arterial, but not venous, endothelial cells. Previously, it was shown that expression of an activated Notch4 transgene in adult mice could induce venous expression of the ephrinB2 gene,47 indicating that Notch signaling can arterialize blood vessels. Our data demonstrate that also constant Notch activity is essential to maintain artery-specific gene expression. In addition to the down-regulation of arterial endothelium-specific genes, expression of smoothelin, a gene predominantly expressed in arterial vascular smooth muscle cells,48 was virtually absent from Dll1 mutant arteries. In addition, in mice mutant for Notch3, which is expressed in vascular smooth muscle cells of arteries but not of veins, smoothelin expression was severely down-regulated, but arterial endothelial markers were expressed normally,49 which suggests that the arterial identity of vascular smooth muscle cells is established independently from the underlying endothelium. Thus, the loss of smoothelin expression in Dll1 mutants might indicate that DLL1 is also a critical ligand for Notch3, rather than reflecting a secondary event arising as a consequence of altered endothelial identity. Loss of DLL1 activity in fetal vessels and down-regulation of arterial markers had remarkably mild morphologic consequences that we could detect. At present, the reason for the observed perinatal lethality of fetuses lacking DLL1 in the vascular endothelium is not completely clear, although the reduced arterial lumen might impair blood transport into the periphery. The DLL1 phenotype is also much milder than sustained ectopic Notch activity in vascular endothelium, which led to enlarged vessels, shunting, and eventually death.47 Loss of Notch(1) activity in early postnatal angiogenesis might reveal its significance only after longer time periods than we analyzed, or under pathophysiologic conditions, as has been observed for heterozygous Dll1 and Notch1 mutants.29,45
Dll1-mediated Notch activation is required for reception of VEGF signals
Studies in zebrafish have first indicated that Notch acts downstream of VEGF-A during formation of the large axial blood vessels of the trunk, determines arterial and venous cell fates in these vessels,25,28 and regulates angiogenesis.50,51 Similarly, studies of mammalian endothelial cells in culture have shown that VEGF-A administration induces Notch1 and Dll4 expression and thus placed the Notch pathway downstream of the VEGF-A pathway.52 During angiogenesis in the postnatal retina in response to VEGF, Dll4 expression is induced in the tip cell, whereas the activation of Notch signaling in neighboring endothelial cells is thought to suppress sprouting of these cells by suppressing the expression of Vegfr2 and thereby limiting the response to VEGF-A.42,53 In addition, Notch mediates the angiogenic effect of VEGF as response to hypoxia.45 Collectively, these data indicate that, in various contexts, Notch mediates VEGF-A signals in the vascular endothelium and, in part, acts in a negative feedback loop with VEGF signaling.
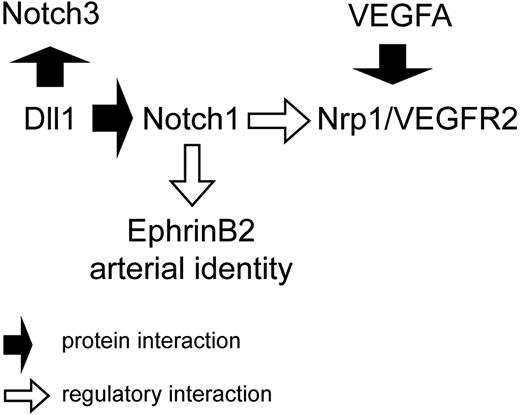
Our analysis has uncovered an additional interrelation between the VEGF-A and Notch pathways in fetal arterial endothelium (Figure 7). Here, Notch activity appears to be required for VEGF-A signaling through VEGFR2 and its coreceptor NRP1, because expression of both receptor and coreceptor was rapidly lost after Notch1 activation ceased. Conversely, inhibition of VEGF-A signaling had virtually no effect on expression of Notch1 and its ligands DLL1 and DLL4, and the generation of NICD, indicating that VEGF-A signals in large fetal arteries are not required for endothelial Notch1 activity. Instead, DLL1-mediated Notch activity appears to be required to render these endothelial cells responsive to VEGF-A signals. This does not exclude that under hypoxic or other physiologic conditions VEGF signals can further increase expression of Notch components and enhance Notch signals as observed in postnatal vessels.29,42,45,53
Proposed interrelation of Notch and VEGF-A signaling in fetal arteries. Dll1-mediated Notch activity maintains the responsiveness of arterial endothelial cells for VEGF-A. For details, see “Discussion.”
Proposed interrelation of Notch and VEGF-A signaling in fetal arteries. Dll1-mediated Notch activity maintains the responsiveness of arterial endothelial cells for VEGF-A. For details, see “Discussion.”
In summary, our results define a specific role for the Notch ligand DLL1 in arterial vascular endothelial cells during fetal stages of development. DLL1 acts nonredundantly to DLL4 and is required to maintain arterial identity, and in this developmental context, acts upstream of VEGF.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Katsuto Hozumi for providing the floxed Dll1 mice, Dietmar Vestweber for the anti-Endomucin antibodies, and Rui Benedito for help with the retina stains.
This work is supported by funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) for the Cluster of Excellence REBIRTH (From Regenerative Biology to Reconstructive Therapy).
Authorship
Contribution: I.S. performed research and collected, analyzed, and interpreted data; R.H.A. contributed vital new reagents; and A.G. designed research, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Achim Gossler, Institute for Molecular Biology OE5250, Medizinische Hochschule Hannover, Hannover, Germany; e-mail: Gossler.Achim@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal