Arteries and veins are structurally and functionally different types of vessels, and they display independent molecular signatures. A genetic program imposes arterial or venous fate onto embryonic precursors prior to formation of patent vessels and onset of blood circulation. This has been shown most elegantly in zebrafish and seems to hold true for mammals as well.1 However, there is enormous plasticity in this system, allowing complete cell fate changes in response to external stimuli during early embryogenesis. This property is progressively lost with developmental age.2
Blood vessels in the embryo are first formed by vasculogenesis, the coalescence of precursors to form vascular tubes and meshworks followed by remodeling and sprouting. The current paradigm of sprouting angiogenesis holds that vascular endothelial growth factor (VEGF) signaling through VEGF receptor 2 (Vegfr2), present on migrating tip cells of newly forming sprouts, induces Dll4 ligands. Dll4 in turn activates Notch signaling in following stalk cells, making them refractory to further VEGF-induced sprouting, in part by reducing Vegfr2 and its coreceptor Nrp1. Dll4 also induces expression of arterial markers like Hey1/2 bHLH transcription factors and Efnb2 ligands, and it promotes maturation and lumen formation. This is paralleled by repression of vein endothelial markers such as Nrp2, EphB4, and Coup-TFII, with the latter being involved in reciprocal antagonism with the Notch pathway components Hey1 and Hey2 to determine arterial versus venous cell fate.
Although this model of sprouting angiogenesis and arterialization has gained broad experimental support, there is increasing evidence that other components may be equally important. Limbourg et al showed that Dll1, a related Notch ligand, is essential for postnatal angiogenesis with heterozygous Dll1+/− mice exhibiting strongly impaired reperfusion after experimental hindlimb ischemia.3 A complete Dll1 knockout is lethal with bleeding around day 11 (E11) of embryonic development, but this seems to be due to defects in surrounding tissues and not related to intrinsic vascular functions. The availability of hypomorphic and conditional knockout alleles of Dll14 now allow Sörensen and colleagues to pinpoint a novel embryonic vascular defect that predicts a revised hierarchy of signaling.5
While Dll4 mutations (as well as mutations of Notch receptors or Hey1/2 bHLH effectors) show lethality after day 9.5 of embryonic development,1 Dll1 hypomorphs or mice with endothelial-specific Dll1 deletion survive until birth. Nevertheless, careful analysis of their blood vessels demonstrated that arterial identity is progressively lost from larger vessels starting at day 13.5, the time point when endothelial Dll1 expression should begin. Surprisingly, Dll4 is still expressed in these vessels, suggesting the existence of a switch making Dll4 incapable to sustain Notch activity and expression of arterial markers like Efnb2, Nrp1, or Hey1 and permitting expression of the vein marker Coup-TFII.
Interestingly, the reduction of Nrp1 expression and thus the impaired capacity of endothelia to respond to VEGF in these mice is not due to up-regulation of the venous regulator Coup-TFII, which gets expressed only later. Sörensen et al instead provide strong evidence that in arteries Nrp1 and perhaps VEGFR2 are directly induced by activated Notch receptors, which are absent from Dll1 negative or hypomorphic endothelia. Thus, VEGF cannot be placed upstream of the Dll/Notch cascade but rather it appears that Notch signaling enables VEGF responsiveness of these cells.
What is striking is the differential severity of endothelial defects in Dll1 versus Dll4 KO mice. While a lack of Dll4 leads to very early defects in angiogenesis and arterialization, this is much less so with Dll1. Although arterial markers are lacking and venous markers are up-regulated, this is still compatible with embryonic development. The perinatal lethality of the conditional endothelial Dll1 knockouts may be due to vascular problems, but this awaits further study. Nevertheless, it seems that arterialization defects may be tolerated in later development or adults for some time, at least as long as the animals are not subject to additional stress. This is reminiscent of the rather limited arterialization of grafted veins in human coronary arteries.6
The present study clearly shows that there are parallel and noncompensating Dll-Notch signals in endothelial cells. Dll4 may be more important early on in the capillary bed, while Dll1 preferentially acts in arteries. Yet, the molecular basis for the differential function of these very similar ligands acting on the same Notch1 receptor remains unresolved. The principle of a VEGF-Dll-Notch signaling cascade in endothelia may have to be revised for arteries with Dll1-Notch1 acting not as a target, but as a facilitator of VEGF sensitivity.
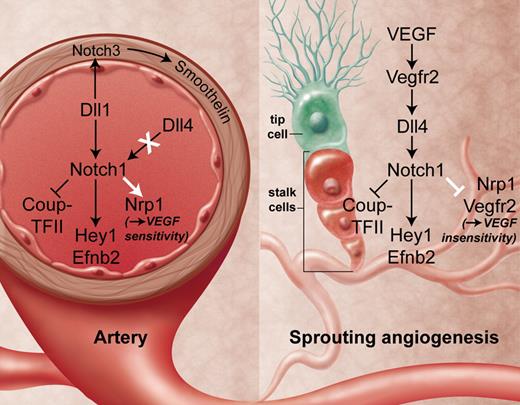
Vascular Dll-Notch signaling. In fetal arteries (left), only Dll1 and not Dll4 appear to signal through Notch1 receptors to block the venous program and to mediate arterial gene expression in endothelia and in the surrounding smooth muscle layer in a VEGF-independent manner. This contrasts with sprouting angiogenesis in the capillary bed (right), where Dll4 is the essential ligand that mediates the arterial program in stalk cells. VEGF induces this pathway and thereby reduces VEGF sensitivity in stalk cells. Professional illustration by A. Y. Chen.
Vascular Dll-Notch signaling. In fetal arteries (left), only Dll1 and not Dll4 appear to signal through Notch1 receptors to block the venous program and to mediate arterial gene expression in endothelia and in the surrounding smooth muscle layer in a VEGF-independent manner. This contrasts with sprouting angiogenesis in the capillary bed (right), where Dll4 is the essential ligand that mediates the arterial program in stalk cells. VEGF induces this pathway and thereby reduces VEGF sensitivity in stalk cells. Professional illustration by A. Y. Chen.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal